Back to Journals » Infection and Drug Resistance » Volume 16
Clinical Observation of Low-Temperature Plasma Ablation Combined with Drug Therapy in the Treatment of Fungal Keratitis
Authors Sun T, Zhang BW, Xiong R, Zhou WT, Qiu JJ
Received 8 December 2022
Accepted for publication 7 March 2023
Published 30 March 2023 Volume 2023:16 Pages 1895—1904
DOI https://doi.org/10.2147/IDR.S399715
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Tao Sun,1– 4 Bo-Wen Zhang,1– 4 Rui Xiong,1– 4 Wen-Tian Zhou,1– 4 Jing-Jing Qiu5
1Affiliated Eye Hospital of Nanchang University, Nanchang, Jiangxi, 330006, People’s Republic of China; 2Jiangxi Clinical Research Center for Ophthalmic Disease, Nanchang City, Jiangxi Province, 330006, People’s Republic of China; 3Jiangxi Research Institute of Ophthalmology and Visual Science, Nanchang City, Jiangxi Province, 330006, People’s Republic of China; 4Jiangxi Provincial Key Laboratory for Ophthalmology, Nanchang City, Jiangxi Province, 330006, People’s Republic of China; 5Department of Ophthalmology of The Second Affiliated Hospital of Nanchang University, Nanchang City, Jiangxi Province, 330006, People’s Republic of China
Correspondence: Jing-Jing Qiu, Department of Ophthalmology of The Second Affiliated Hospital of Nanchang University, No. 1, Minde Road, Nanchang City, Jiangxi Province, 330006, People’s Republic of China, Tel +86 15070001118, Email [email protected]
Objective: To observe the efficacy and prognosis of low-temperature plasma ablation + drug therapy in the treatment of fungal corneal ulcers.
Methods: The present paper presents a retrospective clinical study with a subject base of 34 eyes. Patients with a fungal corneal ulcer who visited the Affiliated Eye Hospital of Nanchang University between August 2019 and December 2021 were selected as the study participants. They were found to have highly reflective fungal hyphae in the corneal stroma layer via confocal microscope examination, which were revealed to be positive on etiology examination, with the ulcer and infiltration depths ≤ 1/2 of the corneal thickness. The efficacy and prognosis were observed after treatment with low-temperature plasma ablation + drug therapy.
Results: A total of 34 cases (34 eyes) had clinical manifestations of corneal infiltration and corneal ulcer formation, with a corneal lesion diameter of 1.31– 8.64 mm (average = 4.79 ± 2.03 mm). The average healing time of corneal ulcers was 6.2 ± 1.7 days. Among a total of 34 cases (34 eyes) in patients with fungal keratitis, the infection was controlled and the ulcers gradually healed after treatment with low-temperature plasma system + drug therapy in a total of 30 cases (30 eyes, 88%). A total of three cases (3 eyes, 9%) exhibited no clear improvement after the treatment, and the patients underwent conjunctival flap covering surgery. One case (one eye, 3%) exhibited no clear improvement after further treatment, with the patient experiencing corneal perforation and ultimately undergoing penetrating keratoplasty.
Conclusion: Low-temperature plasma ablation + drug therapy can effectively control the progression of fungal keratitis infection, as well as significantly shorten the ulcer healing time, and is, therefore, an effective method.
Keywords: low-temperature plasma, keratitis, fungal infection, antimicrobial therapy, ophthalmic surgery
Introduction
Fungal keratitis is a type of blindness-causing keratopathy caused by pathogenic fungal infection and ranks first in the rate of blindness due to infectious keratitis in China.1 Fungal keratitis does not respond well to drugs, and there are few types of antifungal drugs that have shown efficacy, greatly limiting the clinical choice. While on the other, due to the characteristics of fungal corneal ulcers being covered by colonies and the weak permeability of the cornea to the drug, the absorption of drugs is adversely affected. These two factors clearly reduce the antifungal drug response rate. Common surgical treatments include conjunctival flap covering surgery, lamellar keratoplasty, and penetrating keratoplasty. Conjunctival flap covering can be used to effectively apply inflammatory mediators and transport the drug in blood vessels to the surface of corneal ulcers, thus promoting their repair. However, after the ulcer is repaired, the blood vessels of the conjunctiva cannot completely subside, leading to a serious decline in corneal transparency, thereby, affecting vision. Following conjunctival flap covering surgery, most patients require further keratoplasty to improve their vision following inflammation control. Keratoplasty is also an option when fungal corneal ulcers occur. However, when keratoplasty is performed in the inflammatory period, there is the possibility of fungal recurrence. A fungal corneal ulcer increases the chance of transplant rejection since anti-hormone rejection drugs cannot be used following keratoplasty. Therefore, it is critical to research and develop effective and safe treatments for fungal keratitis (FK), to slow down the adverse impact on the vision of patients with FK.
The past decade has seen the rapid application development of low-temperature plasma in the fields of medicine and medical therapy. As a surgical tool, plasma has significant effects in terms of cutting, cauterizing, drying, or coagulating blood and tissues;2 however, in electrosurgery, the high-temperature characteristics of traditional thermal plasma result in unnecessary damage to tissues, often leading to cell water loss, protein denaturation, and tissue inactivation.3 The recently emerging microplasma (low-temperature plasma generated in the tiny-volume cavity gap) has been gradually changing the current treatment status.4 The average temperature of this microplasma is 25°C–28°C, and the ablation depth is approximately 50 μm, eliminating the risk of tissue damage.3 As a result, microplasma and other “cold” plasma technologies have been rapidly introduced into the areas of dermatology, oncology, otorhinolaryngology, gastroenterology, and stomatology.5,6 Relatively speaking, the application of atmospheric pressure low-temperature plasma technology has been significantly slower in ophthalmology than in other medical disciplines. While the antibacterial effect of plasma on various ocular pathogens has been reported, few studies have been conducted on fungal keratitis.4,6 Low-temperature plasma jets have been proven to be a highly effective method in healing rabbit cornea infected with Candida albicans. Generating low-temperature plasma micro-columns, the jet array is particularly suitable for the ophthalmic treatment of fungal infection.7
Therefore, in the present study, we aim to assess the treatment efficacy and recovery prognosis of low-temperature plasma ablation combined with drug therapy in the treatment of fungal keratitis.
Materials and Methods
Participants
A total of 34 patients (34 eyes) with fungal keratitis treated using low-temperature plasma ablation + drug therapy who visited the Affiliated Eye Hospital of Nanchang University between August 2019 and December 2021 were retrospectively observed. The patients comprised 26 males and eight females, who were aged 31–75 (average age = 59.0 ± 10.3) and had a preoperative best corrected visual acuity (BCVA) light perception of ~0.7.
The inclusion criteria included the following under corneal confocal microscope observation: (1) suspected fungal hyphae growth was found between corneal stroma layers; (2) the results of corneal scraping, conjunctival sac discharge, or corneal swab culture were positive; (3) the anterior segment optical coherence tomography (OCT) confirmed an infiltration depth of ≤1/2 of the corneal thickness; and (4) the B-ultrasound indicated no obvious vitreous opacity. The exclusion criteria included the presence of concomitant endophthalmitis, other eye diseases (eg, glaucoma and iridocyclitis), and/or systemic diseases (eg, diabetes or other autoimmune diseases).
All patients signed the informed consent form, thus meeting the ethical requirements.
Methods
Medical History
The patient was asked to provide his/her medical history in detail after admission. This included whether they had a history of trauma caused by plants and/or soil or whether they had undergone long-term local and/or systemic application of glucocorticoids and antibiotics.8
Ocular Examination
After admission to the hospital, the patients underwent routine eye examinations. (1) Corneal scrapers, conjunctival sac secretions, or corneal swabs were taken for culturing; (2) the depth of infection infiltration was confirmed through a slit-lamp microscope and anterior segment OCT examination; and (3) endophthalmitis was ruled out via ocular B-ultrasound examination. First, the mycelium was identified using a confocal microscope before, based on the results of the smear culture, the corresponding antifungal therapy was applied. The effects of the drug treatment were observed under a slit-lamp microscope on a daily basis, which included observing whether (1) the ulceration scope narrowed, (2) the boundary was clear, (3) the depth of infiltration decreased, (4) the hypopyon was absorbed, (5) the pseudopodia had subsided, and (6) the epithelium was scarred.
Treatment methods
Topical Eye Drop Therapy
All patients were treated with 1% voriconazole eye drops (10 mg/mL sterile water for injection) once every 15 min, amphotericin b eye drops (0.25 mg/mL sterile water for injection) once every 15 min, 0.5% levofloxacin eye drops (Santen Pharmaceutical Co., Ltd.) (qid), pranoprofen eye drops (Senju Pharmaceutical Co., Ltd.) (tid), and oral voriconazole tablets (SANDOZ Pharmaceutical Co., Ltd.) (400 mg for the first dose, then 200 mg bid).
In terms of medication, after 3–5 days, if the ulcer exhibited no healing tendency, the infiltration range exhibited no limiting tendency, and the infiltration depth was judged to be less than 1/2 of the corneal thickness through the slit-lamp microscope and anterior segment OCT results, patients who were unwilling to undergo keratoplasty or were not suitable for conjunctival flap covering were treated with the low-temperature plasma system for ablation.
Low-Temperature Plasma Radiofrequency Ablation Surgery Method
The patient was placed in a supine position, and the operative eye was covered by a routine surgical field disinfection drape. Oculomotor nerve block anesthesia was performed on the operative eye, with the eye opened to wash the conjunctival sac with sodium chloride injection. Visible corneal edema (gray in color) signified that the fungal infection had deeply infiltrated the focal area. The fungal coating on the corneal surface was scraped off with a 45 blade, and the focal surface was polished with an MC-409 low-temperature plasma cutter (energy = 40 mW) from the focal center. A voriconazole injection (5 mg/mL sterilized water for injection) was injected into the corneal stroma of the focal area. After the operation, the eye was bandaged.
Postoperative Therapy
On the day of operation, 1% voriconazole eye drops (10 mg/mL sterile water for injection) once every 30 min, amphotericin b eye drops (0.25 mg/mL sterile water for injection) once every 30 min, 0.5% levofloxacin eye drops (Santen Pharmaceutical Co., Ltd.) (qid), pranoprofen eye drops (Senju Pharmaceutical Co., Ltd.) (tid), and oral voriconazole tablets (SANDOZ Pharmaceutical Co., Ltd.) (200 mg bid) were administered.
On postoperative day 1 until discharge, 1% voriconazole eye drops (10 mg/mL sterile water for injection) once every hour, amphotericin b eye drops (0.25 mg/mL sterile water for injection) once every h, 0.5% levofloxacin eye drops (Santen Pharmaceutical Co., Ltd.) (qid), pranoprofen eye drops (Senju Pharmaceutical Co., Ltd.) (tid), and oral voriconazole tablets (SANDOZ Pharmaceutical Co., Ltd.) (200 mg bid) were administered.
Outcome Measures
Visual acuity, intraocular pressure, and slit-lamp examinations were conducted daily for three days after the operation. The international standard Snellen chart was used to record the visual acuity (converted to LogMAR for statistical analysis). Light perception, manual, and index were assigned 2.6, 2.4, and 2.0 LogMAR, respectively.9 The BCVA values and the size of the infiltration or scar, the epithelial re-formation time, the corneal perforation rate, and/or therapeutic penetrating keratoplasty10,11 at postoperative day 3 and week 3 were used to evaluate the treatment outcomes. Re-epithelization is defined as the absence of epithelial defects, determined by fluorescein sodium staining.12
The statistical analysis was conducted using SPSS 23.0 software. Measurement data conforming to normal distribution were expressed as mean ± standard deviation ( ). The paired sample t-test was used to compare the largest transverse meridian of corneal lesion before and after surgery. The measurement data of non-normal distribution was expressed by median (M; P25, P75), and the Wilcoxon signed-rank test was used before and after the operation. A P value of <0.05 was considered statistically significant.
). The paired sample t-test was used to compare the largest transverse meridian of corneal lesion before and after surgery. The measurement data of non-normal distribution was expressed by median (M; P25, P75), and the Wilcoxon signed-rank test was used before and after the operation. A P value of <0.05 was considered statistically significant.
Results
Clinical Manifestations
The present study comprised 34 cases (34 eyes) of fungal keratitis, which included 21 cases (21 eyes, 62%) with a definite history of phytotrauma, 10 cases (10 eyes, 29%) with no obvious inducement, and three cases (three eyes, 9%) with a definite history of dust and sand contact. A total of 34 cases (34 eyes) had clinical manifestations of corneal infiltration and corneal ulcer formation, with the largest corneal lesion diameter of 1.31–8.64 mm (average = 4.79 ± 2.03 mm) (Table 1 and Table 2).
 |
Table 1 Characteristics of Foci in Hospitalized Patients’ Affected Eyes |
 |
Table 2 Culture Characteristics of Pathogens |
Postoperative Efficacy
After day 1 of the low-temperature plasma ablation treatment, the corneal focus boundary in all patients with fungal keratitis cleared, and there was no significant aggravation at the place of infiltration. However, mild edema manifested at the surgical wound of the cornea, which, in general, gradually subsided five days later. After day 1–day 3 of the low-temperature plasma ablation, the corneal lesions gradually re-epithelized. In 30 cases (30 eyes, 88%), the infection was controlled, and the ulcer gradually healed after treatment with low-temperature plasma system + drug therapy. Furthermore, three cases (three eyes) (9%) exhibited no clear improvement following treatment with low-temperature plasma system + drug therapy, but then gradually improved after undergoing conjunctival flap covering surgery. In one case (one eye) (3%), there was no improvement following treatment, and the lesion gradually spread into the deep layer, quickly causing corneal perforation. This patient ultimately underwent penetrating keratoplasty.
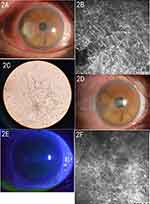
A total of 33 cases (33 eyes) were cured using low-temperature plasma ablation, and in the 1–6-month postoperative follow-up, the patients had completely healed ulcers, as well as re-epithelialization, no infiltration of the corneal stroma, or corned macula or leukoplakia left by scarring in the focus area. The re-examination via corneal confocal microscopy revealed no hyphae. The last postoperative slit-lamp microscope examination of the cured patients revealed a maximum diameter of 1.04–7.98 mm (average = 4.72 ± 2.11 mm) among the ulcers in the focus area, with no significant difference (t = 0.139, P = 0.890) compared with that before surgery (average = 4.79 ± 2.03 mm). Furthermore, the BCVA (log MAR) (0.09 [0.64 ± 0.26]) was improved, with the difference statistically significant (Z = 3.918, P < 0.001) compared with that before operation (0.15 [1.29 ± 0.84]) (Figures 1 and 3, Table 3).
 |
Table 3 Changes in the Visual Acuity of 33 Cases (33 Eyes) at Postoperative Day 3 and Week 3 |
 |
Figure 3 Boxplot of patient’s visual acuity test at postoperative day 3 and week 3. Notes: The postoperative visual acuity was improved with statistical significance. ***P < 0.001. |
Discussion
In this study, 30 patients with fungal keratitis with poor drug efficacy were treated with low-temperature plasma combined with drug therapy. Thirty eyes of 30 patients (88%) were cured, and no cases of infection recurred during 1–6 months postoperative follow-up. The visual acuity of 23 eyes (68%) was improved in different degrees during follow-up compared with that at admission. In this study, the low-temperature plasma radiofrequency ablation technology was applied to the treatment of ophthalmology diseases (mycotic keratitis direction) and combined with antibacterial drugs, and satisfactory results were obtained. The lack of treatment methods for fungal corneal ulcers, as well as the poor treatment effects, has forced many researchers to conduct relevant research and development. At present, to save the residual vision of patients with fungal keratitis, protect their life and health, and improve their quality of life, many research institutions combine images with artificial intelligence or develop various complex and precise instruments to create safer and more efficient treatment methods for the purpose of timely diagnosis and precise treatment.13
One of these methods, the low-temperature plasma ablation technique, is gradually maturing. This technique has been widely used in all kinds of operations in clinical practice due to its merits, which include small trauma to tissues, good safety, minimal invasiveness, convenience, and accuracy. The method’s main working principle is the formation of a highly concentrated plasma area around the electrode through a conductive medium, where highly ionized particles act on human tissue cells, breaking the organic molecular chains in the cells to separate molecules from each other and shrink the tissues. Since the current does not directly pass through the tissue, the treatment temperature is low and controllable, and the tissue fever is minimal, with the tissue often ablated at a fixed point at 40°C–70°C, thus protecting the normal mucosa and surrounding structures to the greatest possible extent, relieving the patient’s postoperative pain, and shortening the postoperative recovery time.14
The MECHAN (MC409) low-temperature plasma ablation system was used for testing. It was simultaneously supplemented with normal saline infusion during the operation to keep the working temperature of the plasma cutter at 25°C–28°C. The surface of the lesion was scraped repeatedly to remove fungal colonies, exfoliate epithelial tissues, and expose the healthy matrix to promote healthy corneal epithelium crawling and growing for re-epithelization, thereby completing the ulcer repair. Moreover, the exposed corneal stroma can absorb antibacterial drugs more fully, which has a supporting effect on the improvement of the antibacterial drugs’ curative effect. In addition, based on the characteristics of breaking molecular bonds by low-temperature plasma, the hyphae of fungi can be directly lyzed to prevent fungi from infiltrating into healthy tissues.
In this study, in the patients with fungal corneal ulcer treated with low-temperature plasma ablation combined with drugs. The visual acuity of the majority (90%) of the patients improved to varying degrees, and the effect of visual acuity was directly related to the size of the ulcer and the range of the covered pupil area. The ulcers in 30 eyes were obviously limited and no longer developed deeper after low-temperature plasma ablation combined with drug treatment. Corneal re-epithelialization in the lesion area was observed—the median time of re-epithelialization was 7 days, and the mean time was 6.2 ± 2.7 days. Moe et al reported in a retrospective study that 103 samples of fungal keratitis were treated with local antifungal drugs only, and the median time for re-epithelialization of affected eyes was 30 days.15 It has also been reported that 5% natamycin and 1% voriconazole are effective primary agents for the treatment of fungal keratitis, and the mean healing time of ulcers is as long as 24.3 days and 27.2 days, respectively.16 In conclusion, the re-epithelialization time of low-temperature plasma ablation combined drug therapy is significantly shorter than that of local drug therapy alone.
The low-temperature plasma radiofrequency ablation caused little damage to the surrounding tissue, and the patients did not complain of obvious discomfort after surgery—the symptoms of eye irritation were less severe than those of previous debridement by mechanical scraping with surgical blades (mainly manifested in postoperative tingling, foreign body sensation, tears, and other symptoms). In three cases, the ulcer covered the pupil area of three eyes, and the lesion had a tendency of deep infiltration. To promptly control the progression of the disease and avoid further damage to the visual quality of the affected eye, the postoperative treatment was combined with conjunctival flap covering, and the ulcer lesions were gradually limited with clear boundaries. Fusarium was cultured in two out of three patients undergoing conjunctival flap covering in three eyes, which may be related to the protective hydrophobic protein and rodlike layer covering Fusarium cells to avoid recognition by immune cells,17 and the special biological activity of Fusarium that requires higher concentration of specific antifungal drugs to inhibit Fusarium growth.18 After low-temperature plasma ablation combined with drug treatment, the lesions did not improve in 1 case and 1 eye, and the infiltration gradually developed deeper. Corneal perforation occurred on the 2nd day after the surgery, and penetrating keratoplasty was finally performed. Although no positive bacteria were cultured in this case, considering the large area of the ulcer lesions and the pyemia in the anterior chamber during hospitalization, it may be related to the fungus penetrating the stroma layer and invading the anterior chamber.
In summary, fungal keratitis has a constantly increasing prevalence and incidence with a long disease course and poor efficacy. The disease mainly occurs in low-income areas, and its high blindness rate brings a heavy burden to individuals and families. The present study revealed that low-temperature plasma ablation + drug therapy has a high recovery rate (97%) with a good prognosis for patients with fungal keratitis with an infiltration depth of <1/2 of the corneal thickness. The treatment method can significantly reduce the blindness rate; however, due to the small sample size, the lack of a control group, and the short follow-up time in some patients in the present study, further studies are required to determine the indication and long-term efficacy of superficial fungal keratitis. The specific mechanism of the efficacy of low-temperature plasma on fungal keratitis requires further exploration.
Conclusions
At present, fungal keratitis has a constantly increasing prevalence and incidence with a long disease course and poor efficacy, bringing a heavy burden to individuals and families. The present study reveals that low-temperature plasma ablation + drug therapy has a high recovery rate (97%) with a good prognosis for fungal keratitis patients with an infiltration depth of <1/2 of the corneal thickness, while it can also significantly reduce the blindness rate. However, due to the small sample size, lack of a control group, and short follow-up time, further studies are required for the indication and long-term efficacy of superficial fungal keratitis.
Ethics Approval and Consent to Participate
This study was conducted in accordance with the declaration of Helsinki. This study was conducted with approval from the Ethics Committee of The Second Affiliated Hospital of Nanchang University (2020-XJS-01). A written informed consent was obtained from all participants.
Funding
General project of key research and development plan of Jiangxi Provincial Department of Science and Technology (20203BBGL73191) Science and Technology Plan Project of Jiangxi Provincial Health Commission (202130556).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Gay-mimbrera J, García MC, Isla-Tejera B, et al. Clinical and biological principles of cold atmospheric plasma application in skin cancer. Adv Ter. 2016;33:894–909.
2. von Woedtke T, Reuter S, Masur K, Weltmann K-D. Plasmas for medicine. Phys Rep. 2013;530:291–320.
3. heaselgrave W, Shama G, Andrew PW, Kong MG. Inactivation of Acanthamoeba spp. and other ocular pathogens by application of cold atmospheric gas plasma. Appl Environ Microbiol. 2016;82:3143–3148. doi:10.1128/AEM.03863-15
4. Park CH, Lee JS, Kim JH, et al. Wound healing with nonthermal microplasma jets generated in arrays of hourglass microcavity devices. J Phys D Appl Phys. 2014;47:435402. doi:10.1088/0022-3727/47/43/435402
5. Eden JG, Park S-J, Ostrom NP, et al. Microplasma devices fabricated in silicon, ceramic, and metal/polymer structures: arrays, emitters and photodetectors. J Phys D Appl Phys. 2003;36:2869. doi:10.1088/0022-3727/36/23/001
6. Ksh PHJ, Ju WJH, Ju HW, et al. Microplasma jet arrays as a therapeutic choice for fungal keratitis. Sci Rep. 2018;8(1):2422. doi:10.1038/s41598-018-20854-8
7. Corneopathy Group. Society of Ophthalmology, Chinese Medical Association Expert consensus on clinical diagnosis and treatment of infectious keratopathy (2011). Chin J Ophthalmol. 2012;48(1):72–75. doi:10.3760/cma.j.issn.0412-4081.2012.01.019
8. schulze-bonsel K, Feltgen N, Burau H, Hansen L, Bach M. Visual acuities “hand motion” and “counting fingers” can be quantified with the Freiburg visual acuity test. Invest Ophthalmol Vis Sci. 2006;47(3):1236–1240. doi:10.1167/iovs.05-0981
9. narayana S, Krishnan T, Ramakrishnan S, et al. Mycotic antimicrobial localized injection. Ophthalmology. 2019;126(8):1084–1089. doi:10.1016/j.ophtha.2019.03.020
10. Prajna NV, Krishnan T, Mascarenhas J, et al. The mycotic ulcer treatment trial. JAMA Ophthalmol. 2013;131(4):422–429. doi:10.1001/jamaophthalmol.2013.1497
11. Srinivasan M, Mascarenhas J, Rajaraman R, et al. Corticosteroids for bacterial keratitis. Arch Ophthalmol. 2012;130(2):143–150. doi:10.1001/archophthalmol.2011.315
12. Liang L, Zhang CY, Zhang C, et al. Pathogenic analysis of suppurative keratitis. New Progress Ophthalmol. 2008;(10). doi:10.13389/j.cnki.rao.2008.10.010
13. Wei ZZ, Liang QF. Progress of clinical diagnosis and treatment in fungal keratitis. Chin J Ophthalmol. 2020;56(8):631–636. doi:10.3760/cma.j.cn112142-20191120-00586
14. Hu YJ, Sun Y, Gu WZ, Dong HJ, Xu H. Application experience of low-temperature plasma ablation in the operation of nasal inverted papilloma. Chin J Otolaryngol Integr Tradit Chin West Med. 2022;30(1):21–25,72. doi:10.16542/j.cnki.issn.l007-4856.2022.01.006
15. Moe CA, Lalitha P, Prajna NV, et al. Outcomes of amoebic, fungal, and bacterial keratitis: a retrospective cohort study. PLoS One. 2022;17(2):e0264021. PMID: 35171970; PMCID: PMC8849599. doi:10.1371/journal.pone.0264021
16. Arora R, Gupta D, Goyal J, Kaur R. Voriconazole versus natamycin as primary treatment in fungal corneal ulcers. Clin Exp Ophthalmol. 2011;39(5):434–440. PMID: 21105974. doi:10.1111/j.1442-9071.2010.02473.x
17. Mills B, Radhakrishnan N, Karthikeyan Rajapandian SG, Rameshkumar G, Lalitha P, Prajna NV. The role of fungi in fungal keratitis. Exp Eye Res. 2021;202:108372. PMID: 33249061. doi:10.1016/j.exer.2020.108372
18. Manikandan P, Abdel-Hadi A, Randhir Babu Singh Y, et al. Fungal keratitis: epidemiology, rapid detection, and antifungal susceptibilities of fusarium and aspergillus isolates from corneal scrapings. Biomed Res Int. 2019;2019:6395840. PMID: 30800674; PMCID: PMC6360544. doi:10.1155/2019/6395840
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.