Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 17
Clinical Misdiagnosis of Cutaneous Malignant Tumors as Melanocytic Nevi or Seborrheic Keratosis: A Retrospective Analysis of a Chinese Population
Authors Zhang J , Wang Y, Zhang W, Cai L, Feng J, Zhu Y, Lu H
Received 22 November 2023
Accepted for publication 18 February 2024
Published 26 February 2024 Volume 2024:17 Pages 465—476
DOI https://doi.org/10.2147/CCID.S451288
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Jun Zhang,1 Yu Wang,1 Wei Zhang,1 Linglong Cai,1 Jianglong Feng,2 Yiwei Zhu,1 Hongguang Lu1
1Department of Dermatology, the Affiliated Hospital of Guizhou Medical University, Guiyang, People’s Republic of China; 2Department of Pathology, the Affiliated Hospital of Guizhou Medical University, Guiyang, People’s Republic of China
Correspondence: Hongguang Lu, Department of Dermatology, the Affiliated Hospital of Guizhou Medical University, No. 28 Guiyi Street, Guiyang, Guizhou, 550001, People’s Republic of China, Tel +86-13885144269, Fax +86-851-86820346, Email [email protected]; [email protected]
Purpose: The rising incidence and mortality associated with cutaneous malignant tumours highlight the importance of early diagnosis of these tumors. In clinical practice, these tumors are often misdiagnosed as benign skin lesions such as melanocytic nevi (MN) and seborrheic keratosis (SK) because of their similar morphologic features. The incidence and clinicopathological subtypes of cutaneous malignancies in East Asia populations significantly differ from those in fair-skinned groups. However, studies on misdiagnoses in Eastern countries are lacking. Therefore, this study focused on the clinical and pathological features of cutaneous malignant tumors misdiagnosed as MN or SK in a Chinese population.
Patients and Methods: A total of 4592 samples clinically diagnosed as MN (n = 3503) or SK (n = 1089) from July 2014 to June 2022 were collected and evaluated retrospectively. The clinical and pathological data were analyzed to identify associated factors.
Results: Pathological assessments showed that 2.5% (86/3503) of the specimens clinically diagnosed as MN were malignancies, predominantly basal cell carcinoma (BCC, 84.9%, 73/86), followed by malignant melanoma (MM, 8.1%, 7/86) and squamous cell carcinoma (SCC, 7.0%, 6/86). Similarly, 5.7% (62/1089) of the specimens clinically diagnosed as SK were malignant tumors, of which BCC (50.0%, 31/62) was the most common, followed by SCC (41.9%, 26/62) and MM (8.1%, 5/62). In both types of specimens, advanced age and facial lesions were risk factors for malignancy misdiagnosis. The malignancy rate, mean age, and proportion of SCC in the specimens clinically diagnosed as SK were higher than those in the specimens clinically diagnosed as MN. Dermoscopy significantly reduced the rate of misdiagnosis of these tumors as MN or SK.
Conclusion: In China, cutaneous malignant tumors misdiagnosed as MN or SK are not uncommon in clinical practice, and active introduction of noninvasive diagnostic techniques is essential to distinguish them.
Keywords: malignant melanoma, squamous cell carcinoma, basal cell carcinoma, skin benign neoplasms, dermoscopy, China
Introduction
The growing prevalence of skin tumors, especially cutaneous malignant tumors, poses a prominent public health challenge worldwide, and has attracted attention from clinicians and researchers.1,2 Early diagnosis is fundamental to reduce the complications and mortality associated with malignant tumors.3 However, cutaneous malignant tumors are frequently misdiagnosed as melanocytic nevi (MN) or seborrheic keratosis (SK), and the misdiagnosis rate is not low.4–8 In particular, special attention should be given to malignant melanoma (MM), the most lethal type of skin cancer, which is often misdiagnosed as MN or SK.4–6,9 MN and SK are the two most prevalent benign neoplasms in dermatology.10–12 In China, many patients with MN or SK visit dermatologists because of concerns regarding malignancy or cosmetic needs,4 and clinicians in primary hospitals and even some tertiary hospitals habitually use minimally invasive methods such as laser treatment or cryotherapy to treat those conditions, which may result in misdiagnosis and inappropriate treatment of malignant tumors. The prevalence and clinicopathologic features of skin cancer vary markedly across different races or skin types.1,13 Previous studies on the misdiagnosis of skin cancer were mainly conducted in Western countries, with a notable gap in research from East Asia.13 This study aimed to identify the risk factors for misdiagnosis of cutaneous malignant tumors as MN or SK in the Chinese population by using data obtained from a tertiary teaching hospital in China from July 2014 to June 2022, to guide clinical practices and reduce misdiagnoses in similar demographic groups.
Materials and Methods
Patients and Methods
This retrospective study examined all surgical and biopsy specimens initially diagnosed as MN or SK at the Department of Dermatology in the Affiliated Hospital of Guizhou Medical University (Guizhou Province, China) from July 2014 to June 2022. The specimens were subjected to pathological examination. If a specimen had multiple suspected diagnoses, it was included in the analysis on basis of the first diagnosis. Congenital MN, halo nevi, and specimens with unclear histopathological diagnosis were excluded. Out of 4986 MN and SK specimens, 4592 were analyzed, which included 3503 MN and 1089 SK specimens. To ensure accuracy of pathological diagnosis, two dermatopathologists (Wang and Cai) reviewed and diagnosed all hematoxylin and eosin (HE)-stained sections independently. In case of disagreement between the assessments by the two dermatopathologists, a third pathologist (Feng) was consulted to reach the final diagnosis after discussion. This study adhered to the diagnostic criteria outlined in the Third Edition of Dermatology edited by Bolognia JL et al.10 General information of the patients, such as sex, age, lesion location, and the date of visit, was collected from the histopathology submission forms. This study was approved by the Ethics Committee of the Affiliated Hospital of Guizhou Medical University. No identifiable patient data were included in this study.
Statistical Analysis
A descriptive analysis was performed to evaluate the clinical and histopathological data, including sex, age, lesion location, and characteristics of the misdiagnosed tumors, in patients with clinically diagnosed MN and SK. Normally distributed data such as age were presented as mean ± standard deviation (95% confidence interval [CI]) and further analyzed using t test, while non-normally distributed data were presented as median (first quartile-third quartile, Q1-Q3) and compared using nonparametric tests. The differences in cutaneous malignant tumor frequencies were assessed using chi-square test and, for comparisons with a small sample size, using Fisher’s exact test. A two-tailed P value less than 0.05 was considered statistically significant. All statistical analyses in this study were performed using the SPSS22.0 software package (IBM, Armonk, NY, USA).
Results
Patient Information Collected from the Histopathology Submission Forms
A total of 4592 specimens from 3942 patients (median age, 38 years; Q1-Q3, 26–55 years; age range, 1–94 years), including 1363 male and 2579 female patients, were analyzed in this study. A substantial proportion of the specimens was obtained from patients aged 21–40 years (40.7%) and 41–60 years (27.1%), followed by those aged >60 years (17.5%) and ≤20 years (14.6%) (Figure 1). The most common site for specimen collection was the face (44.8%), followed by the trunk (20.7%), limbs (17.8%), scalp and neck (13.2%), and buttocks and perineum (3.4%) (Figure 2).
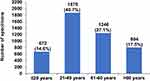 |
Figure 1 Age distribution of the patients whose specimens were submitted. Note: The values are presented as number (%). |
 |
Figure 2 Location distribution of the submitted specimens. Note: The values are presented as number (%). |
Among the specimens submitted for histopathological assessments, 3503 (76.3%) were initially diagnosed as MN, while 1089 (23.7%) were initially diagnosed as SK. A sex-based disparity was observed, with a higher prevalence of female patients in the MN group (P < 0.001, Table 1). In terms of age, the MN group predominantly consisted of individuals aged 21–40 years, and the mean age in the MN group was significantly lower than that in the SK group, which mostly consisted of patients aged >60 years old (all P < 0.001, Table 1). Additionally, a notable difference was found in the distribution of tumor locations between the two groups, with MN primarily located on the face and SK more commonly found on both the face and trunk (P < 0.001, Table 1).
 |
Table 1 Characteristics of the Patients Whose Specimens Were Submitted (Cases, %) |
Cutaneous Malignant Tumors Misdiagnosed as MN
Histopathological review of the specimens clinically diagnosed as MN indicated diseases other than MN in 590 (16.8%) specimens. Notably, 86 (2.5%) specimens showed pathological findings for cutaneous malignant tumors, including BCC, SCC, and MM. Among the diseases misdiagnosed, SK was predominant, accounting for 61.2% (361/590) of the specimens. The next most frequently misdiagnosed diseases were cutaneous malignant tumors, which accounted for 14.6% (86/590) of the specimens. Demographic analysis showed a higher probability of misdiagnosis in male patients (P < 0.001, Table 2), while sex was not significantly associated with the occurrence of cutaneous malignant tumors (P = 0.147, Table 2). Age was linked to the rate of misdiagnosis as MN, with misdiagnosed patients being significantly older than that those who were correctly diagnosed (median age, 50 years [Q1-Q3, 40–62 years] vs median age, 30 years [Q1-Q3, 22–38 years]; P < 0.001). Similarly, among the cases misdiagnosed as MN, the age of patients with cutaneous malignant tumors (median age, 58 years [Q1-Q3, 48–66 years]) was obviously higher than that of patients without cutaneous malignant tumors (median age, 49 years [Q1-Q3, 38–60 years]). Age-group analysis showed that both the incidence of overall misdiagnosis and the rate of cutaneous malignant tumors increased with age, with significant distribution differences (all P < 0.05, Table 2). The lesion location on the body was also correlated with the overall misdiagnosis and incidence of cutaneous malignant tumors. Our findings revealed that overall misdiagnosis occurred more frequently in the specimens from the buttocks, perineum, and trunk (lesion locations, P < 0.001, Table 2) in comparison with specimens from other body areas. Cutaneous malignancies were found more frequently on the face (P < 0.001, Table 2), as was BCC (P < 0.001, Table 2). However, the incidence of SCC and MM did not significantly differ in terms of location (Table 2).
 |
Table 2 Patient Characteristics in Cases of Malignant Tumors That Were Clinically Diagnosed as Melanocytic Nevi (Cases, %) |
Cutaneous Malignant Tumors Misdiagnosed as SK
On the basis of the pathological diagnosis criteria, 238 (21.9%) specimens clinically diagnosed as SK were found to be misdiagnosed. Among these, 62 cases of skin cancer, including BCC, cutaneous SCC, and MM, had been clinically diagnosed as SK, representing 5.7% of the SK specimens. Viral warts constituted the majority of the misdiagnosed diseases, accounting for 22.7% (54/238) of the specimens misdiagnosed as SK, while skin cancers constituted 26.1% (62/238) of these specimens. Statistical analysis suggested that sex was associated with the incidence of misdiagnosis, with female patients being more prone to misdiagnosis than males (P = 0.02, Table 3), although no significant sex-related difference was observed in the misdiagnosis of cutaneous malignancies (P = 0.063, Table 3). The overall age of the misdiagnosed patients (median age, 63 years [Q1-Q3, 50–73 years]) was not significantly higher than that of the accurately diagnosed patients (median age, 61 years [Q1-Q3, 51–70 years], P = 0.161). In the misdiagnosed subgroup, patients with cutaneous malignant tumors were considerably older (median age, 71 years [Q1-Q3, 58–77 years]) than those without malignancies (median age, 60 years [Q1-Q3, 48–70 years], P < 0.001). Although the incidence of skin malignancies misdiagnosed as SK increased with age, significant differences in age distribution were only noted for cutaneous SCC (P = 0.007, Table 3). Significant differences related to lesion locations were observed in the specimens misdiagnosed as SK (P < 0.001, Table 3), with the face being the most common site of such specimens. Likewise, skin cancer occurred more frequently on the face than on other areas (P < 0.001, Table 3). Among all skin cancer types, the location distribution of BCC was similar to the overall distribution of skin cancers (P = 0.009). In addition, the location distribution of SCC did not significantly differ from that of MM (Table 3).
 |
Table 3 Patient Characteristics in Cases of Malignant Tumors That Were Clinically Diagnosed as Seborrheic Keratosis (Case, %) |
Among the specimens clinically diagnosed as SK, 35 (3.2%, 35/1089) were identified as actinic keratosis, a common precancerous skin lesion. These cases showed significant differences in sex, age, and lesion location distributions (P = 0.007, P = 0.005, and P < 0.001, respectively).
Occurrence of Cutaneous Malignant Tumors in the Submitted Specimens
In this study, 828 (18%) specimens were misdiagnosed, of which 148 (17.9%) were cutaneous malignant tumors; these included 104 cases of BCC (70.3%), 32 cases of SCC (21.6%), and 12 cases of MM (8.1%). The specimens clinically diagnosed as SK had a significantly higher overall misdiagnosis rate than those clinically diagnosed as MN (P < 0.001, Table 4) and showed a higher incidence of misdiagnosed cutaneous malignancies (P < 0.001, Table 4). While BCC was predominant among the specimens misdiagnosed as MN (84.9%), the proportions of BCC (50.0%) and SCC (41.9%) were closer among the specimens misdiagnosed as SK. The differences in the proportions of BCC and SCC were significant between the specimens misdiagnosed as MN and SK (P < 0.001, Table 4). Demographic analysis showed that age was associated with the prevalence of cutaneous malignant tumors in the specimens clinically diagnosed as MN and SK (P < 0.001, Table 4), with the average age of patients with skin cancers in the SK group being 67.5 years (95% CI, 63.7–71.1 years) and that in the MN group being 57.3 years (95% CI, 54.5–60.0 years). Subgroup analysis by age indicated that patients with cutaneous malignancies in the MN group were usually aged 41–60 age, while those with cutaneous malignancies in the SK group were typically aged >60 years (Table 4). However, the two groups showed no significant differences in the distribution of skin cancers in terms of sex or location (P = 0.676, P = 0.251, respectively). Additionally, while clinician identified the need for further evaluations to rule out cutaneous malignant tumors in a small number of the submitted MN and SK specimens, the proportion of such specimens was low and did not differ significantly between the two groups (P = 0.258, Table 4).
 |
Table 4 Patient Characteristics in Cases of Malignant Tumors That Were Clinically Diagnosed as Melanocytic Nevi and Seborrheic Keratosis (Cases, %) |
Before the desktop dermoscopy system became operational (July 2014 to June 2021), a total of 3646 specimens were categorized as MN or SK, of which 129 were identified as cutaneous malignant tumors in pathological assessments, indicating a misdiagnosis rate of 3.5%. After the system’s implementation (July 2021 to June 2022), 946 specimens were categorized as MN or SK, of which 19 were diagnosed as cutaneous malignancies in histopathological assessments, reflecting a misdiagnosis rate of 2.0%. Overall, the incidence of misdiagnosis in the latter period was significantly lower than that in the former (P = 0.018).
Discussion
In both East Asia and the Western countries, MN and SK are the two most frequent skin neoplasms10–12 and are common reasons for consultations and surgery in dermatological practice.14,15 Previous studies have confirmed that MN mainly occurs in individuals aged of 20–30 years,10,16,17 and the incidence of SK increases with age,10,12,18 consistent with our findings.
Many researchers have expressed concern regarding MN since they are potential precursors and the main discriminators of MM. Previous studies from Western countries have reported that the rate of misdiagnosis as MN in clinical practice varies from 11.0% to 38.7%.8,19,20 In this study, the overall rate of misdiagnosis as MN was 16.8%, and SK was the most commonly misdiagnosed disease in cases that were clinically diagnosed as MN. These results were similar to a recent investigation conducted in Hunan Province,4 which, like Guizhou, is a southern province in China. However, another study carried out in Beijing, which is located in the north of China, reported a higher rate of 42.1%.14 This discrepancy may stem from regional variations in Chinese dermatologists’ perceptions of MN morphology and patients’ consultation patterns. SK mostly occurs in middle-aged and older individuals, and presents with various clinical manifestations.18 The rate of misdiagnosis as SK is evidently heterogeneous in fair-skinned populations,8,21 and was lower in our study than that in recent reports from China and South Korea.7,14 Karadag et al suggested that this heterogeneity may be due to differences in patients’ populations, insufficient initial diagnosis data, and other factors.22 Some reports have suggested that the overall rate of clinical misdiagnosis as SK was higher than that of clinical misdiagnosis as MN,8,14 although they did not perform statistical analyses. This suggestion was corroborated by our results.
Because of their destructive and lethal nature, cutaneous malignant tumors have become a major concern for dermatologists and patients with skin diseases. In clinical practice, misdiagnosis of these tumors as MN or SK is rather common.4–8,20,22 BCC and cutaneous SCC are the two most important types of cutaneous malignancies.1 The clinical phenotypes of BCC and SCC differ slightly between people of color and fair-skinned individuals.13 However, studies from Asia, Europe, and America have indicated that BCC is more frequent in cases with clinically diagnosed MN,4–6,20 and SCC is more prevalent in cases with clinically diagnosed SK.5–7 In this study, BCC was the most frequent type of cutaneous malignant tumor among the specimens clinically diagnosed as MN, while SCC was not more prevalent than BCC in those clinically diagnosed as SK. Lim et al showed that skin cancers adjacent to or related to SK were mainly BCC.23 Unfortunately, this study did not distinguish whether the malignancies in the submitted specimens were missed as a result of an actual misdiagnosis or because of co-occurrence with SK or the appearance of the malignant tumor neighboring SK, and we also did not analyze the reasons for the higher incidence of BCC in specimens clinically diagnosed as SK. Ultraviolet accumulation is a risk factor for BCC and SCC in both the Eastern and the Western countries.24–26 With the aging of China’s population, the incidence of these cancers is expected to rise, underscoring the importance of identifying them using a variety of excellent tools.
MM is the most lethal tumor in dermatology,1,10 and is often misdiagnosed due to its similarities to other skin tumors.9 Given its low prevalence but increasing incidence and lower survival rate in China,1,2,26,27 early diagnosis of MM is the key to improving the recovery rate.24 The findings of this study suggest that MM should not be overlooked when the skin lesions clinically diagnosed as MN or SK appear in Chinese females, older patients, or on extremities.
In addition, among the specimens clinically diagnosed as SK, the incidence of actinic keratosis was relatively high, especially in older and female patients and among facial lesions. As a precancerous lesion of SCC, actinic keratosis is easily confused with SK,7,28 and should also be handled with caution by the clinicians.
In general, 2.5% of the specimens clinically diagnosed as MN and 5.7% of those clinically diagnosed as SK were histopathologically diagnosed as cutaneous malignant tumors, with a higher incidence of misdiagnosis in specimens clinically diagnosed as SK. These findings were similar to the incidence rates and differences reported in studies from the Eastern and Western countries.4–7,19 Some researchers believe that the differences in the incidence of cutaneous malignancies misdiagnosed as MN and SK may be attributed to the greater number of SK patients undergoing treatments like laser therapy and cryotherapy without histopathological examination.5 Our study also showed that older age and facial lesions were associated with a higher incidence of skin cancers being misdiagnosed as MN or SK, reflecting the fact that cutaneous malignant tumors, especially BCC and SCC, often occur in older people and are associated with ultraviolet exposure.25,26 This also suggests that in clinical practice, for older patients with skin lesions at exposed locations, the possibility of cutaneous malignant tumors should be carefully ruled out, regardless of whether they are suspected to have MN or SK.
Previous studies have shown that dermoscopy, in vivo confocal microscopy, and optical coherence tomography can prominently improve the diagnostic accuracy of skin tumors.29,30 This study also highlighted the effectiveness of dermoscopy in reducing the rate of misdiagnosis of cutaneous malignancies as MN or SK. Our department uses a tabletop dermoscopy system, and only physicians who have mastered the dermoscopy technique can issue examination reports. The reduction in the misdiagnosis rate may be attributable to the reinforcement of clinicians’ understanding of skin tumors such as MN and SK after receiving training for dermoscopy and obtaining feedback on dermoscopic assessments. Currently, dermoscopy is only performed in the dermatology departments of a few hospitals in China. These results indicate the need for wider implementation of dermoscopy and training for this technique in dermatology clinics.
This study had some limitations, including the fact that it only involved a retrospective review of the specimens that underwent pathological examination from a tertiary hospital, and the lack of a detailed analysis to determine whether the misdiagnosed disease existed alone or in conjunction with the clinically diagnosed disease, which may have led to bias in the misdiagnosis rate and potentially inflated the misdiagnosis rate. In addition, we did not categorize or confirm the pathological subtypes of various diseases. More in-depth research is needed to substantiate our findings.
Conclusion
The data indicated that approximately 2.5% of specimens clinically diagnosed as MN and 5.7% of those clinically diagnosed as SK were histopathologically confirmed to be cutaneous malignant tumors. In particular, misdiagnosis as MN or SK was more likely in older patients with skin cancers located on the face. Notably, BCC tended to be ignored and frequently misdiagnosed as MN, while SCC was often mistakenly identified as SK. Clinicians should be highly vigilant about potentially malignant facial lesions in elderly patients to diagnose them accurately and promptly. Furthermore, the use of dermoscopy significantly reduced the misdiagnosis rate of cutaneous malignant tumors, indicating that it was a valuable tool for the diagnosis of these lesions.
Data Sharing Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval
This retrospective study involving human participants was in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The Ethics Committee of the Affiliated Hospital of Guizhou Medical University approved this study (2022-735).
Consent to Participate
All patients from the data source signed the informed consent agreement of “My medical records and samples can be used for medical research without revealing identifiable information” before the surgery or biopsy of the rash.
Acknowledgments
The authors thank Dr. Zhiyun Wang of the School of Public Health of Guizhou Medical University for his guidance on statistics.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
No funding or sponsorship was received for the conduct of this study or the preparation of this manuscript.
Disclosure
Jun Zhang, Yu Wang, Wei Zhang; Linglong Cai, Jianglong Feng, Yiwei Zhu and Hongguang Lu have no conflicts of interest that are directly relevant to the content of this article.
References
1. Urban K, Mehrmal S, Uppal P, Giesey RL, Delost GR. The global burden of skin cancer: a longitudinal analysis from the global burden of disease study, 1990–2017. JAAD international. 2021;2:98–108. doi:10.1016/j.jdin.2020.10.013
2. Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000-14 (Concord-3): analysis of individual records for 37513025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391:1023–1075. doi:10.1016/s0140-6736(17)33326-3
3. Vaz-Luis I, Masiero M, Cavaletti G, et al. ESMO Expert Consensus Statements on Cancer Survivorship: promoting high-quality survivorship care and research in Europe. Ann Oncol. 2022;33:1119–1133. doi:10.1016/j.annonc.2022.07.1941
4. Liu P, Su J, Zheng X, et al. Clinicopathological analysis of melanocytic nevi: a retrospective series. Front Med Lausanne. 2021;8:681668. doi:10.3389/fmed.2021.681668
5. Reeck MC, Chuang TY, Eads TJ, Faust HB, Farmer ER, Hood AF. The diagnostic yield in submitting nevi for histologic examination. J Am Acad Dermatol. 1999;40:567–571. doi:10.1016/s0190-9622(99)70456-1
6. Ferreira P, Vale E, Viana I, Almeida LS. The utility of histologic examination in benign skin lesions. J Eur Acad Dermatol Venereol. 1999;12:79–80. doi:10.1111/j.1468-3083.1999.tb00823.x
7. Roh NK, Hahn HJ, Lee YW, Choe YB, Ahn KJ. Clinical and histopathological investigation of seborrheic keratosis. Ann Dermatol. 2016;28:152–158. doi:10.5021/ad.2016.28.2.152
8. Har-Shai Y, Hai N, Taran A, et al. Sensitivity and positive predictive values of presurgical clinical diagnosis of excised benign and malignant skin tumors: a prospective study of 835 lesions in 778 patients. Plast Reconstr Surg. 2001;108:1982–1989. doi:10.1097/00006534-200112000-00022
9. Klebanov N, Gunasekera NS, Lin WM, et al. Clinical spectrum of cutaneous melanoma morphology. J Am Acad Dermatol. 2019;80:178–188. doi:10.1016/j.jaad.2018.08.028
10. Bolognia JL, Schaffer JV, Cerroni L. Dermatology.
11. Asokan N, Binesh VG. Cutaneous problems in elderly diabetics: a population-based comparative cross-sectional survey. Indian J Dermatol Venereol Leprol. 2017;83(2):205–211. doi:10.4103/0378-6323.190875
12. Plunkett A, Merlin K, Gill D, Zuo Y, Jolley D, Marks R. The frequency of common nonmalignant skin conditions in adults in central Victoria, Australia. Int J Dermatol. 1999;38(12):901–908. doi:10.1046/j.1365–4362.1999.00856.x
13. Karampinis E, Lallas A, Lazaridou E, Errichetti E, Apalla Z. Race-Specific and Skin of Color Dermatoscopic Characteristics of Skin Cancer: a Literature Review. Dermatol Pract Concept. 2023;13:e2023311S. doi:10.5826/dpc.1304S1a311S
14. Jia Q, Jin H, Liu Y, et al. Analysis of clinical and pathological diagnoses of 29987 skin biopsy samples in Peking Union Medical College Hospital. Chin J Dermatol. 2020;53:117–120. doi:10.35541/cjd.20190756
15. Salava A, Oker-Blom A, Remitz A. The spectrum of skin-related conditions in primary care during 2015-2019-A Finnish nationwide database study. Skin Health Dis. 2021;1:e53. doi:10.1002/ski2.53
16. MacKie RM, English J, Aitchison TC, Fitzsimons CP, Wilson P. The number and distribution of benign pigmented moles (melanocytic naevi) in a healthy British population. Br J Dermatol. 1985;113:167–174. doi:10.1111/j.1365-2133.1985.tb02060.x
17. Rokuhara S, Saida T, Oguchi M, Matsumoto K, Murase S, Oguchi S. Number of acquired melanocytic nevi in patients with melanoma and control subjects in Japan: nevus count is a significant risk factor for nonacral melanoma but not for acral melanoma. J Am Acad Dermatol. 2004;50:695–700. doi:10.1016/j.jaad.2003.11.053
18. Gorai S, Ahmad S, Raza SSM, et al. Update of pathophysiology and treatment options of seborrheic keratosis. Dermatol Ther. 2022;35:e15934. doi:10.1111/dth.15934
19. Becker SW. Diagnosis and treatment of pigmented nevi; consideration of some of the pitfalls. Arch Derm Syphilol. 1949;60:44–65. doi:10.1001/archderm.1949.01530010047004
20. Swerdlow M. Nevi; a problem of misdiagnosis. Am J Clin Pathol. 1952;22:1054–1060. doi:10.1093/ajcp/22.11.1054
21. Myroshnychenko MS, Moiseienko TM, Torianyk II, et al. Seborrheic keratosis: current state of the problem. Wiad Lek. 2022;75:172–175. doi:10.36740/wlek202201204
22. Karadag AS, Parish LC. The status of the seborrheic keratosis. Clin Dermatol. 2018;36:275–277. doi:10.1016/j.clindermatol.2017.09.011
23. Lim C. Seborrhoeic keratoses with associated lesions: a retrospective analysis of 85 lesions. Australas J Dermatol. 2006;47:109–113. doi:10.1111/j.1440-0960.2006.00258.x
24. Paulo MS, Adam B, Akagwu C, et al. WHO/ILO work-related burden of disease and injury: protocol for systematic reviews of occupational exposure to solar ultraviolet radiation and of the effect of occupational exposure to solar ultraviolet radiation on melanoma and non-melanoma skin cancer. Environ Int. 2019;126:804–815. doi:10.1016/j.envint.2018.09.039
25. Shin J, Chung KY, Park EC, Nam KA, Yoon JH. Occupational differences in standardized mortality ratios for non-melanotic skin cancer and melanoma in exposed areas among individuals with Fitzpatrick skin types III and IV. J Occup Health. 2019;235–241. doi:10.1002/1348-9585.12040
26. Huang YS, Chen XX, Yang SX, et al. Preliminary exploration of the clinical features of Chinese patients with skin malignancies and premalignancies: a retrospective study of 1420 cases from Peking University First Hospital. J Eur Acad Dermatol Venereol. 2013;27:1114–1119. doi:10.1111/j.1468-3083.2012.04673.x
27. Wu Y, Wang Y, Wang L, Yin P, Lin Y, Zhou M. Burden of melanoma in China, 1990-2017: findings from the 2017 global burden of disease study. Int J Cancer. 2020;147:692–701. doi:10.1002/ijc.32764
28. Han YM, Chen LJ, Dou X, Yang QP. Clinical and pathological study of 328 cases of actinic keratosis in eastern Chinese patients. Dermatology. 2013;227:316–320. doi:10.1159/000354651
29. Xiong YQ, Ma SJ. Comparison of dermoscopy and reflectance confocal microscopy for the diagnosis of malignant skin tumours: a meta-analysis. J Cancer Res Clin Oncol. 2017;143:1627–1635. doi:10.1007/s00432-017-2391-9
30. Xiong YQ, Mo Y, Wen YQ, et al. Optical coherence tomography for the diagnosis of malignant skin tumors: a meta-analysis. J Biomed Opt. 2018;23:1–10. doi:10.1117/1.JBO.23.2.020902
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Syringocystadenoma Papilliferum and Basal Cell Carcinoma Arising in Nevus Sebaceous
Jiang J, Chen Y, He Q, Yang J, Zhang Z, Yang H, Zhang H, Yang C
Clinical, Cosmetic and Investigational Dermatology 2022, 15:2021-2026
Published Date: 23 September 2022
Immunomodulators for Non-Melanoma Skin Cancers: Updated Perspectives
Russomanno K, Abdel Azim S, Patel VA
Clinical, Cosmetic and Investigational Dermatology 2023, 16:1025-1045
Published Date: 18 April 2023
