Back to Journals » Infection and Drug Resistance » Volume 17
Clinical Manifestation, mNGS Based Diagnosis and Treatment of Pulmonary Mucormycosis with Rhizopus delemar in a Diabetic Patient
Authors Cheng X, Li T, Wu F, Liu D
Received 13 December 2023
Accepted for publication 23 March 2024
Published 8 April 2024 Volume 2024:17 Pages 1379—1384
DOI https://doi.org/10.2147/IDR.S454029
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Sandip Patil
Xuesong Cheng,1 Tianyu Li,2 Fengsheng Wu,2 Dandan Liu1
1The Department of Pulmonary and Critical Care Medicine, Anhui No.2 Provincial People’s Hospital, Hefei, People’s Republic of China; 2Genoxor Medical Science and Technology Inc., Shanghai, People’s Republic of China
Correspondence: Dandan Liu, Email [email protected]
Abstract: Pulmonary mucormycosis is a severe and often fatal disease that commonly affects patients with underlying conditions, such as diabetes. Early diagnosis and appropriate treatment are crucial for improving survival rates. However, clinical diagnosis remains challenging due to difficulty in obtaining etiological evidence. In this particular case, the patient presented with a cough-producing bloody sputum, and a chest CT revealed lesions in the right upper lobe of the lung. The patient was ultimately diagnosed with pulmonary mucormycosis caused by Rhizopus delemar through clinical bronchoscopy biopsy and metagenomic next-generation sequencing (mNGS) analysis of bronchoalveolar lavage fluid sample. Subsequently, antifungal therapy using the less toxic Amphotericin B cholesterol Organosulfate complex was initiated, improving the patient’s condition. In conclusion, our findings underscore the potential of mNGS to provide an accurate and rapid etiological diagnosis of pulmonary mucormycosis, offering a foundation for treatment.
Keywords: Rhizopus delemar, pulmonary mucormycosis, metagenomic next generation sequencing, bronchoscopy biopsy
Introduction
Pulmonary mucormycosis is a rare invasive fungal infection caused by a pathogen of the order Mucorales, characterized by an acute onset, rapid progression, and high mortality. The incidence of pulmonary mucormycosis has been steadily increasing over the past few decades, posing a significant risk to human health.1 Pulmonary mucormycosis primarily affects immunocompromised patients, such as those with malignant tumors, organ transplant recipients, and long-term users of immunosuppressants due to diabetes.2,3 The genus Rhizopus of the Mucorales order is the primary pathogen responsible for pulmonary mucormycosis; among these, Rhizopus oryzae and Rhizopus microsporus are the most prevalent, with few reports on Rhizopus delemar as an important pathogen.4,5 Diagnosis of mucormycosis relies on low-sensitivity and time-consuming detection processes, including microscopy, fungal culture, and histopathology.6 Delayed diagnosis can result in missed optimal treatment time for patients with mucormycosis, leading to an increased mortality rate. However, the clinical symptoms and radiographic manifestations of pulmonary mucormycosis are non-specific, making it susceptible to misdiagnosis as invasive aspergillosis, posing a challenge for etiological diagnosis.7 In this case, metagenomic next-generation sequencing (mNGS) detection technology was employed to aid in the clinical diagnosis of pulmonary mucormycosis, leading to an adjustment in the treatment plan based on the mNGS test report and resulting in a favorable therapeutic outcome.
Case Presentation
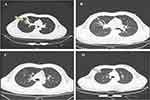
A 34-year-old man was admitted to our hospital on February 5, 2023, due to a two-day cough and hemoptysis. The patient had a four-year history of epilepsy, with no seizures for the past two years, and was not under drug control. Throughout the illness, the patient’s spirit, appetite, and body temperature were normal. The diagnostic and treatment timeline is displayed in Figure 1A. Upon admission, chest CT revealed high-density shadows with blurred margins in the anterior segment of the upper lobe of the right lung. The blood routine test showed a decreased neutrophil ratio, with no abnormalities in other indicators. The inflammatory indicators of WBC count, NE count, and highly-sensitive CRP (Hs-CRP) index of the patient after admission are shown in Figure 1B. The biochemical test results showed that the ALB, ALT, and AST levels decreased significantly, while glucose levels were significantly elevated (Table 1). The changes in these indicators suggested that the patient might have diabetes. On February 6, according to the results of glycated hemoglobin examination and routine urinalysis, the patient was initially diagnosed with diabetes, and the shadow of the lung needed further examination.
 |
Table 1 Results of blood routine and biochemical tests |
On February 7, a bronchoscopy revealed a caseous necrotic material in the right lung’s upper lobe. On February 8, the ultrasound showed no abnormalities, and the result of the sputum bacterial culture was negative. On February 9, the chest CT showed an infectious lesion in the patient’s right lung’s upper lobe and a few fibrous lesions in the middle lobe. Candida albicans was cultured from the sputum at the same time. Pathological results from the bronchoscopy biopsy revealed chronic inflammation of the bronchial mucosa in the right lung’s upper lobe with erosion and mucor hyphae. After staining, wide and diaphragm-free hyphae were observed, and the branches were irregular. (Figure 2). On February 9, the patient’s BALF sample was delivered to Shanghai Genoxor Medical Science and Technology Co., Ltd. for unbiased pathogen detection. A magnetic bead extraction kit (Tiangen, Beijing, China) was used to extract DNA, and the NEB Next Ultra DNA Library Prep Kit (Illumina, USA) was used to construct a metagenomic library. The quality and DNA concentration of the library were detected by the Agilent 2100 bioanalyzer and qubit 2.0, respectively. Finally, the Illumina NextSeq 550 platform was used to sequence the library that passed the quality inspection. After sequencing, the adapter, low-quality base, and short sequences were first removed from the raw data. Then, the remaining reads were not mapped to the Human genome were reserved for comparison with the microbial genome. The pathogenic microorganisms database comes from multiple public databases, covering 27,229 common clinical pathogenic microorganisms, including 14,385 bacteria, 738 fungi, 11,500 viruses, and 128 parasites. The test results were reported the next day, a total of 834 reads were identified to correspond to the Rhizopus delemar genome, and 11 reads were identified to correspond to the Cunninghamella bertholletiae genome.
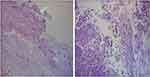 |
Figure 2 The pathological images of bronchoscopy biopsy. The arrows show the position of hyphae. |
Performed blood routine and biochemical tests again on February 11, and the results showed the patient’s inflammatory indicators had increased, but there was no clinical manifestation of bacterial infection, which was considered to be related to phlebitis caused by infusion (Figure 1B). Based on the laboratory tests, pathological biopsy, and BALF-mNGS results, ultimately, the patient was diagnosed as diabetes mellitus combined with right pulmonary mucormycosis caused by Rhizopus delemar. For pulmonary mucormycosis, immediate antifungal therapy targeting Mucorales was initiated, and based on drug availability and cost, Amphotericin B cholesterol Organosulfate complex was selected for antifungal treatment. In particular, the Amphotericin B cholesterol Organosulfate complex has relatively few adverse reactions compared with Amphotericin B. In the meantime, we monitored the patient’s blood sugar fluctuations and sought consultation from the endocrinology department to assist in regulating blood sugar levels.
After 13 days of treatment (February 24), the HRCT results indicated reduced infectious lesions in the anterior segment of the right upper lobe compared to previous findings, albeit with increased inflammatory indicators (Figure 3). After 3 weeks of treatment (March 2), the inflammatory indexes slightly increased but were lower than those on February 24 (Figure 1B); secondary infection was not considered. On March 7, chest HRCT results revealed a diminished and absorbed infectious lesion compared to previous assessments (Figure 3). During the treatment period, there were no adverse reactions as kidney injury or hypokalemia and patient reported no discomfort. The blood sugar level was well controlled, the chest imaging result was improved, and the patient was discharged on March 7. The patient was followed up for 1 year without any further discomfort.
Discussion and Conclusion
According to previous studies, patients with concurrent hematological malignancies were more prone to developing pulmonary mucormycosis, but pulmonary or disseminated infection was uncommon in diabetes-related mucormycosis.8 In contrast, mucormycosis in diabetes mellitus is usually naso-orbito-encephalomycosis.7 The mortality rate of pulmonary mucormycosis is higher than that of infections in other areas, as it can easily spread through the bloodstream and can cause symptoms such as encephalitis, brain abscesses, and gastrointestinal ulcers. Therefore, the etiological diagnosis of pulmonary mucormycosis is the key to early treatment.
The clinical manifestation of pulmonary mucormycosis mainly includes cough, fever, and shortness of breath. The imaging features are diverse and non-specific, including consolidation, cavity, air crescent, and halo sign.7 Currently, fungal culture and histopathological examination are the most traditional diagnostic methods for fungal diseases. Candida albicans was cultured from the patient’s sputum in this case, inconsistent with the histopathological examination results. Candida albicans is a normal flora of the human body, it is isolated in the sputum of 20% to 55% of the healthy population, so the detection of Candida albicans in the sputum is mostly colonization.9 The histopathological examination results indicated the presence of Mucor infection in the patient’s lungs. Histopathological examination is a relatively economical, effective, and quick method for the early diagnosis of invasive fungal diseases, particularly compared to the culture method, which can avoid the interference of background bacteria and distinguish between contamination, colonization, and infection in culture-positive specimens. The results combined with direct microscopic examination and culture method have greater diagnostic significance. However, this method lacks specificity and sensitivity, and cannot accurately determine the fungal strains or subtypes. In particular, when multiple forms of the same species appear under a microscope, distinguishing the types of fungi is challenging.10 Although fungal culture methods can be used for fungal typing, it has a low positivity rate and a lengthy culture period, thus, it cannot provide an early diagnosis for the clinic, and even this delay might miss the optimal time for antifungal therapy.
In this case, it’s difficult to differentiate between invasive aspergillosis infection and mucormycosis infection through the patient’s radiographic features.11 There is a certain risk of missed diagnosis by relying solely on histopathological examination results. mNGS technology, as a novel molecular biology detection method, has been widely used in diagnosing infectious diseases. Compared to traditional fungal detection methods, mNGS technology is faster, has high sensitivity and specificity, and can accurately distinguish species, and even subspecies and covers rare fungi and other types of pathogens.12 This case was initially diagnosed as Mucor infection based on histopathological evidence, but traditional culture methods failed to identify the pathogen. Through mNGS detection, the pathogen was finally identified as Rhizopus delemar of Rhizopus genus, and there was no infection with other types of pathogens. mNGS combined with traditional detection methods (imaging examination, histopathology examination, and culture) can enable rapid and accurate diagnosis of diseases.
In summary, this case report depicts the clinical presentation and treatment experience of a diabetic patient with pulmonary mucormycosis caused by uncommon species (Rhizopus delemar). Using histopathological examination combined with mNGS detection, the pathogen was quickly identified, followed by antifungal treatment and blood sugar level control, resulting in a good prognosis for the patient. As a new method of pathogen diagnosis, mNGS cannot be a definitive basis for etiology, but as an auxiliary diagnostic indicator, combined with the results of traditional fungal detection methods, it can enable precise diagnosis of pulmonary mucormycosis and promote the initiation of early precision treatment.
Abbreviations
mNGS, metagenomic next-generation sequencing technology; BALF, bronchoalveolar lavage fluid; WBC, white blood cell; NE, neutrophil; NE%, neutrophil ratio; Plt, platelets; PCT, procalcitonin; Hs-CRP, hypersensitive-c-reactive-protein; Cre, creatinine; ALB, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; Glu, glucose.
Ethics Approval and Patient Consent
Written informed consent for the publication of the publication of case details and accompanying images was obtained from the patient. Details of the case and any accompanying images can be published without institutional approval.
Acknowledgments
Financial support was provided by University Natural Science Foundation of Anhui Province (2023AH053375). We thank the patient for permitting us to use the data. In addition, we would like to thank the Anhui Province Key Laboratory of Occupation Health for their technical support.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Tianyu Li and Fengsheng Wu are affiliated with Genoxor Medical Science and Technology Inc. All authors state no other conflicts of interest in this work.
References
1. Lin E, Moua T, Limper AH. Pulmonary mucormycosis: clinical features and outcomes. Infection. 2017;45:443–448. doi:10.1007/s15010-017-0991-6
2. Prakash H, Ghosh AK, Rudramurthy SM, et al. A prospective multicenter study on mucormycosis in India: epidemiology, diagnosis, and treatment. Med Mycol. 2019;57:395–402. doi:10.1093/mmy/myy060
3. Kontoyiannis DP, Yang H, Song J, et al. Prevalence, clinical and economic burden of mucormycosis-related hospitalizations in the United States: a retrospective study. BMC Infect Dis. 2016;16:730. doi:10.1186/s12879-016-2023-z
4. Osaigbovo II E, BE DAA, Oladele RO. Mucormycosis in Africa: epidemiology, diagnosis and treatment outcomes. Mycoses. 2023;66:555–562. doi:10.1111/myc.13581
5. Rawas-Qalaji M, Jagal J, Fayed B, Hamdy R, Soliman SSM. Nano-combination for reviving the activity of fluconazole against Rhizopus delemar. Curr Pharm Biotechnol. 2023;24:1568–1575. doi:10.2174/1389201024666230210114632
6. Hamilos G, Samonis G, Kontoyiannis DP. Pulmonary mucormycosis. Semin Respir Crit Care Med. 2011;32:693–702. doi:10.1055/s-0031-1295717
7. Roden MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41:634–653. doi:10.1086/432579
8. Jeong W, Keighley C, Wolfe R, et al. The epidemiology and clinical manifestations of mucormycosis: a systematic review and meta analysis of case reports. Clin Microbiol Infect. 2019;25:26–34. doi:10.1016/j.cmi.2018.07.011
9. Chinese expert consensus group on diagnosis and treatment of adult candidiasis. Chinese expert consensus on the diagnosis and treatment of adult candidiasis. Chin J Med Front. 2020;12(1):35–50.
10. Guarner J, Brandt ME. Histopathologic diagnosis of fungal infections in the 21st century. Clin Microbiol Rev. 2011;24(2):247–280. doi:10.1128/CMR.00053-10
11. Jung J, Kim MY, Lee HJ, et al. Comparison of computed tomographic findings in pulmonary mucormycosis and invasive pulmonary aspergillosis. Clin Microbiol Infect. 2015;21:684.e11–8. doi:10.1016/j.cmi.2015.03.019
12. Li N, Cai Q, Miao Q, Song Z, Fang Y, Hu B. High-throughput metagenomics for identification of pathogens in the clinical settings. Small Methods. 2021;5:2000792. doi:10.1002/smtd.202000792
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.