Back to Journals » Cancer Management and Research » Volume 15
Clinical Insight on Proton Therapy for Paediatric Rhabdomyosarcoma
Authors Vennarini S, Colombo F, Mirandola A, Chiaravalli S, Orlandi E, Massimino M, Casanova M, Ferrari A
Received 17 July 2023
Accepted for publication 5 October 2023
Published 10 October 2023 Volume 2023:15 Pages 1125—1139
DOI https://doi.org/10.2147/CMAR.S362664
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Seema Singh
Sabina Vennarini,1 Francesca Colombo,1,2 Alfredo Mirandola,3 Stefano Chiaravalli,4 Ester Orlandi,5 Maura Massimino,4 Michela Casanova,4 Andrea Ferrari4
1Pediatric Radiotherapy Unit, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy; 2Department of Oncology and Hemato-Oncology (DIPO), University of Milan, Milan, Italy; 3Medical Physics Unit, Clinical Department, National Center for Oncological Hadrontherapy (CNAO), Pavia, Italy; 4Pediatric Oncology Unit, Fondazione IRCCS Istituto Nazionale Tumori, Milano, Italy; 5Radiation Oncology Unit, Clinical Department, National Center for Oncological Hadrontherapy (CNAO), Pavia, Italy
Correspondence: Andrea Ferrari, Pediatric Oncology Unit, Fondazione IRCCS Istituto Nazionale Tumori, Via G. Venezian, Milano, MI, 1 20133, Italy, Tel +39 02 23902588, Fax +39 02 23902648, Email [email protected]
Abstract: This paper offers an insight into the use of Proton Beam Therapy (PBT) in paediatric patients with rhabdomyosarcoma (RMS). This paper provides a comprehensive analysis of the literature, investigating comparative photon-proton dosimetry, outcome, and toxicity. In the complex and multimodal scenario of the treatment of RMS, clear evidence of the therapeutic superiority of PBT compared to other modern photon techniques has not yet been demonstrated; however, PBT can be considered an excellent treatment option, in particular for young children and patients with specific primary sites, such as the head and neck area (and especially the parameningeal regions), genito-urinary, pelvic, and paravertebral regions. The unique depth-dose characteristics of protons can be exploited to achieve significant reductions in normal tissue doses and may allow an escalation of tumour doses and greater sparing of normal tissues, thus potentially improving local control while at the same time reducing toxicity and improving quality of life. However, access of children with RMS (and more in general with solid tumors) to PBT remains a challenge, due to the limited number of available proton therapy installations.
Keywords: rhabdomyosarcoma, pediatric, proton beam therapy, local treatment, radiotherapy
Introduction
Rhabdomyosarcoma (RMS) is the most frequent soft tissue sarcoma in children and adolescents, with around 400 new cases in the 0–19-year-old population each year in Europe,1 and 50–60 in Italy.2,3 RMS is a highly malignant tumour characterised by local invasiveness and a high propensity to metastasize.4 It can occur in any part of the body, with a higher frequency in the head and neck (around 35% of the cases) and genito-urinary (25%) regions, with a high variability of clinical presentation. With current international cooperative protocols and more modern multimodal therapies, overall survival (OS) is reportedly over 70% for patients with localised RMS.5–8 However, patient outcome is influenced by many clinical variables, including tumour size and local invasiveness, regional lymph node involvement, distant metastases, tumour site, extent of residual tumour after initial surgery, and patient’s age. These prognostic factors have been considered in the risk-adapted stratification systems adopted by the international rhabdomyosarcoma community over the years in order to determine how intensively to treat patients.9 In patients whose clinical variables predict a worse prognosis, multimodal treatment is generally intensified to improve the cure rate, while in patients with more favourable features, reduction of treatment intensity is considered to limit possible side effects, without jeopardising outcomes.10 With a better understanding of genomic features and tumorigenesis, risk stratification is becoming more refined. The identification of molecular variables capable of accurately describing intrinsic biological aggressiveness is gradually leading to a more personalised approach to treatment. FOXO1 fusion status is included in all the current RMS stratification.11 Other molecular features will probably be considered in the near future, including MYOD1 mutation and TP53 and MYCN amplification.12 In other words, the use of more intensive chemotherapy or the indication to prescribe radiotherapy (and its dose) will soon be related to genomic and molecular findings rather than to the site of the tumour or the degree of initial surgery.
The treatment of RMS is complex and necessarily multimodal, requiring the involvement of multiple health-care professionals. In principle, all patients with RMS should be considered as having micro-disseminated disease at the time of diagnosis, and should always receive systemic treatment. Complementary local treatment modalities – surgery and radiotherapy – are key parts of multimodal treatment. As a matter of fact, local progressions or relapses represent around 60% of treatment failures:13 local control often represents a challenge in the therapeutic approach to RMS patients, due to the local invasiveness of the tumour but also due to the extreme heterogeneity of clinical presentations, according to the different age of the patients, for example, or the specific site of tumour origin.
Surgery is often considered to be the preferred local treatment option, but feasibility can be challenging due to many variables such as tumour size and local extent.14,15 The clinical approach is particularly complex in specific tumour sites. A good example is RMS arising in a parameningeal site. Parameningeal tumours are often deemed inoperable due to their location, and even in the limited cases where surgery is attempted, achieving negative margins becomes challenging. Resection in these areas may cause important functional damage to vital surrounding organs and can severely compromise cosmesis.16,17 In such cases, radiotherapy is necessarily integrated, supporting an incomplete surgical resection, or mostly having an exclusive role in controlling the disease locally.18 Noteworthy is the historically innovative local treatment approach “AMORE” (Ablative surgery, MOuld technique after loading brachytherapy, and surgical REconstruction), which has shown a lower incidence and severity of adverse events than external beam radiotherapy while maintaining adequate overall survival.19 Moreover, this approach has proved to be feasible and effective in salvage treatment after local recurrence.20 Another specific example is RMS of the bladder/prostate, for which the combination of local treatments for functional organ preservation is currently the gold standard (ie conservative surgery and interstitial brachytherapy).21,22
The combination of conservative surgery and radiotherapy, together with timing and sequence, or radiotherapy techniques and doses aims to optimise disease control and minimise loss of organ function and other late sequelae.23 In this scenario, the continuous technological advancements in radiotherapy techniques have contributed to an increasingly therapeutic landscape for Proton Beam Therapy (PBT).
Proton Beam Therapy: General Aspects
The ongoing pursuit of improved efficacy and reduced toxicity in paediatric oncological treatments has led to significant technological advancements, including PBT, which is a crucial development in this direction. PBT is a sophisticated technology and currently one of the most extensively utilised forms of hadron therapy, which involves the application of external ion beams for treating solid tumours.
Proton beams were first proposed more than 70 years ago by the physicist Robert R. Wilson.24 In the early 1990s, PBT was used in a clinical environment for the first time in Loma Linda (CA, USA). Since then, many facilities have been built or are under construction,25 and PBT can now be considered a solid therapeutic option offered to patients in many places around the world.
The intrinsic physical and biological characteristics make protons (and other ion species like helium, carbon, and oxygen) particularly effective in external radiotherapy.26 In particular, the protons’ inverse depth-dose profile compared to photons and the sharp distal fall-off after the maximum deposition of energy, known as Bragg peak, guarantee that the organs at risks traversed by the beams can be better spared compared to photons.27 This proton characteristic also allows a smaller number of beam ports to be used, thus largely reducing the low doses given to patients. Moreover, the biological characteristic of protons makes the “cell-killing” effect more effective than photons. The Relative Biological Effectiveness (RBE) is 1.1, which means 10% higher than photons. This value, however, is not to be considered fixed, being even higher in the distal part of the dose distribution.28 Most of the facilities in operation adopt the active pencil beam scanning (PBS) technique, in which a pencil beam is scanned in transversal directions (up to 40*40 cm field size) by scanning magnets, while the depth along the beam direction is changed, increasing the beam energy. Cyclotrons or synchrotrons are the main accelerators used in proton therapy, typically spanning the proton energy range in water from 30 to 300 mm, thus consenting to treatments of many tumour sites.
PBT is widely indicated for paediatric neoplasms.29 The growing precision in defining irradiation volumes, aided by dedicated imaging techniques, along with the distinct physical and radiobiological characteristics of proton beams, offer potential advantages that translate into clinical benefits. These benefits include reduced dosimetric exposure of organs at risk near the tumour site and preservation of healthy tissue by delivering the full dose to the target area.30 This latter aspect is strongly associated with the development of late multi-organ toxicity and, more importantly, with the reduction in the occurrence of secondary radiation-induced malignancies resulting from radio-chemotherapy treatments. These concepts hold vital significance in paediatric oncology for the long-term well-being of surviving patients. The importance of Quality of Life (QoL) for long-term survivors has gained increasing recognition, now holding weight comparable to the curative aspect.31–33
The unique physical characteristics of protons enable significant dose escalation, leading to improved cure rates and overall survival in specific paediatric radio-resistant malignancies. This is especially beneficial for localised sarcomas situated in anatomically challenging regions that are difficult to access through surgery, while also minimising radiation exposure to nearby radiosensitive organs.34
Finally, the reduction of acute toxicity during treatment, along with improved therapeutic compliance (which has an impact on the psychological well-being of the patient) and increased tolerance to treatments, enables the implementation of concurrent treatment regimens, combining PBT with surgery and concomitant systemic treatment. This approach shows promising advancements in disease control and overall survival for these neoplasms, which typically require multimodal regimens.35
PBT centres around the world have witnessed significant growth in the past decade.
In the United States, in particular, over 50% of paediatric patients undergo proton therapy for tumours of the central nervous system, bone sarcomas, soft tissue sarcomas (with RMS being the most representative), chordoma and chondrosarcoma, retinoblastoma, neuroblastoma, Hodgkin’s lymphoma, radiation-induced neoplasms, malignancies associated with genetic syndromes, and others.36
The absence of clinical trials comparing photons and protons (which are not feasible for various reasons, including an ethical standpoint), the limited number of patients with sufficient follow-up time, the high costs (resulting in limited treatment access), and the relatively small number of PBT centres worldwide (compared to photons) have been major criticisms voiced by the scientific community regarding the use of PBT. These concerns have called for caution, careful interpretation of results, and the need for comprehensive clinical and radiological follow-up of the provided data.37
The radiotherapy community has made tremendous efforts to date, with the establishment of various consortia and registries that enable the global collection of data. One such example is the Pediatric Proton/Photon Consortium Registry (PPCR),38 where data sheets for photonic and proton treatments of major paediatric neoplasms are shared and collected.
Nonetheless, there has been a notable decrease in costs and a rapid increase in the number of proton therapy centres worldwide. Currently, there are over 60 operational systems globally (25 located in North America and Asia, 16 in Europe, and one in South Africa). As a result, there has been a rise in the number of children receiving proton treatment for elective indications.39
Furthermore, technological advancements in proton therapy facilities, including the introduction of gantry systems and the transition from passive scattering to active scanning techniques, such as PBS and intensity-modulated proton beam therapy (IMPT), have greatly improved the precision and dose conformity for complex targets like RMS.40
Nevertheless, as pointed out in the discussion session, PBT should be managed with extreme care due to the physical, dosimetric, and delivery issues inherent to the technique itself. Only after a solid learning curve should a dedicated and well-trained clinical personnel (in particular physicians and physicists) approach pediatric treatments with particle beams.
Proton Beam Therapy in Rhabdomyosarcoma
PBT has garnered significant attention in the treatment of paediatric RMS in recent years. Current protocols in both the United States and Europe now include provisions for PBT, outlining specific planning methods tailored to the use of protons.41–43
In this review, we have conducted a comprehensive analysis of the literature, starting with a meticulous assessment of comparative photon-proton dosimetric studies. Subsequently, we have thoroughly examined the outcome and toxicity studies involving children treated with protons for RMS across various anatomical sites. The bibliographic study was conducted in the month of August 2023, using the NCBI PubMed, Embase, and Cochrane Library databases. The search terms used were as follows: proton therapy, rhabdomyosarcoma, pediatric, and paediatric. Only manuscripts in English were consulted. Twenty-one studies were identified. Tables 1-3 report the main findings, with a specific focus on comparative dosimetric findings (for 7 studies), outcome (for 13 studies), and toxicity (for 14 studies), respectively.
 |
Table 1 Comparative Dosimetric Studies |
 |
Table 2 Survival Outcomes and Local Control |
 |
Table 3 Acute and Late Toxicities and Incidence of Radiation Induced Neoplasms (Only Toxicities of Grade Greater Than or Equal to 3 Were Reported) |
Advancements in radiotherapy techniques over the past decade have enabled the reduction of irradiation volumes and improved dose conformity to the target area. This has contributed to a reduction in acute toxicities and long-term sequelae associated with the treatment itself. These advancements are particularly significant in the paediatric field and have a profound impact on RMS cases that necessitate high doses of radiotherapy.42
Comparative Dosimetric Studies
Initial comparative studies between photon therapy and PBT for orbital and/or parameningeal RMS were conducted by Ladra et al in the early 2000s.43,49 Although these studies involved a limited number of patients, they quickly demonstrated comparable outcomes in terms of dose coverage and homogeneity within the target volume for both techniques. Moreover, PBT exhibited a clear advantage by significantly reducing the radiation dose to organs at risk.
Of particular interest are the findings from a series reported by Kozak KR et al, focusing on 10 patients with parameningeal RMS. These locations are particularly critical due to their proximity to structures involved in cognitive functions, growth, vision, and hearing. The study compared the treatment plans using intensity-modulated radiotherapy (IMRT) and proton beam therapy (PBT). Both techniques demonstrated optimal coverage of the target volume, with a Mean Clinical Target Volume (CTV) V95 ≥99% for both IMRT and PBT. However, in two cases treated with protons, a Dmax (dose maximum, expressed as percent of prescribed dose) >120% was observed. PBT exhibited dosimetric advantages in all organs evaluated, although statistical significance was not reached for the ipsilateral cochlea and mastoid and was borderline for the ipsilateral parotid gland. It is important to note that the greater dose asymmetry observed in all organs except the crystalline lens warrants further evaluation, as it may potentially lead to an increased risk of late facial asymmetry.47
Cotter et al, on the other hand, presents data from a comparative dosimetric study involving seven male patients with bladder/prostate RMS. The study consistently demonstrates the equivalence between IMRT and PBT in terms of dosimetry on the treatment volume (Mean CTV V95 ≥99% for both techniques). Additionally, PBT offers an advantage by reducing the average radiation dose to critical organs such as the bladder, testicles, femoral heads, growth plates, and pelvic bones.46 These findings hold great significance due to the substantial occurrence of late sequelae reported in literature for these disease sites. In fact, only 40% of patients retain normal bladder function, and there is a risk of long-term sexual and reproductive dysfunctions.64,65
More recently, Ladra et al conducted the first comparative dosimetric study on patients enrolled in a prospective clinical study, which included a substantial series of patients with various disease sites affected by RMS. The extracted data confirmed the similarity in irradiated volume coverage, with Mean CTV V95 = 100% for both techniques and Mean CTV V100 = 98% for PBT and 99% for IMRT. The study also demonstrated a higher sparing rate of surrounding healthy tissues with the use of PBT. Specifically, PBT plans exhibited reduced doses to organs at risk in all tumour sites and in 26 out of 30 examined structures, with a notable advantage observed in both central and lateralised lesions, particularly in the latter. Moreover, a more pronounced benefit was observed for parallel organs (such as temporal lobes, mandibles, and pelvic bones) and organs sensitive to low doses (such as hypothalamus and gonads), compared to organs arranged in series (such as chiasma, brainstem, and spinal cord).44
Outcome and Toxicity
Considering the undeniable superiority of protons in sparing homo- and contralateral critical structures, as evidenced by comparative studies, Ladra et al initiated the first prospective Phase II study in 2004. The study aimed to collect outcome and toxicity data from a large series of patients with RMS treated with PBT.51 The cohort consisted of 57 enrolled patients with diverse disease sites, including 27 with parameningeal RMS and 19 with orbital RMS. In this heterogeneous population, local disease control was comparable to the historical series of patients treated with photons. With a median follow-up of 47 months, the 3-year and 5-year overall survival (OS) rates were 81% and 78%, respectively. The 3-year and 5-year event-free survival (EFS) rates were 73% and 69%, respectively. The local control (LC) rate was 81% at both 3 and 5 years. Orbital neoplasms showed excellent outcomes, with a 5-year EFS of 92% and LC, like those reported in the IRSG-IV (Intergroup Rhabdomyosarcoma Study Group protocol IV) and COG-D9602 (Children Oncology Group) trials.66,67 Among parameningeal RMS patients, the local recurrence rate was 23%, consistent with previously published data from the IRSG-IV and COG-D9803 trials.68 The 5-year EFS was 60%, comparable to the findings reported by Yang et al and Eaton et al.69,70 Regarding treatment tolerance, only 17% of patients experienced grade 3 acute toxicities, including dermatitis, odynophagia, and mucositis, which was significantly lower than the rates reported in the IRSG-IV trial. Additionally, only three patients developed grade 3 late sequelae, including cataract, retinopathy with diffuse acuity reduction, and chronic otitis.
The initial report by the Paul Scherrer Institute,52 which focused on patients treated with PBS-PBT, is of particular significance. The study revealed a 5-year LC and OS rate of 78.5% and 80.6%, respectively, which is comparable to the previously mentioned study by Ladra et al. However, it is important to note that this report also indicated a higher incidence of grade 3 acute and late toxicities. This outcome was likely attributed to the inclusion of patients with advanced disease and larger irradiation volumes, which could have contributed to the increased toxicities observed. Leiser et al also reported the occurrence of second malignant neoplasms (SMN) in two patients, without any established oncological predisposition or genetic syndromes.52 It is worth noting that according to the analysis of the Surveillance, Epidemiology, and End Results (SEER) registries, the risk of SMN was found to be independent of previous radiotherapy treatments.71
A large series reported by Leiser et al (83 RMS cases, 59 arising in unfavourable sites) reported 5-year OS and LC rate of 80.6% and 78.5%, respectively, with no cases of grade 4 acute toxicity and 12/83 grade 3 acute toxicity (mainly mucositis and dermatitis). Regarding late toxicity, no grade 4 and 3 grade 3 toxicity (cataract and hearing loss) were reported.52
Doyen et al retrospectively described a cohort of 46 parameningeal RMS patients, reporting a 2-year OS, EFS, and LC of 88.9%, 76.9%, and 83.8%, respectively.57 The study also highlighted good tolerance to radiotherapy, with only 4.4% of patients experiencing late grade 3 sequelae, including cataracts, dry eye syndrome, sinusitis, and hyperpigmentation. These percentages are comparable to those reported by Weber et al and Child et al.53 Given the high local recurrence rate observed in parameningeal RMS, the authors conducted further evaluation on the location of recurrences in relation to the dosimetry of the initial radiotherapy treatment.50,53 The analysis revealed that the recurrence site received an average D95 (dose to 95% of the target volume) of 52.8 Gy and an average dose of 54.5 Gy. These findings might indicate the potential benefit of dose escalation in overcoming the radio-resistance of these tumours. However, the effectiveness and safety of this therapeutic approach still require further evaluation.
A recent multicenter transatlantic study assessed four distinct local treatments (EBRT, PBT, AMORE, and Paris method) for patients with RMS exhibiting orbital, H-N, and PM localization with a focus on facial dysmorphisms. Patients deemed ineligible for surgical treatment, mainly due to intracranial disease extension, carotid artery encasement, and perineural spread, were not included in the analysis. The entire group of patients displayed a statistically significant decrease in facial growth and an increase in facial asymmetries and deformities when compared with healthy subjects. Patients with orbital RMS exhibited a more favorable profile compared to the other two localizations. The sub-analysis conducted on the RMS PM group indicated a significantly superior toxicity profile for PBT in comparison to EBRT and Paris method. However, it is important to note that the results may have been influenced by the shorter follow-up period and younger age of these patients compared to those treated with other techniques. This is because facial dysmorphisms are age-dependent processes.63
The largest prospective series of RMS patients treated with PBT as part of a multimodal therapeutic approach was recently published by Buszek.60 This series includes a diverse range of sites, with a majority of patients having an unfavourable prognosis (61 out of 94 patients). Survival data, including OS, progression-free survival (PFS), and LC at 4 years (71%, 63%, and 85% respectively), appear to be comparable to modern series using IMRT or PBT. Notably, the absence of marginal relapses in patients treated with PBT provides additional reassurance regarding its effectiveness.60,63,72,73
Discussion
The primary objective of the treatment strategy in RMS protocols has always been to enhance survival rates, despite being aware of the significant toll it takes on young patients in terms of toxicity and the long-term impact of treatment-related toxicities on survivors. In historical European trials, the use of radiotherapy has been approached with caution, with attempts to avoid its use whenever possible in low-risk treatment categories and in children aged 3 years and younger.
In the International Society of Pediatric Oncology – Malignant Mesenchymal Tumour (SIOP-MMT) studies, the extremely conservative approach to radiotherapy aiming to cure a significant proportion of patients without irradiation led to controversial results.74 For patients with orbital RMS, for example, strategies for avoiding radiotherapy showed that children that did not receive radiotherapy had an increased risk of relapse, but they could be salvaged with second-line therapy. On the other hand, a large proportion of cases were treated successfully without the use of radiotherapy, with no radiation-related dysfunction and cosmetic problems.75 However, these favourable results were not achieved in other tumour sites: a study on young children with parameningeal RMS demonstrated how the cure of patients with disease arising in this site remained unlikely without systematic use of radiotherapy.76 More in general, effort to omit irradiation generally produced higher rates of local relapse than in patient populations treated more consistently with radiotherapy, but in many cases also lower overall survival rates.74,77 In general agreement, radiotherapy is considered to be safely withheld in limited subsets of RMS patients, such as those with embryonal RMS completely resected (with microscopically free margins) at diagnosis.
Results from historical European trials led paediatric oncologists and radiotherapists to re-evaluate the role of radiotherapy in the European paediatric Soft Tissue Sarcoma Study Group (EpSSG) RMS 2005 study. The EpSSG RMS 2005 trial (conducted from 2005 to 2016) supported a more systematic use of radiotherapy: as a result, 86% of patients with high- and very high-risk localised RMS received radiotherapy for local disease control, resulting in an increase in 3-year EFS from 55% to 67%6 for high-risk and from 39% to 56% for very high-risk (node-positive alveolar) patients.78
While it is important to recognise the role of systematic use of radiotherapy in achieving these outcomes, it is imperative, on the other hand, to consider the need to reduce medium-to-long-term sequelae and therefore to introduce the concept of advancing radiotherapy techniques.
As an alternative to conventional radiotherapy, PBT has gained prominence due to its radiobiological characteristics in the treatment of various paediatric tumours, including RMS. Published studies, as shown in our review, teach us that comparative photon-proton dosimetric studies have clearly demonstrated a significant advantage in reducing irradiation to organs at risk and a notable decrease in integral dose, while maintaining adequate coverage of the disease target. Outcome analyses have demonstrated comparable disease control when compared to well-established photon series, with lower rates of acute and late toxicities in specific RMS sites.
Despite the limits of published studies (ie limited number of reported series, mostly single-centre experiences, relatively small sample sizes, only one randomised study), available data suggest that PBT has demonstrated effectiveness and safety in the comprehensive local treatment of patients with RMS.
Since it is clear that a formal evidence of superiority of PBT over other radiotherapy modalities is still needed, the indication for protons in RMS and the development of dedicated clinical trials should always be discussed in a multidisciplinary setting and evaluated within the pediatric oncology community.
Despite the increase in the number of proton therapy centres worldwide, access to PBT remains a challenge. A North American study involving 12,101 children with solid malignancies included in the National Cancer Data Base (period 2004–2013) reported that 8% of patients received PBT, with the proportion increasing between 2004 (1.7%) and 2013 (17.5%). The use of PBT was more frequent in younger patients and was affected by socioeconomic factors, ie patients with private/managed care and those with higher median household income and educational attainment were more likely to receive PBT.79
It is therefore extremely important to define shared indications for patients with RMS.
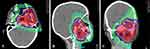
There is a general agreement to consider PBT especially recommended for young children (ie under the age of 3) in order to minimise exposure to medium-to-low radiation doses, which can significantly affect long-term side effects and the overall quality of life for survivors. The devastating side effects of radiotherapy on growing tissues and immature organs of infants and very young children (that often have tumours disproportionate to their body mass) make local control particularly challenging in these patients.80 In the EpSSG RMS 2005 study, 33.6% of infants (less than 12 months of age) received radiotherapy.81 Very young patients should be considered the first candidates for PBT to have the best sparing of organs at risk61 (Figure 1).
Other specific recommendations concern the disease site. In particular, PBT should be indicated, in principle, in unfavourable sites, such as the head and neck area (and especially the parameningeal regions), genito-urinary, pelvic, and paravertebral regions. In these locations, complete surgery may not be feasible in most cases, and surrounding organs at risk may be particularly sensitive to radiation. In these cases, the use of PBT allows for dose escalation. This approach can improve local control while minimising radiation exposure to nearby organs. In addition, the reduced irradiation of surrounding tissues (ie mucosae), especially at medium-low doses, is particularly beneficial in improving compliance with concomitant chemotherapy treatments and reducing the occurrence of acute toxicity (Figure 2).
Unequal and unilateral locations with a favourable prognosis, such as the orbit, were among the first to be investigated in terms of dosimetry.49 Miralbell described four cases with orbital and paraorbital location, showing that – with equal coverage and homogeneity of the target volume – there was a greater sparing of OARs with PBT, particularly in the low- and medium-dose regions. Indeed, the significant advantage of PBT over photons was immediately evident, with the preservation of contralateral organs near the target and a reduction in the integral dose.
The female genital tract and bladder/prostate represent specific and challenging tumour sites. In such cases, brachytherapy should be considered as the primary treatment mode for dose distribution.82 However, given the age of these children, external radiotherapy with protons may play a role and is often utilised. A comprehensive comparison of brachytherapy and PBT is not available.
Further investigations and discussions are needed on the indication of PBT in certain regions, such as the abdomen, retroperitoneum, and specific muscle areas of the trunk adjacent to the chest. In these cases, a specialised team of knowledgeable physicians and physicists plays a crucial role in determining the appropriate use of PBT.
Particular care should be addressed in managing range uncertainties in PBT that could possibly cause suboptimal dose distributions and treatment plans. Unlike photons, two severe consequences can occur due to the potential shift of the proton sharp distal dose fall-off: underestimation of dose to the target or an overdosage to the organs at risk distal to beam direction.83 Range uncertainties arise from organ motion, setup and anatomical variations, dose calculation approximations, and biological considerations. In order to account for both setup errors and range uncertainties, robust plan optimisation84 is highly recommended when using protons. Moreover, in particular for moving targets and when beams are passing through moving regions, an evaluation of the organs motion is needed. For these cases, mitigation strategies should be applied, both in CT simulation and treatment phases, such as breath holding and respiratory gating.
Timing of radiotherapy, and in particular its combination with surgery, represents an important aspect that needs to be investigated. There are very few data comparing pre-operative and post-operative radiotherapy in children with RMS. In adult patients with soft tissue sarcomas, pre-operative radiotherapy can limit the target volume, allowing the surrounding tissue to be spared, and therefore potentially minimising long-term side effects, although more serious wound complications have been reported.85 This aspect is currently being studied in a randomised fashion within the ongoing EpSSG overarching study for children and adults with Frontline and Relapsed RhabdoMyoSarcoma (FaR-RMS) (NCT04625907). Concerning PBT, the role of pre-operative radiotherapy with protons, particularly in certain potentially operable areas, warrants further investigation, as the limitation of irradiation to healthy tissues could potentially reduce acute post-surgical sequelae.
The Far-RMS includes two other randomised questions on radiotherapy, ie to determine whether dose escalation of radiotherapy improves the outcome in patients with a higher local failure risk (ie age ≥ 18 years and tumour arising in unfavourable sites) and to determine whether radiotherapy treatment of all sites of metastases may improve the outcome in metastatic patients.23 In the Far-RMS, there are no specific recommendations on the use of PBT. The protocol states that patients can receive radiotherapy treatment for the primary tumour (and for metastatic sites) using photon-based techniques (including IMRT), or proton therapy/particle therapy. A quality assurance programme is provided to give a real-time review of each case for compliance with protocol target definitions and radiation delivery requirements, ie Quality and Excellence in Radiotherapy and Imaging for Children and Adolescents with Cancer across Europe in Clinical Trials (QUARTET).86
Conclusions
The current paper offers an insight on the use of PBT in RMS patients. With this review, we aim to emphasise that PBT can be an excellent treatment option for patients with RMS, in particular young children and patients with various primary sites. The unique depth-dose characteristics of protons can be exploited to achieve significant reductions in normal tissue doses and may allow the escalation of tumour doses and a greater sparing of normal tissues, thus potentially improving local control while at the same time reducing toxicity and improving quality of life.45,48,54–56,58,59,62 Nevertheless, PBT techniques should always be proposed by well-trained clinical staff with experience in managing particle beams. We also believe that any consideration of dose escalation should be limited to the context of a clinical trial.
In current years, the availability of advanced radiotherapy techniques, on one hand, and the more sophisticated understanding of RMS biology with the possible future identification of novel biomarkers, on the other, are leading to a more targeted and individualised approach to young patients with RMS. The optimal treatment approach for an individual patient may depend on various factors, which include clinical extension and patient’s age, for example, but also the potential risk of late sequelae. The impact of radiotherapy on the quality of life for survivors of RMS can be profound, and the use of PBT may be critical for sparing adjacent organs at risk.
While clear evidence of therapeutic superiority of PBT to other modern photon techniques has not yet been demonstrated, it is evident that an increasing number of children (with RMS and other solid tumours) are being treated with protons in North America and in Europe. However, the number of available proton therapy installations remains unfortunately limited. In Italy, for example, only two proton therapy centres are currently operational (with three estimated installations coming in next years). In a rough estimate, of the 50–60 RMS cases occurring each year in Italy in the 0–19-year population, approximately 10 patients received PBT (that can be seen as about one-third of the cases for which PBT may be indicated according to age, stage, and tumour sites). Access of children with RMS (and more in general with solid tumors) to PBT remains a challenge. This situation restricts the number of patients that can be treated; in addition, it makes it difficult to gather a substantial amount of follow-up clinical data, especially when compared to photon treatments.
Further progress can be achieved, in this research field, by broader collaboration, such as the recently established International Soft Tissue SaRcoma ConsorTium (INSTRuCT) that aims to pool expertise and resources on a broader international level, developing consensus standards to guide diagnosis and treatment and comparing clinical data across different groups and studies.87
Centralisation of care in high-level and high-volume referral hospitals/centres is a recommended strategy for modern and high-quality paediatric radiotherapy. By bringing together various specialists and equipping a select number of treatment centres with state-of-the-art technology, it becomes possible to consolidate efforts and optimise the use of limited national resources. This approach ensures equitable access to treatment for children affected by RMS and promotes optimal outcomes.
Funding
No financial support was received for this submission.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ferrari A, Brecht IB, Gatta G, et al. Defining and listing very rare cancers of pediatric age: consensus of the Joint Action on Rare Cancers (JARC) in cooperation with the European Cooperative Study Group for Pediatric Rare Tumors (EXPeRT). Eur J Cancer. 2019;110:120–126. doi:10.1016/j.ejca.2018.12.031
2. Ferrari A, Trama A, De Paoli A, et al. Access to clinical trials for adolescents with soft tissue sarcomas: enrolment in European pediatric Soft tissue sarcoma Study Group (EpSSG) protocols. Pediatr Blood Cancer. 2017;64(6). doi:10.1002/pbc.26348
3. Ferrari A, Dama E, Pession A, et al. Adolescents with cancer in Italy: entry into the national cooperative pediatric oncology group AIEOP trials. Eur J Cancer. 2009;45(3):328–334. doi:10.1016/j.ejca.2008.12.003
4. Skapek S, Ferrari A, Gupta A, et al. Rhabdomyosarcoma. Nat Rev Dis Primers. 2019;5(1):1. doi:10.1038/s41572-018-0051-2
5. Hawkins DS, Chi YY, Anderson JR, et al. Addition of vincristine and irinotecan to vincristine, dactinomycin, and cyclophosphamide does not improve outcome for intermediate-risk rhabdomyosarcoma: a report from the Children’s Oncology Group. J Clin Oncol. 2018;36(27):2770–2777. doi:10.1200/JCO.2018.77.9694
6. Bisogno G, Jenney M, Bergeron C, et al.; European paediatric Soft tissue sarcoma Study Group. Addition of dose intensified doxorubicin to standard chemotherapy for rhabdomyosarcoma (EpSSG RMS 2005): a multicentre, open-label, randomised controlled, Phase 3 trial. Lancet Oncol. 2018;19(8):1061–1071. doi:10.1016/S1470-2045(18)30337-1
7. Bisogno G, De Salvo GL, Bergeron C, et al. Vinorelbine and continuous low-dose cyclophosphamide as maintenance chemotherapy in patients with high-risk rhabdomyosarcoma (RMS 2005): a multicentre, open-label, randomized, phase 3 trial. Lancet Oncol. 2019;20:1566–1575. doi:10.1016/S1470-2045(19)30617-5
8. Bisogno G, Minard-Colin V, Zanetti I, et al. Non-metastatic rhabdomyosarcoma in children and adolescents: overall results of the European paediatric Soft tissue sarcoma Study Group RMS2005 study. J Clin Oncol. 2023;41(13):2342–2349. doi:10.1200/JCO.22.02093
9. Crane JN, Xue Q. Clinical group and modified TNM stage for rhabdomyosarcoma: a review from the Children’s Oncology Group. Pediatr Blood Cancer. 2022;69(6):e29644. doi:10.1002/pbc.29644
10. Ferrari A, Casanova M. Like apples, rhabdomyosarcomas come in so many kinds. Pediatr Blood Cancer. 2022;69:e29667. doi:10.1002/pbc.29667
11. Hibbitts E, Chi YY, Hawkins DS, et al. Refinement of risk stratification for childhood rhabdomyosarcoma using FOXO1 fusion status in addition to established clinical outcome predictors: a report from the Children’s Oncology Group. CancerMed. 2019;8(14):6437. doi:10.1002/cam4.2504
12. Shern JF, Selfe J, Izquierdo E, et al. Genomic classification and clinical outcome in rhabdomyosarcoma: a report from an international consortium. J Clin Oncol. 2021;39(26):2859–2871. doi:10.1200/JCO.20.03060
13. Sparber-Sauer M, Ferrari A, Kosztyla D, et al. Long-term results from the multicentric European randomized phase 3 trial CWS/RMS-96 for localized high-risk soft tissue sarcoma in children, adolescents, and young adults. Pediatr Blood Cancer. 2022;69:e29691. doi:10.1002/pbc.29691
14. van Scheltinga T, Rogers T, Smeulders N, et al. Developments in the Surgical Approach to Staging and Resection of Rhabdomyosarcoma. Cancers. 2023;15(2):449. doi:10.3390/cancers15020449
15. Rogers TN, Dasgupta R. Management of Rhabdomyosarcoma in Pediatric Patients. Surg Oncol Clin N Am. 2021;30(2):339–353. doi:10.1016/j.soc.2020.11.003
16. Buwalda J, Schouwenburg P, Blank L, et al. A novel local treatment strategy for advanced stage head and neck rhabdomyosarcomas in children: results of the AMORE protocol. Eur J Cancer. 2003;39:1594–1602. doi:10.1016/s0959-8049(03)00363-0
17. Minard-Colin V, Kolb F, Saint-Rose C, et al. Impact of extensive surgery in multidisciplinary approach of pterygopalatine/infratemporal fossa soft tissue sarcoma. Pediatr Blood Cancer. 2013;60:928–934. doi:10.1002/pbc.24374
18. Casey DL, Mandeville H, Bradley JA, et al. Local control of parameningeal rhabdomyosarcoma: an expert consensus guideline from the International Soft Tissue Sarcoma Consortium (INSTRuCT). Pediatr Blood Cancer. 2022;69(7):e29751. doi:10.1002/pbc.29751
19. Schoot RA, Slater O, Ronckers CM, et al. Adverse events of local treatment in long-term head and neck rhabdomyosarcoma survivors after external beam radiotherapy or AMORE treatment. Eur J Cancer. 2015;51(11):1424–1434. doi:10.1016/j.ejca.2015.02.010
20. Vaarwerk B, Hol MLF, Schoot RA, et al. AMORE treatment as salvage treatment in children and young adults with relapsed head-neck rhabdomyosarcoma. Radiother Oncol. 2019;131:21–26. doi:10.1016/j.radonc.2018.10.036
21. Martelli H, Haie-Meder C, Branchereau S, et al. Conservative surgery plus brachytherapy treatment for boys with prostate and/or bladder neck rhabdomyosarcoma: a single team experience. J Pediatr Surg. 2009;44:190–196. doi:10.1016/j.jpedsurg.2008.10.040
22. Chargari C, Haie-Meder C, Guérin F, et al. Brachytherapy Combined with Surgery for Conservative Treatment of Children with Bladder Neck and/or Prostate Rhabdomyosarcoma. Int J Radiat Oncol Biol Phys. 2017;98:352–359. doi:10.1016/j.ijrobp.2017.02.026
23. Mandeville HC. Radiotherapy in the Management of Childhood Rhabdomyosarcoma. Clin Oncol. 2019;31(7):462–470. doi:10.1016/j.clon.2019.03.047
24. Wilson RR. Radiological use of fast protons. Radiology. 1946;47(5):487–491. doi:10.1148/47.5.487
25. Particle Therapy Co-Operative Group. Particle therapy facilities in clinical operation. Available from: https://www.ptcog.ch/index.php/facilities-in-operation.
26. Held KD, Lomax AJ, Troost EGC, et al. Proton therapy special feature: introductory editorial. Br J Radiol. 2020;93(1107):20209004. doi:10.1259/bjr.20209004
27. Newhauser WD, Zhang R. The physics of proton therapy. Review Phys Med Biol. 2015;60(8):R155–209. doi:10.1088/0031-9155/60/8/R155
28. Paganetti H. Relative Biological Effectiveness (RBE) Values for Proton Beam Therapy. Variations as a Function of Biological Endpoint, Dose, and Linear Energy Transfer. Phys Med Biol. 2014;59:R419–R472. doi:10.1088/0031-9155/59/22/R419
29. Mohan R, Grosshans D. Proton therapy - Present and future. Radiotherapy for cancer: present and future. Adv Drug Deliv Rev. 2017;109:26–44. doi:10.1016/j.addr.2016.11.006
30. Mitin T, Zietman AL. Promise and pitfalls of heavy-particle therapy. J Clin on- Col. 2014;32(26):2855–2863. doi:10.1200/JCO.2014.55.1945
31. Greenberger BA, Yock TI. The role of proton therapy in pediatric malignancies: recent advances and future directions. Pediatric Oncology. 2020;47:8–22. doi:10.1053/j.seminoncol.2020.02.002
32. Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–1582. doi:10.1056/NEJMsa060185
33. Bhakta N, Liu Q, Ness KK, et al. The cumulative burden of surviving childhood cancer: an initial report from the St Jude Lifetime Cohort Study (SJLIFE). Lancet. 2017;390:2569–2582. doi:10.1016/S0140-6736(17)31610-0
34. Frisch S, Timmermann B. The Evolving Role of Proton Beam Therapy for Sarcomas Review. Clin Oncol. 2017;29(8):500–506. doi:10.1016/j.clon.2017.04.034
35. Vennarini S, Del Baldo G, Lorentini S, et al. Acute Hematological Toxicity during Cranio-Spinal Proton Therapy in Pediatric Brain Embryonal Tumors. Cancers. 2022;14(7):1653. doi:10.3390/cancers14071653
36. Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review. National Cancer Institute; 2018.
37. Merchant TE. Clinical Controversies: proton therapy for pediatric tumors. J Med. 2013;23:97–108. doi:10.1016/j.semradonc.2012.11.008
38. Lawell MP, Indelicato DJ, Paulino AC, et al. An open invitation to join the pediatric proton/photon consortium registry to standardize data collection in pediatric radiation oncology. BJR. 2020;93:20190673. doi:10.1259/bjr.20190673
39. Journy N, Indelicato DJ, Withrow DR, et al. Patterns of proton therapy use in pediatric cancer management in 2016: an international survey. Radiother Oncol. 2019;132:155–161. doi:10.1016/j.radonc.2018.10.022
40. Langen K, Zhu M. Concepts of PTV and robustness in passively scattered and pencil beam scanning proton therapy. Semin Radiat Oncol. 2018;28(3):248–255. doi:10.1016/j.semradonc.2018.02.009
41. Yechieli RL, Mandeville HC, Hiniker SM, et al. Rhabdomyosarcoma. Pediatr Blood Cancer. 2021;68(Suppl 2):e28254. doi:10.1002/pbc.28254
42. Leiser D, Calaminus G, Malyapa R, et al. Tumour control and Quality of Life in children with rhabdomyosarcoma treated with pencil beam scanning proton therapy. Radiother Oncol. 2016;120(1):163–168. doi:10.1016/j.radonc.2016.05.013
43. Yock T, Schneider R, Friedmann A, et al. Proton radiotherapy for orbital rhabdomyosarcoma: clinical outcome and a dosimetric comparison with photons. Int J Radiat Oncol Biol Phys. 2005. doi:10.1016/j.ijrobp.2005.03.052
44. Ladra MM, Edgington SK, Mahajan A, et al. A dosimetric comparison of proton and intensity modulated radiation therapy in pediatric rhabdomyosarcoma patients enrolled on a prospective phase II proton study. Radiother Oncol. 2014. doi:10.1016/j.radonc.2014.08.033
45. Heinzelmann F, Thorwarth D, Lamprecht U, et al. Comparison of different adjuvant radiotherapy approaches in childhood bladder/prostate rhabdomyosarcoma treated with conservative surgery. Strahlenther Onkol. 2011;187(11):715–721. doi:10.1007/s00066-011-2261-3
46. Cotter SE, Herrup DA, Friedmann A, et al. Proton radiotherapy for pediatric bladder/prostate rhabdomyosarcoma: clinical outcomes and dosimetry compared to intensity-modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2011. doi:10.1016/j.ijrobp.2010.07.1989
47. Kozak KR, Adams J, Krejcarek SJ, et al. A dosimetric comparison of proton and intensity-modulated photon radiotherapy for pediatric parameningeal rhabdomyosarcomas. Int J Radiat Oncol Biol Phys. 2009. doi:10.1016/j.ijrobp.2008.06.1942
48. Fogliata A, Yartsev S, Nicolini G, et al. On the performances of Intensity Modulated Protons, RapidArc and Helical Tomotherapy for selected paediatric cases. Radiat Oncol. 2009;4:2. doi:10.1186/1748-717X-4-2
49. Miralbell R, Cella L, Weber D, et al. Optimizing radiotherapy of orbital and paraorbital tumors: intensity-modulated X-ray beams vs. intensity-modulated proton beams. Int J Radiat Oncol Biol Phys. 2000. doi:10.1016/s0360-3016(00)00494-6
50. Childs SK, Kozak KR, Friedmann AM, et al. Proton radiotherapy for parameningeal rhabdomyosarcoma: clinical outcomes and late effects. Int J Radiat Oncol Biol Phys. 2012;82(2):635–642. doi:10.1016/j.ijrobp.2010.11.048
51. Ladra MM, Szymonifka JD, Mahajan A, et al. Preliminary results of a phase II trial of proton radiotherapy for pediatric rhabdomyosarcoma. J Clin Oncol. 2014. doi:10.1200/JCO.2014.56.1548
52. Leiser D, Calaminus G, Malyapa R, et al. Tumour control and Quality of Life in children with rhabdomyosarcoma treated with pencil beam scanning proton therapy. Radiother Oncol. 2016;120(1):163–168. doi:10.1016/j.radonc.2016.05.013
53. Weber DC, Ares C, Albertini F, et al. Pencil Beam Scanning Proton Therapy for Pediatric Parameningeal Rhabdomyosarcomas: clinical Outcome of Patients Treated at the Paul Scherrer Institute. Pediatr Blood Cancer. 2016;63(10):1731–1736. doi:10.1002/pbc.25864
54. Indelicato DJ, Rotondo RL, Mailhot Vega RB, et al. 45 GyRBE for group III orbital embryonal rhabdomyosarcoma. Acta Oncol. 2019;58(10):1404–1409. doi:10.1080/0284186X.2019.1627412
55. Ludmir EB, Grosshans DR, McAleer MF, et al. Patterns of failure following proton beam therapy for head and neck rhabdomyosarcoma. Radiother Oncol. 2019;134:143–150. doi:10.1016/j.radonc.2019.02.002
56. Bradley JA, Indelicato DJ, Uezono H, et al. Patterns of Failure in Parameningeal Alveolar Rhabdomyosarcoma. Int J Radiat Oncol Biol Phys. 2020;107(2):325–333. doi:10.1016/j.ijrobp.2020.01.035
57. Doyen J, Jazmati D, Geismar D, et al. Outcome and Patterns of Relapse in Childhood Parameningeal Rhabdomyosarcoma Treated With Proton Beam Therapy. Int J Radiat Oncol Biol Phys. 2019;105(5):1043–1054. doi:10.1016/j.ijrobp.2019.08.005
58. Indelicato DJ, Rotondo RL, Krasin MJ, et al. Outcomes Following Proton Therapy for Group III Pelvic Rhabdomyosarcoma. Int J Radiat Oncol Biol Phys. 2020;106(5):968–976. doi:10.1016/j.ijrobp.2019.12.036
59. Suzuki R, Fukushima H, Okuwaki H, et al. Proton beam therapy with concurrent chemotherapy is feasible in children with newly diagnosed rhabdomyosarcoma. Rep Pract Oncol Radiother. 2021;26(4):616–625. doi:10.5603/RPOR.a2021.0082
60. Buszek SM, Ludmir EB, Grosshans DR, et al. Disease Control and Patterns of Failure After Proton Beam Therapy for Rhabdomyosarcoma. Int J Radiat Oncol Biol Phys. 2021;09(3):718–725. doi:10.1016/j.ijrobp.2020.09.050
61. Parekh AD, Indelicato DJ, Vega RBM, et al. Proton radiotherapy for infant rhabdomyosarcoma: rethinking young age as an adverse prognostic factor. Radiother Oncol. 2021;163:215–220. doi:10.1016/j.radonc.2021.05.017
62. Mizumoto M, Murayama S, Akimoto T, et al. Preliminary results of proton radiotherapy for pediatric rhabdomyosarcoma: a multi-institutional study in Japan. Cancer Med. 2018;7(5):1870–1874. doi:10.1002/cam4.1464
63. Hol MLF, Indelicato DJ, Slater O, et al. Facial deformation following treatment for pediatric head and neck rhabdomyosarcoma; the difference between treatment modalities. Results of a trans-Atlantic, multicenter cross-sectional cohort study. Pediatr Blood Cancer. 2023;70(8):e30412. doi:10.1002/pbc.30412
64. Arndt C, Rodeberg D, Breitfeld PP, et al. Does bladder preservation (as a surgical principle) lead to retaining bladder function in bladder/prostate rhabdomyosarcoma? Results from Intergroup Rhabdomyosarcoma Study IV. J Urol. 2004. doi:10.1097/01.ju.0000127752.41749.a4
65. Ferrer FA, Isakoff M, Koyle MA, et al. Bladder/prostate rhabdomyosarcoma: past, present and future. J Urol. 2006;176(4 Pt 1):1283–1291. doi:10.1016/j.juro.2006.06.019
66. Raney RB, Walterhouse DO, Meza JL, et al. Results of the Intergroup Rhabdomyosarcoma Study Group D9602 protocol, using vincristine and dactinomycin with or without cyclophosphamide and radiation therapy, for newly diagnosed patients with low-risk embryonal rhabdomyosarcoma: a report from the Soft Tissue Sarcoma Committee of the Children’s Oncology Group. J Clin Oncol. 2011;29:1312–1318. doi:10.1200/JCO.2010.30.4469
67. Crist WM, Anderson JR, Meza JL, et al. Intergroup Rhabdomyosarcoma Study-IV: results for patients with nonmetastatic disease. J Clin Oncol. 2001;19:3091–3102. doi:10.1200/JCO.2001.19.12.3091
68. Spalding AC, Hawkins DS, Donaldson SS, et al. The effect of radiation timing on patients with high-risk features of parameningeal rhabdomyosarcoma: an analysis of IRS-IV and D9803. Int J Radiat Oncol Biol Phys. 2013;87:512–516. doi:10.1016/j.ijrobp.2013.07.003
69. Yang JC, Wexler LH, Meyers PA, et al. Parameningeal rhabdomyosarcoma: outcomes and opportunities. Int J Radiat Oncol Biol Phys. 2013;85:e61–e66. doi:10.1016/j.ijrobp.2012.08.019
70. Eaton BR, McDonald MW, Kim S, et al. Radiation therapy target volume reduction in pediatric rhabdomyosarcoma: implications for patterns of disease recurrence and overall survival. Cancer. 2013;119:1578–1585. doi:10.1002/cncr.27934
71. Archer NM, Amorim RP, Naves R, et al. An Increased risk of second malignant neoplasms after rhabdomyosarcoma: population-based evidence for a cancer predisposition syndrome? Pediatr Blood Cancer. 2015;63(2):196–201. doi:10.1002/pbc.25678
72. Ladra MM, Mandeville HC, Niemierko A, et al. Local failure in parameningeal rhabdomyosarcoma correlates with poor response to induction chemotherapy. Int J Radiat Oncol Biol Phys. 2015;92:358–367. doi:10.1016/j.ijrobp.2015.01.049
73. Curtis AE, Okcu MF, Chintagumpala M, et al. Local control after intensity-modulated radiotherapy for head-and-neck rhabdomyosarcoma. Int J Radiat Oncol Biol Phys. 2009;73:173–177. doi:10.1016/j.ijrobp.2008.03.029
74. Stevens MC, Rey A, Bouvet N, et al. Treatment of nonmetastatic rhabdomyosarcoma in childhood and adolescence: third study of the International Society of Paediatric Oncology--SIOP Malignant Mesenchymal Tumor 89. J Clin Oncol. 2005;23:2618–2628. doi:10.1200/JCO.2005.08.130
75. Oberlin O, Rey A, Andersn J, et al. Treatment of orbital rhabdomyosarcoma: survival and late effects of treatment--results of an international workshop. J Clin Oncol. 2001;19(1):197–204. doi:10.1200/JCO.2001.19.1.197
76. Defachelles AS, Rey A, Oberlin O, et al. Treatment of nonmetastatic cranial parameningeal rhabdomyosarcoma in children younger than 3 years old: results from international society of pediatric oncology studies MMT 89 and 95. J Clin Oncol. 2009;27(8):1310–1315. doi:10.1200/JCO.2008.19.5701
77. Reguerre Y, Martelli H, Rey A, et al. Local therapy is critical in localised pelvic rhabdomyosarcoma: experience of the International Society of Pediatric Oncology Malignant Mesenchymal Tumor (SIOP-MMT) committee. Eur J Cancer. 2012;48(13):2020–2027. doi:10.1016/j.ejca.2011.11.011
78. Gallego S, Zanetti I, Orbach D, et al. Fusion status in patients with lymph node-positive (N1) alveolar rhabdomyosarcoma is a powerful predictor of prognosis: experience of the European Paediatric Soft Tissue Sarcoma Study Group (EpSSG). Cancer. 2018;124(15):3201–3209. doi:10.1002/cncr.31553
79. Shen CJ, Hu C, Ladra MM, et al. Socioeconomic factors affect the selection of proton radiation therapy for children. Cancer. 2017;123(20):4048–4056. doi:10.1002/cncr.30849
80. Ferrari A, Casanova M, Bisogno G, et al. Rhabdomyosarcoma in infants younger than one year old: a report from the Italian Cooperative Group. Cancer. 2003;97(10):2597–2604. doi:10.1002/cncr.11357
81. Slater O, Gains JE, Kelsey AM, et al. Localised rhabdomyosarcoma in infants (<12 months) and young children (12–36 months of age) treated on the EpSSG RMS 2005 study. Eur J Cancer. 2021;160:206–214. doi:10.1016/j.ejca.2021.10.031
82. Akkary R, Guérin F, Chargari C, et al. Long-term urological complications after conservative local treatment (surgery and brachytherapy) in children with bladder-prostate rhabdomyosarcoma: a single-team experience. Pediatr Blood Cancer. 2022;69(8):e29532. doi:10.1002/pbc.29532
83. Paganetti H. Range uncertainties in proton therapy and the role of Monte Carlo simulations. Phys Med Biol. 2012;57(11):R99–117. doi:10.1088/0031-9155/57/11/R99
84. Unkelbach J, Paganetti H. Robust Proton Treatment Planning: physical and Biological Optimization. Semin Radiat Oncol. 2018;28(2):88–96. doi:10.1016/j.semradonc.2017.11.005
85. O’Sullivan B, Davis AM, Turcotte R, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet. 2002;359:2235–2241. doi:10.1016/S0140-6736(02)09292-9
86. Kelly SM, Effeney R, Gaze MN, et al. QUARTET: a SIOP Europe project for quality and excellence in radiotherapy and imaging for children and adolescents with cancer. Eur J Cancer. 2022;172:209–220. doi:10.1016/j.ejca.2022.05.037
87. Hawkins DS, Bisogno G, Koscielniak E, et al. Introducing INSTRuCT: an international effort to promote cooperation and data sharing. Pediatr Blood Cancer. 2020:e28701. doi:10.1002/pbc.28701
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.