Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 18
Clinical Implications of Peak Inspiratory Flow in COPD: Post Hoc Analyses of the TRONARTO Study
Authors Mahler DA , Watz H, Emerson-Stadler R, Ritz J, Gardev A, Shaikh A , Drummond MB
Received 10 January 2023
Accepted for publication 24 July 2023
Published 10 August 2023 Volume 2023:18 Pages 1729—1740
DOI https://doi.org/10.2147/COPD.S404243
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Donald A Mahler,1,2 Henrik Watz,3 Rachel Emerson-Stadler,4 John Ritz,5 Asparuh Gardev,4 Asif Shaikh,6 M Bradley Drummond7
1Geisel School of Medicine at Dartmouth, Hanover, NH, USA; 2Valley Regional Hospital, Claremont, NH, USA; 3Pulmonary Research Institute at LungenClinic Grosshansdorf, Grosshansdorf, Germany; 4Boehringer Ingelheim International GmbH, Ingelheim am Rhein, Germany; 5Syneos Health, Somerset, NJ, USA; 6Boehringer Ingelheim Pharmaceuticals, Inc., Ridgefield, CT, USA; 7Pulmonary Diseases and Critical Care Medicine, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA
Correspondence: Donald A Mahler, Tel +1 603 542-6777, Email [email protected]
Background: In patients with COPD, inhalation ability should be assessed when considering inhaler choice. To evaluate whether the soft mist inhaler (SMI) is suitable for COPD patients irrespective of inhalation ability, the TRONARTO study investigated the efficacy of dual long-acting bronchodilator therapy delivered via the Respimat® SMI on lung function in patients with COPD stratified by inhalation ability. Tiotropium/olodaterol delivered via the SMI was effective both in patients with peak inspiratory flow (PIF) < 60 L/min and PIF ≥ 60 L/min, measured against medium-low resistance.
Methods: This congress compilation summarizes post hoc analyses from the TRONARTO study presented at the annual American Thoracic Society 2022 and European Respiratory Society 2022 meetings. These analyses evaluated PIF in over 200 patients, with PIF measurements taken daily at home for 4 weeks, and in the clinic at baseline, Weeks 2 and 4.
Results: Overall, 57.9% of patients had a PIF range (difference between lowest and highest PIF measurements) < 20 L/min (12.4% of patients had PIF range < 10 L/min). At-home PIF range decreased over the study period, suggesting that inhaler training/repeated PIF measurements may help to make patients’ inspiratory effort more consistent. Some patient characteristics correlated with lower PIF (female gender, shorter stature, more severe disease, worse airflow obstruction) and lower PIF range (more severe disease). PIF measurements differed between medium-low and high-resistance settings, highlighting the importance of measuring PIF at the resistance of a patient’s inhaler. PIF correlated poorly with spirometry measurements.
Conclusion: As indicated in COPD management guidelines, choice of inhaler is essential to optimize pharmacologic therapies for COPD. Poor inspiratory ability should be viewed as a treatable trait that can help to inform inhaler choice. Inhaler training and consideration of PIF (if patients use a dry powder inhaler) can reduce patient-to-inhaler mismatch, with potential consequences for health status and exacerbation risk.
Keywords: COPD, dry powder inhaler, DPI, PIF, suboptimal, congress, variability, characteristics
Introduction
Chronic obstructive pulmonary disease (COPD) is a respiratory condition that requires long-term maintenance treatment for symptom relief and to reduce the risk of exacerbations.1,2 Inhaled bronchodilator therapy is currently the mainstay of COPD treatment, with long-acting muscarinic antagonists (LAMAs) and long-acting β2-agonists (LABAs) in combination being recommended as the initial therapy for the majority of symptomatic patients with COPD.2
The three main types of handheld inhalation devices to deliver treatment to patients with COPD are dry powder inhalers (DPIs), pressurized metered-dose inhalers (pMDIs) and soft mist inhalers (SMIs).3,4 The delivery and deposition of medication in the lungs by these devices can be affected by a range of patient-related factors, including, for DPIs, a patient’s peak inspiratory flow (PIF).4,5 If a patient uses their inhaler incorrectly, or has suboptimal PIF, they may not receive the clinically relevant dose to alleviate their symptoms and reduce the risk of exacerbations.3,6
In order to overcome the internal resistance of the inhaler and to separate the drug from its carrier molecule, most DPIs require patients to achieve a PIF of 30–60 L/min depending on the internal resistance of the device, although a PIF of 60 L/min is considered optimal for most devices to disaggregate the powder and produce drug particles of an optimal size.4,7–13 For patients with inadequate PIF, this can result in ineffective drug delivery from DPIs, impacting disease control and health status.6,8,14 pMDIs operate independently of PIF but rely on patients coordinating inhaler activation with the intake of breath.3 SMIs use mechanical energy to generate a slow-moving mist of drug and require slow, coordinated inhalation,3,15,16 though coordination is less of an issue for SMIs as the duration of the aerosol is approximately four times longer than pMDIs.16
The TRONARTO study evaluated the efficacy of drug delivery via the SMI in patients with COPD and different inhalation abilities.17 TRONARTO (NCT04223843) was a Phase IV, randomized, double-blind, placebo-controlled, multicenter, parallel-group study that investigated the efficacy of tiotropium/olodaterol (TIO/OLO) 5 μg/5 μg delivered via the SMI, Respimat® (Boehringer Ingelheim, Ingelheim am Rhein, Germany), on lung function in patients with COPD stratified by inhalation ability (<60 L/min or ≥60 L/min), measured with the In-Check DIAL G16 (Clement Clarke Ltd, Harlow, UK) set at medium-low resistance.17 Included patients were aged ≥40 years, current or ex-smokers, with a spirometry-confirmed diagnosis of moderate-to-severe COPD.17 The TRONARTO study (n=213) showed that treatment with TIO/OLO via the SMI results in significant lung function improvements versus placebo, irrespective of the PIF that a patient can generate.17
This article summarizes post hoc analyses from the TRONARTO data set, which addresses the variation in PIF over time, comparisons between at-home and in-clinic measurements of PIF, associations between PIF and expiratory spirometry measures, and patient characteristics. Daily PIF measurements from over 200 patients participating in TRONARTO were included in these post hoc analyses.17
Presentation 1: The Variability of Peak Inspiratory Flow: Analyses of the TRONARTO Population
Mahler DA, Watz H, Ritz J, Gardev A, Shaikh A, Drummond MB.18
This post hoc analysis evaluated PIF variability in the 209 patients (of 213 randomized) who had available at-home PIF measurements in the TRONARTO study. Patients measured their PIF daily for 4 weeks at home, using an In-Check DIAL G16 device set to medium-low resistance. All PIF values were analyzed and the variability between PIF values (all patients, all days) was assessed, as well as within-patient PIF range. Data were pooled for all patients irrespective of treatment (TIO/OLO or placebo) or PIF stratum at screening.
From this analysis, three patient subgroups were identified based on the recorded daily PIF values. One in five patients consistently had a PIF <60 L/min (“can’t do”, where patients had consistently poor inspiratory ability to optimally operate DPIs of medium-low resistance), one in three patients had values above and below 60 L/min (“can and sometimes do”, where patients’ inspiratory ability was sufficient to operate DPIs of medium-low resistance on some but not all days) and half of patients consistently achieved a PIF ≥60 L/min (“can and consistently do”, where patients had consistently sufficient inspiratory ability to operate DPIs of medium-low resistance) (Figure 1).
 |
Figure 1 Patient subgroups based on self-measured PIF readings. Abbreviation: PIF, peak inspiratory flow. Note: Data from Mahler et al.18 |
Analysis of the overall distribution of PIF measurements showed that individual PIF values most commonly fell between 50 and <60 L/min (Figure 2a). In addition, over the 4-week study period, the most common PIF range (difference between a patient’s highest and lowest PIF values) was 10–<20 L/min (Figure 2b). Overall, 12.4% of patients had a PIF range of <10 L/min over the 4-week period; 57.9% had a PIF range of <20 L/min, 86.1% had a PIF range of <30 L/min, and 13.9% had a PIF range of >30 L/min.
 |
Figure 2 (a) overall distribution of PIF values and (b) range between highest and lowest PIF value (by PIF subgroup based upon self-measured PIF readings over 28 days). Abbreviation: PIF, peak inspiratory flow. Note: Data from Mahler et al.18 |
Clinical Implications
In patients with COPD, PIF can be variable in the home setting. Some patients have consistently adequate PIF, whereas others have variable or consistently low PIF, which is suboptimal for medium-low-resistance DPI devices. Considering this variability, if a patient uses a DPI, it is advisable to assess PIF at each clinic visit to ensure that patients are matched with their inhalers effectively. Patients with variable PIF could be considered for inhaler training to avoid potential inadequate dosing. Patients with consistently low PIF should be offered an alternative device with an active drug delivery mechanism, such as an SMI, pMDI, or nebulizer.
Presentation 2: TRONARTO Post Hoc Analysis: Factors Affecting Peak Inspiratory Flow (PIF) Variability
Mahler DA, Watz H, Emerson-Stadler R, Ritz J, Shaikh A, Drummond MB.19
This post hoc analysis evaluated the relationship between patient characteristics and PIF variability in 213 patients with COPD randomized in the TRONARTO study. Patients were stratified into quartiles according to their PIF range (ie, the difference between their minimum and maximum PIF values), derived from at-home daily PIF measurements. These quartiles were tested for trend against baseline characteristics including age, gender, stature and disease severity (Global Initiative for Chronic Obstructive Lung Disease [GOLD] stage 2 vs 3). Additionally, differences between in-clinic PIF (single measurements at baseline, Week 2 and Week 4) and at-home PIF (mean average of aggregated at-home PIF values during the week closest to each clinic visit) were compared, overall and by PIF strata from the original TRONARTO study (<60 L/min or ≥60 L/min). Lastly, weekly PIF range (using the 28 days of at-home measurements), and weekly population means and standard errors (SEs) were calculated. For PIF range, a test for trend with study duration was performed.
Patients with more severe disease were found to have a significantly lower PIF range than those with moderate disease (GOLD 3 vs 2; P=0.01), but no other baseline characteristic showed a trend with PIF range (Table 1).
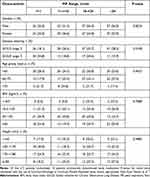 |
Table 1 Trend Analysis for Baseline Characteristics Across the PIF Range Quartiles |
The mean (SE) difference between PIF measured at home versus in the clinic decreased between clinic visits (Figure 3). Over the study period, the mean PIF in the overall population increased slightly from baseline (65.97 L/min) to Week 4 (69.52 L/min), though the change was not statistically significant. In contrast, the weekly PIF range (difference between minimum and maximum daily PIF measurements for each patient) and variance (SE) declined significantly with study week (11.35 L/min [SE 0.50] at Week 1 to 8.78 L/min [SE 0.39] at Week 4 [test-of-trend P=0.0001]) (Figure 4).
 |
Figure 3 Difference in PIF between at-home and in-clinic visits.a Abbreviations: BL, baseline; PIF, peak inspiratory flow. Notes: aA single measurement was taken in the clinic and a mean value for the respective week at home (seven measurements per patient). Data from Mahler et al.19 Error bars depict standard error. |
 |
Figure 4 Mean PIF, weekly PIF range and variance (SE) in the overall population by study week. Abbreviations: BL, baseline; PIF, peak inspiratory flow; SE, standard error. Notes: Baseline PIF was measured in the clinic at first visit. Numbers in parentheses represent SE for both mean PIF and PIF range. At-home PIF measurements over respective weeks for each patient were used in the calculation of weekly PIF mean and range (difference between the highest and lowest PIF values). Data from Mahler et al.19 |
Clinical Implications
Previous research has shown that certain COPD patient phenotypes are more likely to have lower PIF,20 but to our knowledge, there is little known about factors associated with PIF variability. In our analysis, with the exception of disease severity constraining a patient’s PIF range, no other baseline characteristic was found to predict PIF range. This finding highlights the difficulty in predicting which patients will have variable PIF performance by the usual clinical characteristics available to the healthcare professional.
A comparison of in-clinic versus at-home PIF measurements showed that if a patient had low PIF at their clinic visit (before regular PIF measurements were made), they were likely to generate an even lower PIF reading when at home. If a physician is concerned that a patient’s PIF at home will not be sufficient for their inhaler, they should offer the patient an alternative device to avoid a potential mismatch between a patient’s inspiratory ability and their inhaler’s airflow requirement, thereby avoiding potentially compromising a patient’s health status. Within-patient PIF range reduced over the 4-week analysis period, suggesting that with training/repeated PIF measurements, patients could become more consistent in their inspiratory effort. Appropriate training in a real-world setting warrants further exploration.
Presentation 3: Relationship Between Peak Inspiratory Flow and Patient Characteristics: Analysis of Trends from the TRONARTO Population
Drummond MB, Watz H, Ritz J, Gardev A, Shaikh A, Mahler DA.21
This post hoc analysis investigated the potential association between baseline characteristics and PIF in the 213 patients with COPD who were randomized in the TRONARTO study. At baseline, post-bronchodilator PIF was measured in the clinic with the patient in a seated position using the In-Check DIAL G16 at medium-low and high resistance. Triplicate readings were taken and the highest PIF value was recorded at each resistance setting. Patients were then stratified into six groups (PIF: <30, 30–<45, 45–<60, 60–<80, 80–<100 and ≥100 L/min) according to their highest PIF against medium-low resistance, and a test of trend was carried out against various baseline characteristics.
Female gender (P=0.0013), shorter stature (P=0.0008), more severe disease (GOLD stage 3 vs GOLD 2; P=0.0056), worse airflow obstruction (post-bronchodilator forced expiratory volume in 1 second [FEV1] [P<0.0001] and percent predicted FEV1 [P=0.0122]) were found to be significantly associated with lower PIF; no trends were observed for age or body mass index (Table 2).
 |
Table 2 Trend Analysis for Baseline Characteristics Across the PIF Subcategories |
In a linear regression analysis, a strong correlation was observed between baseline PIF measurements at the medium-low- and high-resistance settings (correlation coefficient, R=0.766) (Figure 5). However, PIF measurements were not equal at the different resistance settings, with high-resistance measurements having 64% of the value of medium-low-resistance measurements. In a separate regression analysis, a weak correlation was observed between baseline PIF and FEV1 or forced vital capacity (FVC) (correlation coefficients, 0.303 and 0.365, respectively [Figure 6]). Figures 5 and 6 were created using locally estimated scatterplot smoothing.
 |
Figure 5 Correlation of PIF recorded at medium-low and high resistance. Abbreviation: PIF, peak inspiratory flow. Notes: Figure created using locally estimated scatterplot smoothing. Data from Drummond et al.21 |
 |
Figure 6 Correlation of PIF with FVC and FEV1. Abbreviations: FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; PIF, peak inspiratory flow. Notes: aPost-bronchodilator. Figure created using locally estimated scatterplot smoothing. Data from Drummond et al.21 |
Clinical Implications
In this population of patients with moderate-to-severe COPD, factors such as female gender, shorter stature, more severe disease and worse airflow obstruction were significantly associated with lower PIF. Although PIF at medium-low resistance strongly correlated with PIF at high resistance, the PIF measurements at the two resistance settings were not equal. Hence, PIF readings measured at different resistances are not interchangeable, highlighting the importance of measuring PIF at the resistance of the patient’s inhaler in order to ensure a good match between patients and their DPIs. In linear regression, PIF measurements showed a poor correlation with FVC and FEV1, indicating that patient expiratory ability (ie, spirometry measurements) should not be used to predict inspiratory ability.
Discussion
COPD is a complex, heterogeneous condition that is associated with a high personal and societal burden.2 There is increasing recognition that a precision-medicine approach would be beneficial in COPD to deliver treatments that are targeted to the individual needs of patients.22–25 To this end, it is valuable to identify “treatable traits”, which have been defined as traits that are clinically relevant and associated with specific outcomes, easily identifiable and measurable, and treatable.23 Key to the success of this approach is that any treatable trait should be evaluable objectively by means of a biomarker.23
In COPD, airflow limitation and muscle deconditioning are treatable traits, which can be assessed using the biomarkers of FEV1 and exercise testing (eg, 6-minute walk test), and treated with bronchodilators and pulmonary rehabilitation, respectively.22,23,26 Similarly, poor inspiratory ability can be considered a treatable trait, assessed using PIF as a biomarker, and optimally managed by matching a patient’s inspiratory ability with the most appropriate inhaler device and offering self-management education and digital behavioral change interventions (including electronic monitoring devices or automated text messages) to improve the consistency of inhalation effort.22,27
The GOLD report 2023 highlights the importance of inhalation ability, with DPIs described as unsuitable for patients unable to perform a forceful and deep maneuver.2 Suboptimal PIF is associated with poor outcomes, as shown by results from previous studies.6,8,28 The PIFotal cross-sectional observational study assessed the impact of PIF, inhalation technique errors and medication adherence on patient-reported outcomes in ~1400 patients with COPD in the primary care setting who had been using a DPI for COPD maintenance therapy for ≥3 months.6 In the PIFotal cohort, 29% had suboptimal PIF; 16% were able to generate optimal PIF when inhaling maximally but not through typical inhalation maneuvers (“can, but will not do”) and 13% were incapable of generating the sufficient PIF required for their device under either maximal or typical inhalation maneuvers (“cannot do”).6 Suboptimal PIF in the PIFotal study was associated with significant reductions in health status (lower scores on the Clinical COPD questionnaire).6 Although no significant association was found between suboptimal PIF and moderate and severe exacerbations, participants with suboptimal PIF had a 73% higher rate of severe exacerbations compared with those with optimal PIF.6 These results are in line with previous studies, which found an association between reduced PIF and an increased risk of acute exacerbations of COPD.7,8
Results from the TRONARTO post hoc analyses support the need to identify patients with low PIF, if a DPI is being considered, in order to make the most informed choice of inhaler that matches the patient’s clinical inspiratory phenotype. In our analyses, some patients had consistently adequate inspiratory ability as assessed by daily measurement of PIF; however, as many as one in three had variable PIF readings and one in five had consistently low PIF readings,18 which are suboptimal for medium/low-resistance DPI devices. Patients with variable PIF should be considered for inhaler training in order to avoid inadequate dosing and consequent poor outcomes. In patients with consistently low PIF, inhalers requiring a specific degree of effort should be avoided and an active device preferred, such as an SMI, pMDI, or nebulizer. In one study comparing delivery of LAMA monotherapy via SMI versus DPIs, SMI users had fewer exacerbations and a lower risk of re-admission following hospitalization,29 suggesting the potential benefits of an active drug delivery system. Similarly, another study found that the LAMA/LABA combination of TIO/OLO was associated with higher therapeutic effects on lung function and symptoms compared with LAMA/LABA combinations delivered by DPI.30 Given the differences in lung deposition between SMIs and DPIs,31 it is possible that the higher drug deposition associated with SMIs may translate into better clinical outcomes for patients who are not well suited to DPI devices.
Reinforcing the need for inhaler training or switching of inhaler where appropriate, our research showed that if patients have low PIF readings in the clinic, their PIF is likely to be even lower at home, when not being observed or supervised by their healthcare provider.19 We also found that inspiratory ability is weakly correlated with expiratory ability, and that the PIF required for one device is not interchangeable for another device with different internal resistance.21 Consistent with our findings, a previous study did not find a strong association between PIF and expiratory ability.9 In a recent systematic review, however, low PIF did show a positive correlation with reduced FEV1 in 9 of 14 papers.20
From our data, PIF variability was not associated with specific patient characteristics, except for disease severity (more severe disease associated with lower PIF range).19 Low PIF, however, was found to be associated with specific characteristics (female gender, shorter stature, more severe disease and worse airflow obstruction).21 These data are in agreement with a recent review of the literature, which evaluated the relationship between PIF and patient and disease characteristics.20 According to this literature review, low PIF correlated with female gender, shorter height and greater disease severity, as well as other factors such as increased age and decreased handgrip/inspiratory muscle strength.20 Importantly, these clinical characteristics have no clear thresholds that can be used to clearly identify an individual with low PIF, highlighting the need to measure PIF directly and repeatedly.
Conclusion
These post hoc analyses of findings from the TRONARTO study provide insights supporting the important role of PIF as an indicator of inspiratory ability, which we believe should be considered a treatable trait informing inhaler choice in patients with COPD.
We have demonstrated that PIF can be variable in the home setting and that some patients consistently achieve optimal or suboptimal inspiratory effort required for DPIs. Unlike the PIF value (L/min), which can be associated with patient characteristics, such as female gender, shorter stature, more severe disease and worse airflow obstruction, PIF variability is harder to predict. Consideration of PIF if the patient is using a DPI can therefore help to inform inhaler choice to optimize clinical benefit from inhaled therapies by avoiding mismatches between patients and their inhalers.
Abbreviations
COPD, chronic obstructive pulmonary disease; DPI, dry powder inhaler; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; GOLD, Global Initiative for Chronic Obstructive Lung Disease; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist; PIF, peak inspiratory flow; pMDI, pressurized metered-dose inhaler; SE, standard error; SMI, soft mist inhaler; TIO/OLO, tiotropium/olodaterol.
Data Sharing Statement
To ensure independent interpretation of clinical study results and enable authors to fulfil their role and obligations under the ICMJE criteria, Boehringer Ingelheim grants all external authors access to relevant clinical study data. In adherence with the Boehringer Ingelheim Policy on Transparency and Publication of Clinical Study Data, scientific and medical researchers can request access to clinical study data after publication of the primary manuscript and secondary analyses in a peer-reviewed journals and regulatory and once reimbursement activities are completed, normally within 1 year after the marketing application has been granted by major Regulatory Authorities. Researchers should use the https://vivli.org/ link to request access to study data and visit https://www.mystudywindow.com/msw/datasharing for further information.
Ethics Approval and Informed Consent
The TRONARTO study protocol was reviewed and approved by the respective independent review boards and ethics committees of the participating sites: 26 in Germany and the United States of America beginning January 8, 2020 and ending September 29, 2020 (for full details, see Supplementary Table 1). The TRONARTO study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. All patients provided written informed consent.
Consent for Publication
All authors provide their consent for publication of this manuscript and all related contents. All patients provided their informed consent when entering the TRONARTO study.
Acknowledgments
These post hoc analyses were sponsored by Boehringer Ingelheim International GmbH. Medical writing assistance, in the form of the preparation and revision of the manuscript, was supported financially by Boehringer Ingelheim and provided by Paul Todd, PhD, at Meditech Media, under the authors’ conceptual direction and based on feedback from the authors.
Author Contributions
The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors. All authors 1) made a significant contribution to the work reported, whether through conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; 2) took part in drafting, revising or critically reviewing the article; 3) gave final approval of the version to be published; 4) agreed on the journal to which the article was submitted; and 5) agreed to be accountable for all aspects of the work. The authors received no compensation related to the development of the manuscript.
Disclosure
MBD reports research grants from the National Institutes of Health, Department of Defense, American Lung Association, Boehringer Ingelheim, Midmark and Teva unrelated to this work. He reports personal consulting fees from Boehringer Ingelheim, GlaxoSmithKline, AstraZeneca, Teva, Midmark, Verona, Optimum Patient Care, Chiesi, Becker Pharma and Polarean unrelated to this work. HW reports grants, personal fees and other support from Boehringer Ingelheim during the conduct of the study. Outside the submitted work, HW received grants, personal fees and other support from AstraZeneca, Chiesi, Bayer, Boehringer Ingelheim, GlaxoSmithKline, Sanofi, and Novartis. JR is an employee of Syneos Health working on behalf of Boehringer Ingelheim. AG, RE-S and AS are employees of Boehringer Ingelheim. DAM reports fees for advisory boards from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Teva, Theravance, Verona and Viatris; royalties from Salem Media Group – COPD: Answers to Your Questions (2015); work with pharmaceutical companies on the use of BDI/TDI; participation in speaker’s bureau for AstraZeneca, Boehringer Ingelheim and Teva; and https://www.donaldmahler.com – an educational website for those with COPD and their families. The authors report no other conflicts of interest in this work.
References
1. Lopez-Campos JL, Calero C, Quintana-Gallego E. Symptom variability in COPD: a narrative review. Int J Chron Obstruct Pulmon Dis. 2013;8:231–238. doi:10.2147/COPD.S42866
2. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: 2023 report; 2022. Available from: https://goldcopd.org/wp-content/uploads/2022/11/GOLD-2023-ver-1.0-14Nov2022_WMV.pdf.
3. Usmani OS. Choosing the right inhaler for your asthma or COPD patient. Ther Clin Risk Manag. 2019;15:461–472. doi:10.2147/TCRM.S160365
4. Mahler DA. The role of inspiratory flow in selection and use of inhaled therapy for patients with chronic obstructive pulmonary disease. Respir Med. 2020;161:105857.
5. Represas-Represas C, Aballe-Santos L, Fernandez-Garcia A, et al. Evaluation of suboptimal peak inspiratory flow in patients with stable COPD. J Clin Med. 2020;9(12):3949. doi:10.3390/jcm9123949
6. Kocks J, Wouters H, Bosnic-Anticevich S, et al. Factors associated with health status and exacerbations in COPD maintenance therapy with dry powder inhalers. NPJ Prim Care Respir Med. 2022;32(1):18. doi:10.1038/s41533-022-00282-y
7. Ghosh S, Pleasants RA, Ohar JA, Donohue JF, Drummond MB. Prevalence and factors associated with suboptimal peak inspiratory flow rates in COPD. Int J Chron Obstruct Pulmon Dis. 2019;14:585–595. doi:10.2147/COPD.S195438
8. Loh CH, Peters SP, Lovings TM, Ohar JA. Suboptimal inspiratory flow rates are associated with chronic obstructive pulmonary disease and all-cause readmissions. Ann Am Thorac Soc. 2017;14(8):1305–1311. doi:10.1513/AnnalsATS.201611-903OC
9. Ghosh S, Ohar JA, Drummond MB. Peak inspiratory flow rate in chronic obstructive pulmonary disease: implications for dry powder inhalers. J Aerosol Med Pulm Drug Deliv. 2017;30(6):381–387. doi:10.1089/jamp.2017.1416
10. Virchow JC, Crompton GK, Dal Negro R, et al. Importance of inhaler devices in the management of airway disease. Respir Med. 2008;102(1):10–19.
11. Capstick TG, Clifton IJ. Inhaler technique and training in people with chronic obstructive pulmonary disease and asthma. Expert Rev Respir Med. 2012;6(1):91–101; quiz 102–103. doi:10.1586/ers.11.89
12. Newman SP. Inhaler treatment options in COPD. Eur Resp Rev. 2005;14(96):102–108. doi:10.1183/09059180.05.00009605
13. Mahler DA. Peak inspiratory flow rate as a criterion for dry powder inhaler use in chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2017;14(7):1103–1107. doi:10.1513/AnnalsATS.201702-156PS
14. Sulaiman I, Cushen B, Greene G, et al. Objective assessment of adherence to inhalers by patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;195(10):1333–1343.
15. Dhand R, Eicher J, Hansel M, Jost I, Meisenheimer M, Wachtel H. Improving usability and maintaining performance: human-factor and aerosol-performance studies evaluating the new reusable Respimat inhaler. Int J Chron Obstruct Pulmon Dis. 2019;14:509–523. doi:10.2147/COPD.S190639
16. Hochrainer D, Holz H, Kreher C, Scaffidi L, Spallek M, Wachtel H. Comparison of the aerosol velocity and spray duration of Respimat Soft Mist inhaler and pressurized metered dose inhalers. J Aerosol Med. 2005;18(3):273–282. doi:10.1089/jam.2005.18.273
17. Mahler DA, Ludwig-Sengpiel A, Ferguson GT, et al. TRONARTO: a randomized, placebo-controlled study of tiotropium/olodaterol delivered via soft mist inhaler in COPD patients stratified by peak inspiratory flow. Int J Chron Obstruct Pulmon Dis. 2021;16:2455–2465. doi:10.2147/COPD.S324467
18. Mahler D, Watz H, Ritz J, Gardev A, Shaikh A, Drummond M. The variability of peak inspiratory flow: analyses of the TRONARTO population.
19. Mahler DA, Watz H, Emerson-Stadler R, Ritz J, Shaikh A, Drummond MB. The variability of peak inspiratory flow: analyses of the TRONARTO population.
20. Leving MT, Kocks J, Bosnic-Anticevich S, Dekhuijzen R, Usmani OS. Relationship between peak inspiratory flow and patient and disease characteristics in individuals with COPD-a systematic scoping review. Biomedicines. 2022;10(2):458. doi:10.3390/biomedicines10020458
21. Drummond M, Watz H, Ritz J, Gardev A, Shaikh A, Mahler D. Relationship between peak inspiratory flow and patient characteristics: analysis of trends from the TRONARTO population.
22. Agusti A, Bel E, Thomas M, et al. Treatable traits: toward precision medicine of chronic airway diseases. Eur Respir J. 2016;47(2):410–419. doi:10.1183/13993003.01359-2015
23. Cardoso J, Ferreira AJ, Guimaraes M, Oliveira AS, Simao P, Sucena M. Treatable traits in COPD - a proposed approach. Int J Chron Obstruct Pulmon Dis. 2021;16:3167–3182.
24. McDonald VM, Fingleton J, Agusti A, et al. Treatable traits: a new paradigm for 21st century management of chronic airway diseases: Treatable Traits Down Under International Workshop report. Eur Respir J. 2019;53(5):1802058. doi:10.1183/13993003.02058-2018
25. McDonald VM, Osadnik CR, Gibson PG. Treatable traits in acute exacerbations of chronic airway diseases. Chron Respir Dis. 2019;16:1479973119867954. doi:10.1177/1479973119867954
26. Agusti A, Bafadhel M, Beasley R, et al. Precision medicine in airway diseases: moving to clinical practice. Eur Respir J. 2017;50(4):1701655. doi:10.1183/13993003.01655-2017
27. Blakey JD, Bender BG, Dima AL, Weinman J, Safioti G, Costello RW. Digital technologies and adherence in respiratory diseases: the road ahead. Eur Resp J. 2018;52(5):1801147.
28. Mahler DA, Demirel S, Hollander R, et al. High prevalence of suboptimal peak inspiratory flow in hospitalized patients with COPD: a real-world study. Chronic Obstr Pulm Dis. 2022;9(3):427–438. doi:10.15326/jcopdf.2022.0291
29. Singer D, Bengtson LGS, Elliott C, Buikema AR, Franchino-Elder J. Healthcare resource utilization, exacerbations, and readmissions among medicare patients with chronic obstructive pulmonary disease after long-acting muscarinic antagonist therapy initiation with soft mist versus dry powder inhalers. Int J Chron Obstruct Pulmon Dis. 2020;15:3239–3250. doi:10.2147/COPD.S284678
30. Cheng S-L. Comparison of effectiveness using different dual bronchodilator agents in chronic obstructive pulmonary disease treatment. J Clin Med. 2021;10(12):2649. doi:10.3390/jcm10122649
31. Ciciliani AM, Langguth P, Wachtel H. In vitro dose comparison of Respimat® inhaler with dry powder inhalers for COPD maintenance therapy. Int J Chron Obstruct Pulmon Dis. 2017;12:1565–1577. doi:10.2147/COPD.S115886
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
