Back to Journals » OncoTargets and Therapy » Volume 15
Clinical Impact of Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor Associated Clostridioides difficile Infection Among Patients with Lung Cancer
Authors Chung YS, Lin YC , Hung MS, Ho MC, Fang YH
Received 19 August 2022
Accepted for publication 21 December 2022
Published 28 December 2022 Volume 2022:15 Pages 1563—1571
DOI https://doi.org/10.2147/OTT.S386807
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Arseniy Yuzhalin
Ying-Shan Chung,1,2 Yu-Ching Lin,3,4 Ming-Szu Hung,3– 5 Meng-Chin Ho,3 Yu-Hung Fang2,3
1Department of Pharmacy, Chang Gung Memorial Hospital, Puzi City, Taiwan, Republic of China; 2Department of Nursing, Chang Gung University of Science and Technology, Puzi City, Taiwan, Republic of China; 3Division of Thoracic Oncology, Department of Pulmonary and Critical Care Medicine, Chang Gung Memorial Hospital, Puzi City, Taiwan, Republic of China; 4Department of Respiratory Care, Chang Gung University of Science and Technology, Puzi City, Taiwan, Republic of China; 5School of Medicine, College of Medicine, Chang Gung University; Guishan Township, Taoyuan, Taiwan, Republic of China
Correspondence: Yu-Hung Fang, Division of Thoracic Oncology, Department of Pulmonary and Critical Care Medicine, Chang Gung Memorial Hospital, Chiayi Branch, No. 6, W. Sec., Jiapu Road, Puzi City, Chiayi County, 61363, Taiwan, Republic of China, Tel +886-5-362-1000 ext. 2762, Fax +886-5-362-3005, Email [email protected]
Aim: Epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKIs)-associated Clostridioides difficile infection (CDI) among lung cancer patients have been reported in case reports and adverse events reporting system databases in the United States and Japan, but clinical data remained insufficient. This study aims to evaluate CDI in lung cancer patients receiving EGFR-TKIs.
Methods: We conducted a retrospective cohort study using multi-institutional electronic medical records database. We included patients aged older than 20 years diagnosed with lung cancer and treated with EGFR-TKIs (gefitinib, erlotinib, afatinib). We defined EGFR-TKI initiation date as the index date and occurrence of diarrhea with CDI or without CDI as the event date. We followed patients from the index date until the event date, ICU admission, death, or 12/31/2019.
Results: We included 2242 diarrhea patients, 51 were EGFR-TKI with CDI cohort, and 2191 were diarrhea without CDI cohort. Patients who were concurrently taking antibiotics (hazard ratio [HR], 3.30; 95% CI, 1.67– 6.5) and systemic steroids (HR, 4.9; 95% CI, 2.65– 9.06) had an increased risk of CDI. First-generation EGFR-TKIs tended to be associated with an increased risk of CDI compared with afatinib (HR, 1.81, 95% CI, 0.94– 3.47). EGFR-TKI with CDI had a higher ICU admission rate (HR, 3.42, 95% CI, 1.98– 5.91) and mortality rate (HR, 2.34, 95% CI, 1.67– 3.28) than diarrhea without CDI.
Conclusion: Patients with CDI had higher ICU admission rates and mortality rates than those without CDI. Concurrent use of antibiotics and systemic steroids were risk factors for CDI among patients with lung cancer receiving EGFR-TKIs. Afatinib was not associated with a higher risk of CDI than first-generation EGFR-TKIs.
Keywords: Clostridioides difficile infection, CDI, epidermal growth factor receptor tyrosine kinase inhibitors, EGFR-TKIs, lung cancer
Introduction
Lung cancer is the leading cause of cancer-related death worldwide.1 Tyrosine kinase inhibitors (TKIs) are one of the most important advances in lung cancer treatment in recent times and have resulted in improved survival outcomes for patients with advanced stage lung cancer harboring epidermal growth factor receptor (EGFR)-sensitizing mutations.2 Many clinical trials have revealed that EGFR-TKIs, especially second-generation TKIs such as afatinib or dacomitinib, cause considerable gastrointestinal (GI) toxicities such as nausea, vomiting, and diarrhea.3 The incidence of all-grade diarrhea without any antidiarrheal prophylaxis ranges between 18% to 95%, and up to 25% of patients experience grade 3 or higher diarrhea.4 Severe diarrhea can lead to dehydration, electrolyte imbalances, acute kidney injury, and even death.
Differential diagnoses for EGFR-TKI-associated diarrhea include infection, inflammatory bowel disease, irritable bowel disease, ischemic enteritis, or diarrhea caused by other medications.4 Clostridioides difficile infection (CDI) is a common concern for patients with cancer. In a prospective multicenter study, the incidence of CDI among patients with lung cancer receiving chemotherapy was 22.7% of all diarrhea cases.5 Studies have also reported that even patients who had not used any antibiotics developed CDI after chemotherapy.6 Early identification of TKI-associated diarrhea, diet instructions, and treatment with antimotility agents such as loperamide can effectively reduce TKI treatment interruptions.7 However, the use of antimotility agents in patients with active CDI has traditionally been avoided because of possible GI complications.8 Therefore, it is necessary to distinguish whether a patient is suffering from CDI or TKI-associated diarrhea so that the appropriate treatment can be administered to reduce unnecessary complications.
A few clinical reports have shown that the use of EGFR-TKIs may be associated with CDI,9,10 but the correlation and clinical patterns among patients with lung cancer remains insufficient. A study calculating the reporting odds ratios (RORs) using the Food and Drug Administration (FDA) Adverse Event Reporting System (AERS) database between January 2004 and March 2018 reported that the incidences of EGFR-TKI-associated pseudomembranous colitis were the highest with afatinib (ROR: 14.56).11 However, no previous studies have analyzed whether afatinib is a risk factor for CDI.
This study aims to evaluate the risk factors for CDI in patients with lung cancer receiving EGFR-TKIs. We hypothesized that afatinib would be associated with a higher risk of CDI in patients with lung cancer than first-generation EGFR-TKIs such as gefitinib or erlotinib.
Methods
Data Source
The Chang Gung Research Database (CGRD), a large multi-institutional electronic medical record (EMR) database in Taiwan, was the basis for this retrospective cohort study. The CGRD covers seven Chang Gung Memorial Hospitals throughout northern and southern Taiwan and contains the EMRs of 1.3 million patients (6.1% of Taiwan’s population). Disease identification in the CGRD follows the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) in the period prior to 2016, followed by the ICD-10-CM thereafter. The database includes pathological and laboratory examination data, which are useful to confirm patient diagnoses and disease status. The CGRD covers approximately 6.1% and 10.2% of the entire Taiwanese outpatient and inpatient population, respectively. The CGRD data structures and representativeness have been described in more detail elsewhere,12,13 and many diagnostic codes used within the CGRD have been validated.14,15 This study was approved by the Institutional Review Boards of Chang Gung Medical Foundation (202101121B0) and conformed to the Helsinki Declaration.
Study Population
In this retrospective study, we included patients aged 20 years or older with lung cancer who had ever been treated with EGFR-TKIs, including gefitinib, erlotinib, afatinib, or osimertinib, between 2011 and 2019. The diagnosis of lung cancer was identified according to the ICD-9-CM code 162 and the ICD-10-CM code C34 and ascertained by the Registry of Catastrophic Illness Database. The Registry of Catastrophic Illness Database is a subset of the National Health Insurance Research Database (NHIRD), and pathological confirmation of lung cancer is required to apply for this certification.16 To prevent preexisting events from confounding the analyses, we excluded patients with any history of inflammatory bowel disease, intestinal obstruction, ischemic bowel disease, radiation colitis, or GI cancer (Figure 1).
 |
Figure 1 Flow Chart. |
We defined the first date of an EGFR-TKI prescription as the index date and occurrence of diarrhea with CDI (cases) or diarrhea without CDI (controls) as the event date. CDI was defined as a CDI diagnosis based on ICD-9-CM or ICD-10-CM codes, a positive test for C. difficile toxin A or B, or a positive stool culture for C. difficile. Diarrhea without CDI was defined as a prescription for anti-diarrheal medications, such as loperamide or dioctahedral smectite, for longer than 14 days. We followed up patients from the index date to the event date, death, or 12/31/2019.
Covariates
We retrieved data from one year prior to the index date for baseline patient characteristics, including age, gender, prior lines of lung cancer treatment, type of EGFR-TKI, intensive care unit (ICU) admission, comorbidities (ie, hypertension, diabetes mellitus, coronary artery disease, ischemic stroke, chronic obstructive pulmonary disease, peptic ulcer, or chronic kidney disease). Definitions of prior lines of lung cancer treatment were any one use of the following medication will be one line: chemotherapy: docetaxel, paclitaxel, gemcitabine, vinorelbine, pemetrexed, TS-1; target therapy: afatinib, erlotinib, gefitinib, osimertinib; alectinib, brigatinib, ceritinib, crizotinib, loratinib; immunotherapy: pembrolizumab, nivolumab, atezolizumab. Data from six months before the index date were retrieved for concomitant medications, including antibiotics, systemic steroids, and proton pump inhibitors (PPIs).17,18 We defined the wash-out periods for antibiotics, systemic steroids, and PPIs for 7 days, 14 days, and 14 days before the event date, respectively, to avoid coexisting CDI/diarrhea events confounding the analyses.19–21
Statistical Analysis
The baseline characteristics of the study patients are presented as numbers and percentages, means and standard deviations (SDs), or medians and interquartile ranges (IQRs), if appropriate. We used multivariate Cox proportional hazards regression models to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) for the risk of CDI. Afatinib users were considered the reference group because afatinib is a newer generation TKI associated with more diarrhea events.22 We considered results with a two-sided P value < 0.05 to be statistically significant. Statistical analyses were performed using the SAS Enterprise Guide (Version 7.1; SAS Institute Inc., Cary, North Carolina).
Results
We identified a total of 5759 patients with lung cancer aged 20 years or older who received EGFR-TKIs, including gefitinib, erlotinib, afatinib, or osimertinib, between 2011 and 2019 (Figure 1). We totally excluded 3517 patients, among whom three were diagnosed with inflammatory bowel disease; 50 with intestinal obstruction; six with ischemic bowel disease; seven with radiation colitis; and 68 with GI cancer one year prior to the index date. We excluded 3380 patients did not meet the criteria for diarrhea with a prescription for antidiarrheal medications for longer than 14 days. Only 20 patients received osimertinib or dacomitinib, and only one of them developed CDI, so we did not include them in the analysis because small sample size and too short observation period of these 2 drugs (Taiwan’s national health insurance reimbursed osimertinib in 2020/04 and dacomitinib in 2020/10). Finally, we included 2242 diarrhea patients in our study cohort, among whom 51 were included in the EGFR-TKI with CDI cohort and 2191 of whom were in the EGFR-TKI with diarrhea cohort. The incidence rate of EGFR-TKI associated CDI among was only 0.8%. Among patients with EGFR-TKI related diarrhea, the incidence rate of EGFR-TKI associated CDI was 2.2%.
Table 1 presents the baseline patient characteristics of the two cohorts. In the EGFR-TKI with CDI cohort, the mean age was 66.5 (± 11.1) years, and 64.7% of the patients were female; in the EGFR-TKI with diarrhea cohort, the mean age was 65.4 (± 11.5) years, and 60.9% of the patients were female. In the EGFR-TKI with CDI cohort and EGFR-TKIs with diarrhea cohort, 68.6% and 75.0% of the patients, respectively, were treated with first-line EGFR-TKIs. Most patients in both cohorts suffered from comorbidities such as hypertension (56.9% vs 42.4), diabetes mellitus (33.3% vs 21.1%), and peptic ulcers (33.3% vs 22.2%). Patients in the EGFR-TKI with CDI cohort suffered from a higher incidence of stroke (27.5% vs 11.2%) and chronic kidney disease (15.7% vs 6.1%) than those in the EGFR-TKI with diarrhea cohort. In the six months before initiation of EGFR-TKI therapy, the EGFR-TKI with CDI cohort had higher ICU admission rate (26.9% vs 9.6%) than the EGFR-TKI with diarrhea cohort. Patients in the EGFR-TKI with CDI cohort also received more medications including antibiotics (37.3% vs 8.3%), PPI (31.4% vs 16.2%), and systemic steroids (56.9% vs 15.5%) in the six months prior to treatment using EGFR-TKIs than those in the EGFR-TKI with diarrhea cohort. Among patients with CDI, 34 received gefitinib or erlotinib, and 17 received afatinib. There was a longer period of time from index date to event date in the EGFR-TKI with CDI cohort, and the median period was 75 days (range, 25 to 376 days) and 7 days (range, 0 to 49 days) in the EGFR-TKI with CDI and EGFR-TKI with diarrhea cohorts, respectively. Huge disparity between the mean and median demonstrates that data is not normal distribution but seriously skewed.
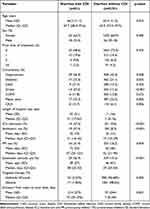 |
Table 1 Baseline Characteristics |
Multivariate analyses of CDI risk from six months before the index date were conducted (Table 2). We found that patients who received antibiotics (HR, 3.30; 95% CI, 1.67–6.50) and systemic steroids (HR, 4.90; 95% CI, 2.65–9.06) had an increased risk of CDI. Patients with stroke also tended to have an increased risk of CDI (HR: 2.00; 95% CI, 0.99–4.05) with a p-value > 0.05. First-generation EGFR-TKIs (gefitinib and erlotinib) tended to have be associated with an increased risk of CDI compared with afatinib (HR, 1.81; 95% CI, 0.94–3.47). Afatinib was not associated with an increased risk of CDI. Patients with CDI had higher ICU admission rates (adjusted HR, 2.34, 95% CI, 1.68–3.28) and mortality rates (Adjusted HR, 3.42, 95% CI 1.98–5.91) than patients without CDI in the succeeding three years. (Table 3)
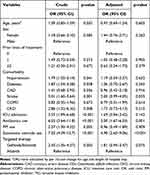 |
Table 2 Multivariate Analyses of CDI Risk from 6 Months Before the Index Date |
 |
Table 3 Comparison of ICU Admission and Mortality Between the Two Groups for 3 Years |
Discussion
This is the first study to evaluate the risk factors for EGFR-TKI-associated CDI among patients with lung cancer. We found that CDI occurred much later than the usual adverse effect of diarrhea caused by EGFR-TKIs. Patients who received antibiotics and systemic steroids had an increased risk of CDI. Conversely, afatinib was not a risk factor for CDI, but first-generation TKIs tended to be associated with an increased risk of CDI. Patients with CDI had a much higher ICU admission rate and mortality rate than those without CDI.
A study using US National Hospital Discharge surveys showed that the CDI incidence rate among patients with cancer was 0.86% per cancer discharge, with a high mortality rate (9.4%). CDI was associated with significantly increased mortality and longer hospital stays among patients with cancer.23 A multicenter study reported that the rate of hospital-acquired CDI in patients with cancer was more than twice the rate of general hospitalization in the US (15.8 vs 7.4 per 10,000 patient-days).24 The presence of malignancy also has an important impact on CDI treatment and is an independent risk factor for recurrent CDI.25 In the present study, CDI was also independently associated with high mortality and a high ICU admission rate. We also evaluate if ICU admission within 6 months prior to EGFR-TKIs treatment could be a risk factor for CDI. But in multivariate analyses, ICU admission had higher HR for CDI, p value showed no statistical significance. Systemic steroid and antibiotic use within six months prior to initiation of EGFR-TKIs increased the risk of CDI. Restricting the unnecessary use of systemic steroids or antibiotics may help to reduce the occurrence of CDI.
Research on EGFR-TKI-associated CDI is still limited. Case reports on the use of TKIs (dasatinib) to treat leukemia have shown EGFR-TKI-related CDI.8,9 A study using the JADER showed that 375 cases of C. difficile colitis and 903 cases of pseudomembranous colitis were reported between April 2004 and September 2017.10 The case proportions of CDI and pseudomembranous colitis ascribed to EGFR-TKIs (gefitinib, erlotinib, and afatinib) were 0.8% (3/375) and 0.4% (4/903), respectively. Another study, which calculated RORs using the FDA AERS database between January 2004 and March 2018, reported that the incidence of EGFR-TKI-associated pseudomembranous colitis was the highest with afatinib (ROR: 14.56), followed by erlotinib (ROR: 5.11), gefitinib (ROR: 3.92), and osimertinib (ROR: 3.09).11 However, afatinib, a second-generation EGFR-TKI, is known to have a higher rate of diarrhea side effects than first-generation EGFR-TKIs such as gefitinib and erlotinib. Higher ORs of CDI with afatinib could be caused by a notoriety bias.26 In the present study, conversely, we found an unexpected trend of an increased CDI risk in patients receiving first-generation EGFR-TKIs (gefitinib and erlotinib) compared with those receiving afatinib. Given the relatively small sample size and short follow-up period, the results of the analysis were not significant.
In a retrospective study,18 44 CDI cases were diagnosed among 188 patients with lung cancer receiving chemotherapy. Poor performance status and lower serum albumin level were associated with CDI. Other medications except antibiotics and underlying comorbidities were not evaluated in the study. In a retrospective study, 27 among 345 patients who received 492 terms of chemotherapy regimens, 36 (7.3%) had grade 2 or higher diarrhea, and eight had CDI without prior exposure to antibiotics. In a prospective study,27 five cases of CDI were diagnosed among 263 patients with lung cancer who received chemotherapy, and the incidence rate of CDI was 22.7% in all-grade diarrhea cases. Poor performance status and paclitaxel were identified as risk factors for CDI. Systemic steroids and underlying comorbidities were not evaluated in the study. Patients who developed CDI tended to have a later onset date (7 days vs 38 days). Our studies showed similar results: patients with adverse effects of diarrhea associated with EGFR-TKIs usually experienced those symptoms earlier (median, 7 days; range, 0–49 days). For patients with a delayed onset of diarrhea, CDI is a serious concern.
In a recent retrospective study,28 among 421 patients with cancer receiving immunotherapy including programmed death-1 or its ligand inhibitors or cytotoxic T-lymphocyte-associated antigen 4 inhibitors, 41 patients had CDI (9.7%). Prior use of antibiotics and PPIs were significantly more common among individuals who developed CDI after immune checkpoint inhibitors exposure. The incidence rate of CDI was much lower in patients who received EGFR-TKIs (only 2.2% in the present study) than in those receiving chemotherapy or immunotherapy with adverse effects of diarrhea. The etiological mechanisms of TKI-associated CDI remain uncertain, but the mechanism of EGFR-TKI-associated diarrhea is quite different from that of chemotherapy- or immunotherapy-related diarrhea. According to previous studies, the calcium-activated chloride channel is the trigger of EGFR-TKI-associated diarrhea.29,30 Chemotherapy-induced intestinal damage may facilitate the proliferation of C. difficile.30 Patients who have immune-related adverse events, including immune checkpoint inhibitor-induced diarrhea, usually have to be treated with corticosteroids, which has been shown to be a risk factor for CDI.
Strengths and Limitations
The strengths of this study include the large real-world cohort to evaluate the possible effects of EGFR-TKIs on CDI risk by adopting active comparator (diarrhea) controls. Furthermore, the present study included important laboratory measurements, such as positivity for C. difficile toxin A or B and stool culture, which were not available in the FDA AERS. Finally, a range of sensitivity analyses confirmed our study results.
Nonetheless, we acknowledge some limitations to the present study. First, we were unable to assess actual medication adherence, which may cause a possible bias toward null. Second, because diarrhea events occur commonly during TKI treatment, healthcare providers may tend to prescribe prophylactic antidiarrheal medications without recording the diagnoses. We reviewed the medical records manually with more clinical information such as laboratory and examination reports to confirm the events. Third, we did not obtain medical record data from outside the CGRD in Taiwan, which may have led to loss to follow-up. To address this issue, our as-treated analyses indicated the robustness of our results. Fourth, the population in the CGRD had more severe disease condition than those in general hospitals; researchers should pay special attention to selection biases because the patient characteristics from the CGRD differ from those of the national database. Forth, several clinical information without structured data like performance status, abdominal CT, and colonoscope were unavailable in this database cohort study using CGRD. Lab data without routinely checked were also missing among part of patients, we could not further analysis because of limited number of patients. Finally, even if our study results indicated a lower risk of CDI in patients taking afatinib, more studies are needed to clarify the role of EGFR-TKIs in the pathophysiology of CDI differ from chemotherapy or immunotherapy and to replicate our findings, especially other EGFR-TKIs like osimertinib and dacomitinib, which were excluded in this analysis due to small sample size and too short observational period.
Conclusions
In conclusion, our findings suggest that patients with CDI had higher mortality and ICU admission rates than without CDI. The use of antibiotics and systemic steroids were risk factors for the development with CDI after EGFR-TKI treatment in patients with lung cancer. Afatinib was not associated with an increased risk of CDI compared with first-generation EGFR-TKIs. Physicians should be aware of CDI in these patients to attain an early diagnosis, proper treatment, and reduced complications.
Data Sharing Statement
No additional data available.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2013.
Acknowledgments
We would like to thank the Health Information and Epidemiology Laboratory of Chang Gung Memorial Hospital, Chiayi Branch, for their comments on and assistance with data analysis. This study was supported by a grant from Chang Gung Memorial Hospital, Chia-Yi branch, Taiwan (CGRPG6L0071). This funding body played no role in the study design, analysis, or interpretation of data in this study.
Disclosure
The authors have no conflicts of interest relevant to this article.
References
1. Thandra KC, Barsouk A, Saginala K, et al. Epidemiology of lung cancer. Contemp Oncol. 2021;25(1):45–52.
2. Liao BC, Lin CC, Yang JC. Second and third-generation epidermal growth factor receptor tyrosine kinase inhibitors in advanced nonsmall cell lung cancer. Curr Opin Oncol. 2015;27(2):94–101. doi:10.1097/CCO.0000000000000164
3. Tripathi SK, Pandey K, Rengasamy KRR, et al. Recent updates on the resistance mechanisms to epidermal growth factor receptor tyrosine kinase inhibitors and resistance reversion strategies in lung cancer. Med Res Rev. 2020;40(6):2132–2176. doi:10.1002/med.21700
4. Hirsh V, Blais N, Burkes R, et al. Management of diarrhea induced by epidermal growth factor receptor tyrosine kinase inhibitors. Curr Oncol. 2014;21(6):329–336. doi:10.3747/co.21.2241
5. Toi Y, Kobayashi T, Harada T, et al. Prospective multicenter study of chemotherapy-induced clostridium (clostridioides) difficile infection in patients with lung cancer: north japan lung cancer study group trial 1204. Front Oncol. 2021;11:685320. doi:10.3389/fonc.2021.685320
6. Centre. AOGoHUTHNTQs. HUTH cancer guidelines for the management of chemotherapy and/or radiotherapy induced acute mucositis version 1.4; 2019.
7. Yang JC, Reguart N, Barinoff J, et al. Diarrhea associated with Afatinib: an oral ErbB family blocker. Expert Rev Anticancer Ther. 2013;13(6):729–736. doi:10.1586/era.13.31
8. Jung YJ, Han JJ, Lee CK. Tyrosine kinase inhibitor-induced colitis as a rare cause of bloody diarrhea. Clin Gastroenterol Hepatol. 2021;19(4):e30–e31. doi:10.1016/j.cgh.2020.02.023
9. Datta AK, Debnath P, Chakraborty U, et al. Clostridioides difficile-induced diarrhoea following dasatinib therapy. BMJ Case Rep. 2021;14:1. doi:10.1136/bcr-2020-239394
10. Risako T, Kana M, Sho T, et al. Factorial analysis of clostridioides difficile colitis and pseudomembranous colitis using JADER. BPB Reports. 2020;3(1):1–6. doi:10.1248/bpbreports.3.1_1
11. Huang J, Meng L, Yang B, et al. Safety profile of epidermal growth factor receptor tyrosine kinase inhibitors: a disproportionality analysis of FDA adverse event reporting system. Sci Rep. 2020;10(1):4803. doi:10.1038/s41598-020-61571-5
12. Tsai MS, Lin MH, Lee CP, et al. Chang Gung Research Database: a multi-institutional database consisting of original medical records. Biomed J. 2017;40(5):263–269. doi:10.1016/j.bj.2017.08.002
13. Shao SC, Chan YY, Kao Yang YH, et al. The Chang Gung Research Database-A multi-institutional electronic medical records database for real-world epidemiological studies in Taiwan. Pharmacoepidemiol Drug Saf. 2019;28(5):593–600. doi:10.1002/pds.4713
14. Su YC, Shao SC, Lai EC, et al. Risk of diabetic macular oedema with sodium-glucose cotransporter-2 inhibitors in type 2 diabetes patients: a multi-institutional cohort study in Taiwan. Diabetes Obes Metab. 2021;23(9):2067–2076. doi:10.1111/dom.14445
15. Fang WF, Douglas IS, Chen YM, et al. Development and validation of immune dysfunction score to predict 28-day mortality of sepsis patients. PLoS One. 2017;12(10):e0187088. doi:10.1371/journal.pone.0187088
16. Hsieh CY, Su CC, Shao SC, et al. Taiwan’s National health insurance research database: past and future. Clin Epidemiol. 2019;11:349–358. doi:10.2147/CLEP.S196293
17. Rodriguez Garzotto A, Merida Garcia A, Munoz Unceta N, et al. Risk factors associated with Clostridium difficile infection in adult oncology patients. Support Care Cancer. 2015;23(6):1569–1577. doi:10.1007/s00520-014-2506-7
18. Hwang KE, Hwang YR, Seol CH, et al. Clostridium difficile Infection in lung cancer patients. Jpn J Infect Dis. 2013;66(5):379–382. doi:10.7883/yoken.66.379
19. Hensgens MP, Goorhuis A, Dekkers OM, et al. Time interval of increased risk for Clostridium difficile infection after exposure to antibiotics. J Antimicrob Chemother. 2012;67(3):742–748. doi:10.1093/jac/dkr508
20. Das R, Feuerstadt P, Brandt LJ. Glucocorticoids are associated with increased risk of short-term mortality in hospitalized patients with clostridium difficile-associated disease. Am J Gastroenterol. 2010;105(9):2040–2049. doi:10.1038/ajg.2010.142
21. Arriola V, Tischendorf J, Musuuza J, et al. Assessing the risk of hospital-acquired clostridium difficile infection with proton pump inhibitor use: a meta-analysis. Infect Control Hosp Epidemiol. 2016;37(12):1408–1417. doi:10.1017/ice.2016.194
22. Rugo HS, Di Palma JA, Tripathy D, et al. The characterization, management, and future considerations for ErbB-family TKI-associated diarrhea. Breast Cancer Res Treat. 2019;175(1):5–15. doi:10.1007/s10549-018-05102-x
23. Delgado A, Reveles IA, Cabello FT, et al. Poorer outcomes among cancer patients diagnosed with Clostridium difficile infections in United States community hospitals. BMC Infect Dis. 2017;17(1):448. doi:10.1186/s12879-017-2553-z
24. Chopra T, Chandrasekar P, Salimnia H, et al. Recent epidemiology of Clostridium difficile infection during hematopoietic stem cell transplantation. Clin Transplant. 2011;25(1):E82–E87. doi:10.1111/j.1399-0012.2010.01331.x
25. Chung MS, Kim J, Kang JO, et al. Impact of malignancy on Clostridium difficile infection. Eur J Clin Microbiol Infect Dis. 2016;35(11):1771–1776. doi:10.1007/s10096-016-2725-6
26. Neha R, Subeesh V, Beulah E, et al. Existence of Notoriety Bias in FDA adverse event reporting system database and its impact on signal strength. Hosp Pharm. 2021;56(3):152–158. doi:10.1177/0018578719882323
27. Toi Y, Sugawara S, Kobayashi T, et al. Observational study of chemotherapy-induced Clostridium difficile infection in patients with lung cancer. Int J Clin Oncol. 2018;23(6):1046–1051. doi:10.1007/s10147-018-1304-5
28. Vasavada S, Panneerselvam K, Amin R, et al. Clostridioides difficile infection in cancer patients receiving immune checkpoint inhibitors. Ann Gastroenterol. 2022;35(4):393–399. doi:10.20524/aog.2022.0722
29. Harada Y, Sekine H, Kubota K, et al. Calcium-activated chloride channel is involved in the onset of diarrhea triggered by EGFR tyrosine kinase inhibitor treatment in rats. Biomed Pharmacother. 2021;141:111860. doi:10.1016/j.biopha.2021.111860
30. Kim Y, Quach A, Das S, et al. Potentiation of calcium-activated chloride secretion and barrier dysfunction may underlie EGF receptor tyrosine kinase inhibitor-induced diarrhea. Physiol Rep. 2020;8(13):e14490. doi:10.14814/phy2.14490
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
