Back to Journals » Infection and Drug Resistance » Volume 16
Clinical Impact and Risk Factors of Intensive Care Unit-Acquired Nosocomial Infection: A Propensity Score-Matching Study from 2018 to 2020 in a Teaching Hospital in China
Authors Wang Y, Ren J, Yao Z, Wang W, Wang S, Duan J, Li Z, Zhang H , Zhang R, Wang X
Received 20 October 2022
Accepted for publication 5 January 2023
Published 26 January 2023 Volume 2023:16 Pages 569—579
DOI https://doi.org/10.2147/IDR.S394269
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Yanhui Wang,1,* Jian Ren,2,* Zhiqing Yao,1 Wei Wang,3 Siyang Wang,2 Junfang Duan,3 Zhen Li,4 Huizi Zhang,1 Ruiqin Zhang,2 Xiaoru Wang3
1College of Pharmacy, Shanxi Medical University, Taiyuan, Shanxi, People’s Republic of China; 2Department of Pharmacy, Second Hospital of Shanxi Medical University, Taiyuan, Shanxi, People’s Republic of China; 3Intensive Care Unit, Second Hospital of Shanxi Medical University, Taiyuan, Shanxi, People’s Republic of China; 4College of Pharmacy, Chonnam National University, Gwangju, Korea
*These authors contributed equally to this work
Correspondence: Ruiqin Zhang; Xiaoru Wang, Email [email protected]; [email protected]
Purpose: Nosocomial infection (NI) is associated with poor prognosis. The present study assessed the clinical and microbiological characteristics of NI patients in the intensive care unit (ICU) and investigated the clinical impact and risk factors for NI in ICU patients.
Patients and Methods: An observational study was conducted in an adult general ICU. The electronic medical records of all patients admitted to the ICU for > 2 days from 2018– 2020 were analyzed retrospectively. Multivariate regression models were used to analyze the risk factors for NI in ICU patients. Propensity score-matching (PSM) was used to control the confounding factors between the case and control groups, thus analyzing the clinical impact of NIs.
Results: The present study included 2425 patient admissions, of which 231 (9.53%) had NI. Acinetobacter baumannii (33.0%) was the most common bacteria. Long-term immunosuppressive therapy, disturbance of consciousness, blood transfusion, multiple organ dysfunction syndromes (MODS), treatment with three or more antibiotics, mechanical ventilation (MV), tracheotomy, the urinary catheter (UC), nasogastric catheter, and central venous catheter (CVC) were risk factors for NI in the ICU patients. After PSM, patients with NI had a prolonged length of stay (LOS) in the ICU and hospital, significant hospitalization expenses (all p< 0.001), increased mortality (p=0.027), and predicted mortality (p=0.007). The differences in the ICU and hospital LOSs among three pathogens were statistically significant (p< 0.001); the results of the Escherichia coli infection group were lower than the other two pathogenic groups.
Conclusion: NI was associated with poor outcomes. The risk factors for NI identified in this study provided further insight into preventing NI.
Keywords: nosocomial infection, epidemiology, North China, propensity score-matching, intensive care unit, retrospective study
Introduction
Nosocomial infection (NI) is defined as an infection occurring in a patient admitted to a healthcare facility for >48 h but without any evidence that the infection was present or incubating at the time of admission.1 The incidence rates of NI in China from 2018–2020 were 1.91%, 1.86%, and 1.65%, respectively, which decreased steadily.2 However, the NI rate in the intensive care unit (ICU) was about 22%.3 It increased the hospitalization costs for patients, reduced the health-related quality of life, had a substantial effect on morbidity and mortality, prolonged the length of stay (LOS), reduced bed turnover rates, and seriously affected the quality of medical care, thus becoming a major global public health concern.4 Reportedly, 33% of the NIs are preventable.5 Therefore, studying clinical and preventive medicine related to NI is a challenging issue in modern hospital management but needs to be investigated in depth.
The current studies are primarily focused on the epidemiology and economics of nosocomial infection; however, statistics present a wide variation in the clinical outcomes and methods used for estimation. A few studies on the economic losses of NIs are descriptive studies matching a few variables such as age and gender, which may have a significant confounding bias.6–8 Therefore, we used a propensity score-matching analysis (PSM) to evaluate the direct economic burden of NIs to minimize the influence of confounding factors. In addition, we also analyzed the risk factors of NI. This would help us comprehend the clinical characteristics of hospital-infected patients in the ICU, quickly and accurately identifying those who are susceptible to the infection and implementing protection and prevention measures to reduce the physical and financial burden of patients while improving the level of diagnosis and treatment of hospital infections.
Materials and Methods
Study Design and Settings
A retrospective observational cohort study was conducted in the ICU of the Second Hospital of Shanxi Medical University, a tertiary hospital in Shanxi Province, China. It is a 2700-bed teaching hospital with an 18-bed ICU. The study complied with the Declaration of Helsinki and was carried out from January 1, 2018 to December 31, 2020. The control group comprised patients without NIs who had a length of stay >48 h and were hospitalized during the same period. This study was approved (2021YX −161) by the Ethics Committee of the Second Hospital of Shanxi Medical University, with a waiver of informed consent from the patients.
Data Collection
NI was developed 48 h after ICU admission and diagnosed according to the standards for NI surveillance issued by the Ministry of Health of China.1 NI was diagnosed based on the following parameters: (1) Conform to both clinical symptoms and pathogenic diagnosis; (2) Reports by clinicians and pharmacists; (3) Confirmation by staff members of the hospital’s infection-control department. The exclusion criteria were as follows: (1) Only culture results of pathogenic bacteria without clinical signs and symptoms; (2) Patients with repeat ICU admissions during a single hospital stay, the first admission information was recorded. Patient data included demographic information, illness severity, comorbidities on ICU admission, invasive procedures, drug usage, and clinical outcomes. Illness severity was evaluated by Acute Physiology and Chronic Health Evaluation (APACHE) II score during the first 24 h of ICU admission. The comorbidities on the ICU admission, including chronic underlying diseases (respiratory diseases, cardiovascular diseases, malignancies, diabetes, hypertension, liver diseases, and renal diseases), consciousness disorder, blood transfusion, surgical operation, trauma, shock, hypoproteinemia, immunological diseases, pneumonia, and multiple organ dysfunction syndrome (MODS), were assessed during the diagnoses. Pneumonia in the comorbidities on ICU admission included both community-associated pneumonia and hospital-acquired pneumonia before ICU admission. The invasive procedures included mechanical ventilation (MV), tracheal intubation, tracheotomy, the urinary catheter (UC), nasogastric catheter, drainage catheter, central venous catheter (CVC), and continuous renal replacement therapy (CRRT).
Definition of Outcome Indicators
The clinical outcomes consist of the LOS in the ICU and hospital, costs of hospitalization and antimicrobial drugs, death in the ICU (all-cause mortality), and predicted death in the ICU. Hospitalization costs are the direct medical costs incurred by patients during their stay in the hospital. Predicted death in the ICU included death in the ICU and after discharge against medical advice because of critical conditions and the patient’s desire to die at home.9 Hospitalization costs are the direct medical costs incurred by patients during their stay in the hospital.
PSM
To minimize the impact of potential confounding variables, we employed PSM using R Package Matching version 4.0.4 (CRAN.R-project.org/package = Matching). Based on previous reports, consultation with the relevant experts, and in conjunction with the ICU-targeted monitoring database, factors that may affect patient prognostic indicators were identified for inclusion as adjustment factors. Herein, we input variables that included the patient’s demographics (age and sex), APACHE II score on ICU admission, malignancies, a disorder of consciousness, surgical operation, trauma, shock, MV, CVC, CRRT, and MODS.9,10 We also used the predicted probabilities of each potential confounding variable for PSM. The propensity score was balanced between the two groups; therefore, nearest-neighbor matching was employed to obtain the matched pairs of subjects and controls at a 1:2 ratio and a 0.02 calipers value. Variables with an absolute value of standard deviation (SD) ≤0.1 after PSM indicated a balance between the groups. The resultant pairs were subjected to additional clinical outcome analyses.
Statistical Analysis
Statistical analyses were performed using SPSS 25.0. Continuous variables were described as means and standard deviations (SD) when fitting a normal distribution, otherwise as medians and interquartile range (IQR), while categorical variables were expressed as percentages. Chi-square and Fisher’s exact tests were used to compare the categorical variables. If the two groups of continuous variables showed normal distribution, the independent sample’s t-test was used; otherwise, the non-parametric test of two independent samples (Mann–Whitney U-test) was employed. Kruskal–Wallis test was used to analyze the differences in clinical outcomes between groups of infection sites and causative organisms. For evaluating the risk factors of NIs, the variables with a p-value <0.05 in the univariate analysis were selected for multivariate logistic regression models. All tests were two-sided, and p<0.05 was considered statistically significant.
Results
Population
A total of 2425 patients, including 1298 patients who stayed at least 48 h in our ICUs, were enrolled during the study period. A total of 231 patients acquired NIs after ICU admission, at a rate of 9.53%. The baseline characteristics of the study patients are shown in Table 1. The 231 patients with NI were 60-years-old, mostly males (163/231, 70.6%), and had a median APACHE II score of 10.0 at the time of ICU admission. Compared to the patients without NIs, those with NIs had a significantly higher in-ICU mortality rate (12.1% vs 2.2%, p<0.05).
 |
Table 1 Comparison of Patients with Nosocomial Infections and Without Nosocomial Infections Groups in Terms of the Baseline Characteristics and Risk Factors |
Acquired Infection
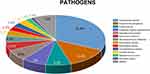
A total of 389 pathogens were isolated from 231 infections: 293 Gram-negative bacilli, 78 Gram-positive cocci, and 18 fungi. The respiratory tract, bloodstream, urinary tract, and intra-abdominal events accounted for the majority of the ICU-acquired infections (56.3%, 22.4%, 6.2%, and 6.2%, respectively). Acinetobacter baumannii (31.6%), Pseudomonas aeruginosa (13.4%), and Escherichia coli (8.2%) were the most frequently isolated pathogens and A. baumannii had the highest rate of drug resistance (Table 2). Enterobacter cloacae also ranked in the top 10 in terms of isolation rate (Figure 1).
 |
Table 2 Pathogens Associated with Selected Nosocomial Infection Types |
Risk Factors for NIs
The risk factors associated with NIs in the ICU patients were long-term immunosuppressive therapy [odds ratio (OR) 2.57, 95% confidence interval (CI): 1.21–5.50, p=0.015)], surgical operation (OR 0.50, 95% CI: 0.35–0.71, p<0.001), disturbance of consciousness (OR 1.80, 95% CI: 1.20–2.71, p=0.005), blood transfusion (OR 1.91, 95% CI: 1.31–2.78, p=0.001), MODS (OR 3.29, 95% CI: 1.51–7.17, p<0.001), and treatment with three or more antibiotics (OR 1.84, 95% CI: 1.28–2.63, p<0.001). Moreover, patients with invasive procedures were at high risk for developing NIs. These included MV (OR 3.67, 95% CI: 2.26–5.96, p<0.001), tracheotomy (OR 4.07, 95% CI: 2.56–6.48, p<0.001), UC (OR 3.79, 95% CI: 1.08–13.33, p=0.038), a nasogastric catheter (OR 2.44, 95% CI: 1.57–3.81, p<0.001), and CVC (OR 1.61, 95% CI: 1.07–2.44, p=0.024). Finally, the current results showed that surgical operations have advantages in these patients in lowering the risk of infection (OR 0.5, 95% CI: 0.35–0.71, p<0.001) (Table 1).
Impact of NIs on the Outcomes
After PSM, 196 cases in the case group were successfully matched (Table 3), and the distributions of covariates in the NIs group and the non-infection group were balanced (Figure 2). Compared with the control group, patients with NIs have prolonged LOS in the ICU (median 12.0 days, p<0.001) and hospital (median 14.0 days, p<0.001) and increased hospitalization (median 73,596.62-yuan, p<0.001), antibacterial drug costs (median 7612.44-yuan, p<0.001), mortality in the ICU (5.8%, p=0.005), and predicted mortality in the ICU (11.3%, p=0.003) compared to those without NI. The antimicrobial drug costs account for 1/10th of the overall hospital costs (Table 4).
 |
Table 3 The Baseline Charities of the Two Groups of Patients Before and After Matching |
 |
Table 4 Excess Outcomes of Nosocomial Infection After PS Matching |
Impact of Different Infection Sites and Pathogens on the Outcomes
Several studies have identified the association of NI with poor clinical outcomes, including LOS in hospitals, healthcare costs, and mortality. As shown in Table 5, the length of ICU stay in the A. baumannii, P. aeruginosa, and the E. coli groups were 20.0, 35.0, and 16 days, respectively. The length of hospital stay in the A. baumannii group, the Pseudomonas aeruginosa group and the Escherichia coli group were 33.5, 49, and 24 days, respectively. The differences in the ICU and hospital LOSs among the three pathogens were statistically significant (p<0.001), and that in the E. coli infection group was lower than in the other two pathogenic groups (LOS in ICU: E. coli vs A. baumannii, p<0.001, E. coli vs P. aeruginosa, p<0.001; LOS in hospital: E. coli vs A. baumannii, p<0.001, E. coli vs P. aeruginosa, p=0.001).
 |
Table 5 Clinical Outcomes of Nosocomial Infection with Different Clinical Forms and Pathogens After PS Matching |
Discussion
A targeted surveillance of NI in the ICU is beneficial for decreasing the incidence of NI. Compared to other domestic studies, this survey based on the retrospective cohort data showed that the proportion (9.53%) of NIs was significantly higher than in studies from Jiangsu Province (7.23%),11 Inner Mongolia Autonomous Region (3.62%),12 and Hubei Province (5.51%)13 and lower than in studies from Heilongjiang Province (12.87%)14 and Anhui Province (15.11%).15 The incidence of NIs in our hospital was much lower than in other countries.3,16,17 The primary causes of infection rates vary according to the characteristics of the patient’s condition, regional economic conditions, hospital size, medical treatment capacity, application of antimicrobial drugs, infection control, and case diagnosis level. Thus, our hospital should take comprehensive measures to reduce the incidence of NIs, such as enhanced training, information disclosure, and supervisory feedback.
The current study showed that respiratory tract infection is the most common type of infection, which is in accordance with the data published in the National Bacterial Resistance Surveillance Report in 2020.18 Gram-negative bacteria were dominant, and A. baumannii (31.6%), P. aeruginosa (13.4%), and E. coli (8.2%) were the main pathogens. Consistent with other studies, A. baumannii is becoming a major cause of NIs in critically ill patients.19,20 Previously, Acinetobacter was recognized as a low-virulent organism that causes infections primarily among immunocompromised hosts. Nonetheless, it has also acquired resistance to antibiotics over the past several years, and therefore its virulence is becoming an increasing concern. Multidrug-resistant A. baumannii (MDR-AB) has been reported worldwide and is now recognized as one of the most difficult NIs to be treated and controlled.21,22
Long-term immunosuppressive therapy, comorbidities on ICU admission, invasive procedures, and using three or more antibiotics were the main factors associated with a high prevalence of NIs among ICU patients in this study. A single-center point-prevalence survey in an American hospital showed that 96.8% of the hospitalized adult patients had at least one indwelling device;23 however, the invasive operation destroys the normal defense of the body barrier, facilitating pathogen invasion and colonization.24 The current study pointed out that tracheotomy with OR 4.07 and urinary catheters (UC) with OR 3.79 were the strongest independent risk factors for NI; these results were similar to the previously reported conclusions in adults about the risk factors of NIs.25,26 In addition, the long-term use of a variety of antibiotics will inhibit the immune function of the body, disturb the normal flora of the patient body, affect the stability of the internal environment, provide adequate conditions for the breeding and reproduction of pathogens, and increase the possibility of NI occurrence.27,28 However, some studies showed that previous broad-spectrum antibiotics are associated with the acquisition of MDR bacterial infections.29,30 This phenomenon indicated that prudent and high-quality antibiotics prescription and rational use of antibiotics are essential to restrict the overuse of antibiotics, thereby reducing the occurrence of NIs among ICU patients. Some studies have shown that a large number of blood transfusions and stale storage components in the blood can promote the activation of inflammatory cytokines in the lung endothelium and induce NIs.31 Several studies have shown an association between blood transfusions and subsequent ICU-acquired infections,32–34 with platelet transfusions having a higher risk than other blood components.35,36 A meta-analysis by Rohde et al demonstrated that a restrictive blood transfusion policy significantly reduces the incidence of infection.37 Therefore, restrictive transfusion strategies are essential for preventing and controlling hospital infections. Furthermore, patients with consciousness disorders have an increased risk of aspiration due to long-term bed rest, the disappearance of cough reflex, and insufficient food intake, which become high-risk factors for nosocomial infection.38,39 Finally, patients with MODS have a combination of risk factors. Therefore, in all patients, MODS is associated with exceptionally high rates of infection.40,41
Conversely, surgical operations exhibited a protective effect on ICU inpatients to prevent NIs. Tai showed that compared to the non-surgery group, the surgery group has less diabetes, tracheotomy, and ICU LOS, lower APACHE II score, and younger age.42 In the current study, we found that the number of patients with disturbed consciousness and tracheotomy in the surgery group was significantly lower than that in the non-surgery group. In summary, the risk of NIs was lower due to fewer comorbidities and risk factors. In addition, surgical site infections (SSIs) are the most common type of infection in surgical patients;43 and the surgeries in our study were mainly orthopedic surgeries, hence, clinical active debridement surgery and the application of local bone cement can effectively reduce the occurrence of NI.44,45 Nevertheless, the protective factors of surgery in ICU infection during hospitalization need to be substantiated in the future.
Several studies have shown that NIs prolong the LOS of patients from 10–20 days, with additional hospital costs ranging from $7000–$15,000 and mortality rates 20–30%.46–48 PSM is one of the most commonly used clinical tools to analyze the economic burden of hospital-acquired infections and avoid the impact of confounding factors on the economic burden and provide an objective and accurate assessment.49,50 Therefore, in the present study, the economic burden of hospital-acquired infections on ICU patients was analyzed using PSM in a 1:2 matching exercise. In the present study, the direct economic burden of hospital-acquired infections was 73,596.62 yuan, which was much higher than that reported previously. The mortality rate (9.2%) was significantly lower in this study but similar to the predicted mortality rate (29.1%). This phenomenon could be attributed to the serious condition of hospitalized patients in China and poor prognosis. Moreover, due to the economic level and the traditional concept of dying at home, many patients and their families choose to give up treatment and be discharged from the hospital; however, these patients are likely to die in the short term. A few studies have estimated the difference in the clinical outcomes between different sites and pathogens of infection. The current study showed that LOS in the hospital, the cost of hospitalization, and the mortality for UTIs were higher than those of the other two types. This finding was not consistent with that of the other studies.47,51,52 Another review showed that all SSIs were not similar. Although by definition, all SSIs were costly; however, the principal determinants of the cost of an SSI were geographic locale, the type of surgery performed, and the depth of the infection.53 Next, we analyzed the clinical outcomes of different pathogens, which showed that E. coli is less abundant compared to the other two pathogens and P. aeruginosa has poor clinical outcomes. Moreover, the extra economic burden of NIs caused by MDR bacteria deserves significant attention. Although the current study did not match MDR bacteria, a retrospective study in Spain found that the hospitalization costs for patients hospitalized with MDR P. aeruginosa were 1.7 times more than those for non-NI patients.54 Therefore, NI should be under intensive focus, especially in ICU patients.
Nevertheless, the current study has several limitations. First, we used retrospective data in a single hospital, which caused inevitable biases. Secondly, this study assessed the overall cost of hospitalization to NI patients in the ICU but did not evaluate the classified costs, such as the treatment, nursing, or direct expenses after developing NI. Finally, the generalizability of our findings could be limited by the type of ICU (a general ICU always admits critically ill patients) and the high prevalence of NIs in this unit.
Conclusions
In conclusion, NI has a high prevalence in ICU patients and is associated with poor clinical outcomes, which include LOS in the ICU and the hospital, hospitalization costs, and all-cause mortality in the ICU. Long-term immunosuppressive therapy, disturbance of consciousness, blood transfusion, MODS, three or more antibiotics treatment, MV, tracheotomy, UC, nasogastric catheter, and CVC were risk factors for developing NI in ICU patients. These factors provided potential measures for preventing NI, such as aseptic techniques, promoting the rational use of antimicrobial drugs, and the importance of accurate determination of the underlying disease status.
Ethics Approval and Consent to Participate
This study was approved by the Ethics Committee of the Second Hospital of Shanxi Medical University (Reference Number 2021YX −161). The Ethics Committee granted permission to access the raw data and approved the waiver of informed consent to participate in this study due to its retrospective design. All patient data were anonymous prior to the analysis.
Acknowledgments
We thank the staff of the Second Hospital of Shanxi Medical University for supporting this study.
Funding
This study was supported by the Shanxi Province Natural Science Foundation (Grant number 202104031402140).
Disclosure
The authors declare that they have no competing interests in this work.
References
1. MoH. Diagnostic criteria for nosocomial infections. Chin Med J. 2001;2001(81):314–320.
2. Zhao JH, Qin B, Yan RN, Ma J, Hu LL, Liu YL. Status and trend of the main healthcare-associated infection indicators in tertiary public hospitals in China (2018–2020). Chin J Infect Control. 2022;21(06):524–531.
3. Vincent JL, Sakr Y, Singer M, et al. Prevalence and outcomes of infection among patients in intensive care units in 2017. JAMA. 2020;323(15):1478–1487. doi:10.1001/jama.2020.2717
4. WHO. Health care without avoidable infections: the critical role of infection prevention and control; 2016. Available from: https://www.who.int/publications/i/item/health-care-without-avoidable-infections-The-critical-role-of-infection-prevention-and-control.
5. Lobdell KW, Stamou S, Sanchez JA. Hospital-acquired infections. Surg Clin North Am. 2012;92(1):65–77. doi:10.1016/j.suc.2011.11.003
6. Baier C, Linke L, Eder M, et al. Incidence, risk factors and healthcare costs of central line-associated nosocomial bloodstream infections in hematologic and oncologic patients. PLoS One. 2020;15(1):e0227772. doi:10.1371/journal.pone.0227772
7. Lee NY, Lee HC, Ko NY, et al. Clinical and economic impact of multidrug resistance in nosocomial Acinetobacter baumannii bacteremia. Infect Control Hosp Epidemiol. 2015;28(6):713–719. doi:10.1086/517954
8. Pada SK, Ding Y, Ling ML, et al. Economic and clinical impact of nosocomial meticillin-resistant Staphylococcus aureus infections in Singapore: a matched case-control study. J Hosp Infect. 2011;78(1):36–40. doi:10.1016/j.jhin.2010.10.016
9. Zhu S, Kang Y, Wang W, Cai L, Sun X, Zong Z. The clinical impacts and risk factors for non-central line-associated bloodstream infection in 5046 intensive care unit patients: an observational study based on electronic medical records. Crit Care. 2019;23(1):52. doi:10.1186/s13054-019-2353-5
10. An YJ, Yang H, Mu X, Yu FX, An YZ, Zhan L. Evaluation of economic losses due to carbapenem-resistant Klebsiella pneumoniae nosocomial infection based on propensity index matching. Chin J Nosocomiol. 2022;32(09):1410–1414.
11. Li Y, Cao X, Ge H, Jiang Y, Zhou H, Zheng W. Targeted surveillance of nosocomial infection in intensive care units of 176 hospitals in Jiangsu province, China. J Hosp Infect. 2018;99(1):36–41. doi:10.1016/j.jhin.2017.10.009
12. Liu WP, Tian YQ, Cao QL, Qin LL, Li ZH. Targeted surveillance of nosocomial infections in ICUs of 40 hospitals of inner mongolia autonomous region in 2014. Chin J Nosocomiol. 2015;25(11):2492–2494.
13. Xu C, Xiong W, Lai XQ, Xu M. Targeted surveillance of nosocomial infection in ICUs of 47 hospitals of Hubei Province in 4 consecutive years. Chin J Nosocomiol. 2019;29(21):3334–3338.
14. Xiao JQ, Lin L, Ren H, Li J, Zhang XX. Survey of prevalence rates of nosocomial infections in 74 hospitals of Heilongjiang province in 2018. Chin J Nosocomiol. 2020;30(10):1569–1573.
15. Peng H, Tao XB, Li Y, et al. Health care-associated infections surveillance in an intensive care unit of a university hospital in China, 2010–2014: findings of international nosocomial infection control consortium. Am J Infect Control. 2015;43(12):e83–e85.
16. Ali S, Birhane M, Bekele S, et al. Healthcare associated infection and its risk factors among patients admitted to a tertiary hospital in Ethiopia: longitudinal study. Antimicrob Resist Infect. 2018;7:2. doi:10.1186/s13756-017-0298-5
17. Agarwal R, Gupta D, Ray P, Aggarwal AN, Jindal SK. Epidemiology, risk factors and outcome of nosocomial infections in a respiratory intensive care unit in North India. J Infect. 2006;53(2):98–105. doi:10.1016/j.jinf.2005.10.021
18. System CARS. 2020 national antimicrobial resistance surveillance report. Chin J Lab Med. 2022;45(02):122–136.
19. Rafa E, Walaszek MZ, Walaszek MJ, Domanski A, Rozanska A. The incidence of healthcare-associated infections, their clinical forms, and microbiological agents in intensive care units in Southern Poland in a multicentre study from 2016 to 2019. Int J Environ Res Public Health. 2021;18(5):2238. doi:10.3390/ijerph18052238
20. Peng GX, Zhang HG, Huang XX, Luo WJ, Peng S. Distribution of pathogenic bacteria causing nosocomial infection and drug-resistance in ICU from 2018 to 2020. Chin J Biomed Eng. 2021;27(06):667–673.
21. Inchai J, Liwsrisakun C, Theerakittikul T, Chaiwarith R, Khositsakulchai W, Pothirat C. Risk factors of multidrug-resistant, extensively drug-resistant and pandrug-resistant Acinetobacter baumannii ventilator-associated pneumonia in a medical intensive care unit of university hospital in Thailand. J Infect Chemother. 2015;21(8):570–574. doi:10.1016/j.jiac.2015.04.010
22. Chan MC, Chiu SK, Hsueh PR, Wang NC, Wang CC, Fang CT. Risk factors for healthcare-associated extensively drug-resistant Acinetobacter baumannii infections: a case-control study. PLoS One. 2014;9(1):e85973. doi:10.1371/journal.pone.0085973
23. Chen S, O’Malley M, Chopra V. How common are indwelling devices in hospitalized adults? A contemporary point prevalence study in a tertiary care hospital. Am J Infect Control. 2021;49(2):194–197. doi:10.1016/j.ajic.2020.06.205
24. Liu P, Li X, Luo M, et al. Risk factors for carbapenem-resistant Klebsiella pneumoniae infection: a meta-analysis. Microb Drug Resist. 2018;24(2):190–198. doi:10.1089/mdr.2017.0061
25. Despotovic A, Milosevic B, Milosevic I, et al. Hospital-acquired infections in the adult intensive care unit-epidemiology, antimicrobial resistance patterns, and risk factors for acquisition and mortality. Am J Infect Control. 2020;48(10):1211–1215. doi:10.1016/j.ajic.2020.01.009
26. Klavs I, Kolman J, Zupanc TL, et al. The prevalence of and risk factors for healthcare-associated infections in Slovenia: results of the second national survey. Zdr Varst. 2016;55(4):239–247. doi:10.1515/sjph-2016-0033
27. Chen J, Pan QS, Hong WD, et al. Use of an artificial neural network to predict risk factors of nosocomial infection in lung cancer patients. Asian Pac J Cancer Prev. 2014;15(13):5349–5353. doi:10.7314/APJCP.2014.15.13.5349
28. Jiao P, Jiang Y, Jiao J, Zhang L. The pathogenic characteristics and influencing factors of health care-associated infection in elderly care center under the mode of integration of medical care and elderly care service: a cross-sectional study. Medicine. 2021;100(21):e26158. doi:10.1097/MD.0000000000026158
29. Zheng YL, Wan YF, Zhou LY, et al. Risk factors and mortality of patients with nosocomial carbapenem-resistant Acinetobacter baumannii pneumonia. Am J Infect Control. 2013;41(7):e59–e63. doi:10.1016/j.ajic.2013.01.006
30. Dent LL, Marshall DR, Pratap S, Hulette RB. Multidrug resistant Acinetobacter baumannii: a descriptive study in a city hospital. BMC Infect Dis. 2010;10:196. doi:10.1186/1471-2334-10-196
31. Balvers K, Wirtz MR, van Dieren S, Goslings JC, Juffermans NP. Risk factors for trauma-induced coagulopathy- and transfusion-associated multiple organ failure in severely injured trauma patients. Front Med. 2015;2:24. doi:10.3389/fmed.2015.00024
32. Robert W, Taylor M, Lisa Manganaro R, et al. Impact of allogenic packed red blood cell transfusion on nosocomial infection rates in the critically ill patient. Crit Care Med. 2002;30(10):2249–2254.
33. Deng S, Feng S, Wang W, Zhu H, Gong Y. Bacterial distribution and risk factors of nosocomial blood stream infection in neurologic patients in the intensive care unit. Surg Infect. 2019;20(1):25–30. doi:10.1089/sur.2018.085
34. Wylie MC, Graham DA, Potter-Bynoe G, et al. Risk factors for central line-associated bloodstream infection in pediatric intensive care units. Infect Control Hosp Epidemiol. 2010;31(10):1049–1056. doi:10.1086/656246
35. Lai YC, Lyu Y, Liu M, Mao W. Risk factors for allogeneic blood transfusion-related nosocomial infection. Chin J Nosocomiol. 2021;31(18):2788–2792.
36. Aubron C, Flint AW, Bailey M, et al. Is platelet transfusion associated with hospital-acquired infections in critically ill patients? Crit Care. 2017;21(1):2. doi:10.1186/s13054-016-1593-x
37. Rohde JM, Dimcheff DE, Blumberg N, et al. Health care-associated infection after red blood cell transfusion: a systematic review and meta-analysis. JAMA. 2014;311(13):1317–1326. doi:10.1001/jama.2014.2726
38. Huang H, Niu Z, Liu G, et al. Early consciousness disorder in acute large hemispheric infarction: an analysis based on quantitative EEG and brain network characteristics. Neurocrit Care. 2020;33(2):376–388. doi:10.1007/s12028-020-01051-w
39. Li J, Wang D, Tao W, et al. Early consciousness disorder in acute ischemic stroke: incidence, risk factors and outcome. BMC Neurol. 2016;16(1):140. doi:10.1186/s12883-016-0666-4
40. Shepherd JM, Cole E, Brohi K. Contemporary patterns of multiple organ dysfunction in Trauma. Shock. 2017;47(4):429–435. doi:10.1097/SHK.0000000000000779
41. Barea-Mendoza JA, Chico-Fernandez M, Molina-Diaz I, et al. Risk factors associated with early and late posttraumatic multiorgan failure: an analysis from RETRAUCI. Shock. 2021;55(3):326–331. doi:10.1097/SHK.0000000000001628
42. Tai YY. Study on MDROs Infection Prediction Model of ICU Patients Based on Random Forest [Master]. Huzhou University; 2022.
43. Li K, Li X, Si W, et al. Preoperative and operation-related risk factors for postoperative nosocomial infections in pediatric patients: a retrospective cohort study. PLoS One. 2019;14(12):e0225607. doi:10.1371/journal.pone.0225607
44. Han T, Hao JQ, Li WB, Shi J, Gao QM. Advantages and problems of antibiotic-loaded bone cements for bone and joint infections. Chin J Tissue Eng Res. 2023;27(03):470–477.
45. Schwarz EM, McLaren AC, Sculco TP, et al. Adjuvant antibiotic-loaded bone cement: concerns with current use and research to make it work. J Orthop Res. 2021;39(2):227–239. doi:10.1002/jor.24616
46. Chacko B, Thomas K, David T, Paul H, Jeyaseelan L, Peter JV. Attributable cost of a nosocomial infection in the intensive care unit: a prospective cohort study. World J Crit Care Med. 2017;6(1):79–84. doi:10.5492/wjccm.v6.i1.79
47. Leal MA, Freitas-Vilela AA. Costs of healthcare-associated infections in an intensive care unit. Rev Bras Enferm. 2021;74(1):e20200275. doi:10.1590/0034-7167-2020-0275
48. Nangino Gde O, Oliveira CD, Correia PC, Machado Nde M, Dias AT. Financial impact of nosocomial infections in the intensive care units of a charitable hospital in Minas Gerais, Brazil. Rev Bras Ter Intensiva. 2012;24(4):357–361. doi:10.1590/s0103-507x2012000400011
49. Kulaylat AN, Rocourt DV, Podany AB, et al. Costs of Clostridium difficile infection in pediatric operations: a propensity score-matching analysis. Surgery. 2017;161(5):1376–1386. doi:10.1016/j.surg.2016.10.020
50. Punekar YS, Naya I, Small M, et al. Bronchodilator reliever use and its association with the economic and humanistic burden of COPD: a propensity-matched study. J Med Econ. 2017;20(1):28–36. doi:10.1080/13696998.2016.1223085
51. Chen YY, Wang FD, Liu CY, Chou P. Incidence rate and variable cost of nosocomial infections in different types of intensive care units. Infect Control Hosp Epidemiol. 2009;30(1):39–46. doi:10.1086/592984
52. Lv Y, Chen L, Yu JW, et al. Hospitalization costs due to healthcare-associated infections: an analysis of propensity score matching. J Infect Public Health. 2019;12(4):568–575. doi:10.1016/j.jiph.2019.01.069
53. Urban JA. Cost analysis of surgical site infections. Surg Infect. 2006;7(Suppl 1):S19–S22. doi:10.1089/sur.2006.7.s1-19
54. Morales E, Cots F, Sala M, et al. Hospital costs of nosocomial multi-drug resistant Pseudomonas aeruginosa acquisition. BMC Health Serv Res. 2012;12:122. doi:10.1186/1472-6963-12-122
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.