Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 15
Clinical Impact and Healthcare Resource Utilization Associated with Early versus Late COPD Diagnosis in Patients from UK CPRD Database
Authors Kostikas K , Price D , Gutzwiller FS , Jones B , Loefroth E, Clemens A , Fogel R , Jones R , Cao H
Received 31 March 2020
Accepted for publication 27 June 2020
Published 16 July 2020 Volume 2020:15 Pages 1729—1738
DOI https://doi.org/10.2147/COPD.S255414
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Konstantinos Kostikas, 1 David Price, 2 Florian S Gutzwiller, 3 Bethan Jones, 4 Emil Loefroth, 3 Andreas Clemens, 3, 5 Robert Fogel, 6 Rupert Jones, 7 Hui Cao 6
1Respiratory Medicine Department, University Hospital of Ioannina, Ioannina, Greece; 2Centre of Academic Primary Care, University of Aberdeen, Aberdeen, UK; 3Novartis Pharma AG, Basel, Switzerland; 4Pharmatelligence, Cardiff, UK; 5Department of Cardiology and Angiology I, Heart Center Freiburg University, Faculty of Medicine, University of Freiburg, Freiburg, Germany; 6Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA; 7Plymouth University Peninsula School of Medicine and Dentistry, Plymouth, UK
Correspondence: Konstantinos Kostikas
Respiratory Medicine Department, University Hospital of Ioannina, Ioannina, Greece
Email [email protected]
Purpose: Previous studies have shown that opportunities to diagnose chronic obstructive pulmonary disease (COPD) early are often missed in primary care. This retrospective study aimed to utilize secondary data from the United Kingdom (UK) healthcare system to understand the impact of early versus late diagnosis of COPD.
Patients and Methods: Newly diagnosed COPD patients were identified in the UK Clinical Practice Research Database from 2011 to 2014. Patients whose 5-year medical data before diagnosis revealed ≥ 3 counts of eight indicators of early COPD were deemed as late-diagnosed, whereas others were deemed as early-diagnosed. We assessed patients’ characteristics; time-to-first, risk, and rates of exacerbation; and healthcare resource utilization (COPD-related clinic visits, Accident and Emergency visits, and hospitalizations) in late- versus early-diagnosed patients.
Results: Of 10,158 patients included in the study, 6783 (67%) were identified as late-diagnosed and 3375 (33%) as early-diagnosed. The median time-to-first exacerbation was shorter in late-diagnosed (14.5 months) versus early-diagnosed (29.0 months) patients, with a significant risk of exacerbation (hazard ratio 1.46 [95% confidence interval: 1.38– 1.55]). Additionally, the exacerbation rate (per 100 person-years) over 3 years was higher in late (108.9) versus early (57.2) diagnosed patients. Late-diagnosed patients had a significantly higher rate of COPD hospitalizations (per 1000 patient years) compared with early-diagnosed patients during 2 and 3 years of follow-ups (P = 0.0165 and P < 0.0001, respectively).
Conclusion: Results showed that a significant percentage of COPD patients in UK primary care are diagnosed late. A late COPD diagnosis is associated with a shorter time-to-first exacerbation and a higher rate and risk of exacerbations compared with early diagnosis. Additionally, late diagnosis of COPD is associated with a higher rate of COPD-related hospitalizations compared with early diagnosis.
Keywords: chronic obstructive pulmonary disease, COPD, clinical practice research datalink, UK-CPRD, early diagnosis of COPD, late diagnosis of COPD, healthcare utilization
Corrigendum for this paper has been published
Introduction
Chronic obstructive pulmonary disease (COPD) is the second most common lung disease in the United Kingdom (UK), after asthma.1,2 An estimated 1.2 million people in the UK are living with COPD, which constitutes around 2% of the overall population.1,2 An earlier retrospective study conducted by Jones et al revealed that 85% of patients in the UK remain undiagnosed despite presenting COPD symptoms in the past 5 years.3 Missing an opportunity to diagnose COPD translates to missing an opportunity to treat a patient early and potentially avoid adverse patient outcomes.3,4 We hypothesized that early treatment of COPD is likely to lead to better control of the disease and reduced healthcare resource utilization (HCRU).4 In this study, we have utilized data from the UK healthcare system to understand how the clinical and economic consequences are associated with the timing of COPD diagnosis in patients with COPD. Here, we report the clinical and economic outcomes associated with early versus late diagnosis of COPD in primary care.
Patients and Methods
Study Design
This was a retrospective cohort study conducted to compare the outcomes in patients with an early versus late diagnosis of COPD, among newly diagnosed COPD patients managed in primary care settings in the UK from 01 January 2006 through 31 March 2016 (Figure 1). Index date was the time of the first recorded physician’s diagnosis of COPD during the identification time frame. The follow-up period for each patient was objective-specific with maximum 3 years. The study protocol was approved by the Clinical Practice Research Datalink (CPRD), Independent Scientific Advisory Committee for Medicines and Healthcare products Regulatory Agency database research, UK (ISAC 17_213), and the study was conducted in accordance with the principles of the Declaration of Helsinki. The results of this study were reported adhering to the STROBE (Strengthening The Reporting of OBservational Studies in Epidemiology) guidelines.
 |
Figure 1 Study design. *Index date was the time of the first recorded physician’s diagnosis of COPD during the identification time frame. Abbreviation: COPD, chronic obstructive pulmonary disease. |
Database
The data for this study were retrieved from the CPRD linked to the Hospital Episode Statistics (HES) database. The UK CPRD is a high-quality clinical primary care database, whereas the HES database contains high-quality data from admitted patients, outpatients, and Accident and Emergency (A&E) records in England. Both databases are in part linked and we focused our investigation only on CPRD patients who are eligible for the linkage to evaluate acute and chronic outcomes most appropriately in primary and secondary care. CPRD is a longitudinal, anonymized research database derived from nearly 700 primary care practices in the UK.5 By January 2015, CPRD contained more than 13 million research-quality patients registered at 684 practices.6 Data include demographics, diagnoses, symptoms, investigations, referrals, and prescriptions. HES database contains details of all admissions, A&E attendances, and outpatient appointments at the National Health Service hospitals in England. Inpatient data are recorded in HES using the International Classification of Diseases 10th Revision (ICD-10) classification.7
Study Patients
Inclusion Criteria
Patients with a COPD diagnosis during the identification period,6 eligible for data linkage to HES, age (≥40 years at index date) and gender information not missing, acceptable flag criterion in CPRD, and their practices flagged as up-to-standard during the pre-index period were included in the study. All patients were required to have a minimum of practice data of 5 years before and 1 year after their first COPD diagnosis. The registration date and a practice up-to-standard date 5 years prior to the index date were captured to ensure a minimum of 5 years of pre-index follow-up.
Exclusion Criteria
A diagnosis of both COPD and asthma in the pre-index period (as the respiratory signs and symptoms in a patient with asthma would make the algorithm to identify early and late diagnosis in COPD invalid).
Early and Late-Diagnosed Cohorts
Patients who had fewer than three prior indicators of COPD within the 5-year pre-index period were included in the early diagnosis of COPD category. Patients who had three or more prior indicators of COPD within the 5-year pre-index period or had at least one prior indicator of COPD per year in the 2 years prior to the index date were included in the late diagnosis of COPD category. Selected indicators of COPD were pneumonia, respiratory diseases other than pneumonia, chest radiograph, prescription of oral steroids, prescription of antibiotics for airway or lung infections, prescriptions for respiratory disease targeted to relieve respiratory symptoms, and lung function measurements. Indicators recorded fewer than 28 days apart were considered as the same event and assigned to the earliest occurrence date. These indicators suggested possible early COPD respiratory signs, symptoms or exacerbations that patients experienced and led to prescriptions managing symptoms or diagnostic tests to investigate by their physicians.
Outcomes
Baseline characteristics were reported for these two cohorts, namely early- and late-diagnosed COPD patients. The clinical impact of early-diagnosed COPD compared with late-diagnosed COPD was assessed in terms of the time to, risk of, and rate of exacerbations. Economic burden was measured in terms of HCRU based on COPD-related clinic visits, A&E visits, and hospitalizations. To estimate both the clinical burden and HCRU, all patients were followed up for 3 years after the index date.
Diagnosis of Chronic Obstructive Pulmonary Disease
COPD diagnosis was defined by the presence of at least one specific code for COPD (ICD-10 code: J44), at least two prescriptions for a medication indicated for COPD within 4 weeks subsequent to the COPD diagnosis, and at least one spirometry exam within 18 months from the COPD diagnosis.6 Severity of COPD was assessed according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) updated in 2015 criteria presented in Supplementary Table S1.
Exacerbations
Episodes of COPD exacerbation treated in primary care were defined according to a validated algorithm that takes into account the drug codes and diagnosis codes in the CPRD database8 and integrated with in-hospital data from the HES database. Moderate exacerbations were defined as treatment with oral corticosteroids (OCS) and antibiotics on the same day (but no hospitalization) or a lower respiratory tract infection except pneumonia, or exacerbation symptoms and prescription of OCS or oral antibiotics on the same day. Severe exacerbations were defined as COPD-related hospitalizations (Admitted Patient Care) or emergency visits (Emergency and Admission) or an episode of acute exacerbation recorded by a general practitioner (details of the exacerbation symptoms, algorithm, read codes, and definition are provided in Supplementary Section S1). Recurrent exacerbations occurring within 14 days were considered as a unique event.
Comorbidities
Comorbidities were defined based on the diagnosis codes in the primary or secondary care setting and by medications according to the National Prescription Register.9
Statistical methods
Categorical data for baseline characteristics are presented as counts (n) and proportions (%). Continuous data are presented as summary statistics (mean, standard deviation, median, interquartile range, minimum, maximum). A Kaplan–Meier cumulative incidence curve was calculated to estimate the time and risk of first exacerbation. Adjusted hazard ratios and 95% confidence intervals (CIs) for the time-to-first exacerbation were estimated using a multivariable Cox model. A negative binomial regression model was used to calculate unadjusted and adjusted rate ratios of exacerbations between the early and late-diagnosed COPD patients. The models were adjusted for all “a priori” variables that would provide the best Akaike information criterion in the model, which is an estimator of the relative quality of statistical models for a given set of data. The “a priori” variables were age, Charlson Comorbidity Index (CCI), body mass index (BMI), smoking status, GOLD severity, and adherence to COPD treatment. Severity of the disease and adherence to medication were included as covariates in this analysis to avoid the lead-time bias.
Results
Baseline Characteristics
A total of 135,739 HES-eligible patient data of acceptable research quality were identified in CPRD. After applying inclusion and exclusion criteria, 10,158 patients were remaining for the evaluation, of which 6783 (67%) were identified as late-diagnosed and 3375 (33%) as early-diagnosed. Attrition of patients is presented in Supplementary Figure S1. The baseline characteristics were comparable and are presented in Table 1. Early-diagnosed patients were relatively younger and more often male and current smokers compared with late-diagnosed patients (P < 0.001). Patients with late-diagnosed COPD had more comorbidities than those with early-diagnosed COPD. The most commonly recorded comorbidity was hypertension in both the cohorts.
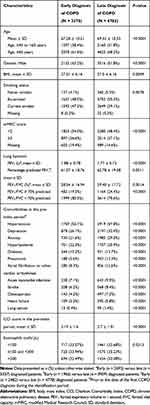 |
Table 1 Baseline Characteristics of Early- versus Late-Diagnosed COPD Patients |
Clinical Burden
Time-to-First Exacerbation
The median (95% CI) time-to-first exacerbation from the index date in the late-diagnosed COPD patients was 14.5 months (13.80–15.10) compared with 29.0 months (27.40–30.70) in the early-diagnosed COPD patients (P < 0.0001; Figure 2). The hazard ratio for the first exacerbation was significantly higher (1.46; P < 0.0001) in late-diagnosed COPD patients compared with early-diagnosed COPD patients (Table 2). Patients aged 40–64 years who were adherent to their COPD medication were less likely to have an exacerbation compared with patients who were non-adherent. Patients in GOLD group D had a hazard ratio of 1.83 for the first exacerbation compared with patients in GOLD group A (Table 2).
 |
Table 2 Hazard Ratio of First Exacerbation for Early versus Late COPD Diagnosis |
 |
Figure 2 Time-to-first exacerbation in the early and late-diagnosed COPD patients. Abbreviation: COPD, chronic obstructive pulmonary disease. |
Rate of Exacerbation
The rate of exacerbations was consistently higher in the late-diagnosed patients versus early-diagnosed patients when compared over a 3-year period (Table 3).
 |
Table 3 Annual Rate of Exacerbations in the 1-, 2-, and 3-Year Periods Following the Index Date |
Healthcare Resource Utilization
COPD-Related Clinic Visits
The rate of clinic visits (per 1000 person-years) in late-diagnosed patients was lower than that of early-diagnosed patients during 1 and 2 years of follow-up. However, comparable rates of clinic visits were observed in both the early- and late-diagnosed patients during 3 years of follow-up (Table 4).
 |
Table 4 Annual Rate of COPD-Related Clinic Visits, A&E Visits, and Hospitalizations After 1, 2, and 3 Years from the Index Date |
COPD-Related A&E Visits
The rate of A&E visits was numerically higher in the late-diagnosed patients than in the early-diagnosed patients during all 3 years of follow-up (Table 4).
COPD-Related Hospitalization
The rate of hospitalization was higher in patients in the late-diagnosed COPD patients than in the early-diagnosed patients during all 3 years of follow-up (Table 4).
Overall Healthcare Resource Utilization
Overall, the HCRU in terms of annual rates of COPD-related clinic visits, A&E visits, and hospitalizations was higher in the late-diagnosed patients in all 3 years of follow-up compared with the early diagnosed patients. However, for clinical visits the HCRU was lower in patients in the late-diagnosed patients versus early diagnosed patients during year 1 and year 2 of follow-up.
Medication Use in Post-Index Period
Overall, the proportion of patients receiving GOLD appropriate therapy after 2 years of follow-up was low in both late- (55.7%) and early (46.2%) diagnosed COPD patients (Supplementary Table S2). The median time-to-first inhaled corticosteroid-long-acting β2-agonist-long-acting muscarinic antagonist (ICS-LABA-LAMA) combination therapy was slightly lower in patients with late-diagnosed COPD (60.1 months) compared with those with early-diagnosed COPD (63.5 months; Supplementary Figure S2). Short-acting β2-agonist (SABA) was most commonly prescribed in combination in both the early (57.5%) and late(59.3%) diagnosed COPD cohorts; however, it was not possible to discriminate whether SABA was administered as rescue medication or as maintenance therapy. In the late-diagnosed patients, ICS was prescribed in combination with 50.9% compared with 43% in the early-diagnosed patients. The combination of ICS-LABA-LAMA was prescribed in 17.1% of early-diagnosed patients compared to 22.5% of late-diagnosed patients during the follow up (Supplementary Table 3).
Discussion
In this retrospective study, we showed that the opportunity for an early diagnosis of COPD was missed in two-thirds of the patients in the UK primary care setting, despite the previous reports.3,10,11 Our results also showed that late diagnosis of COPD was associated with worse clinical outcomes and higher HCRU compared with an early diagnosis. Our findings are in agreement with the outcomes of a retrospective study in UK primary care conducted by Jones et al3 which showed that majority of the patients (85%) presented symptoms of COPD during the past 5 years leading to diagnosis of the disease. The fact that the missed opportunity for COPD diagnosis was present in a smaller proportion of patients (67%) in our study may be due to the differences in the study period and the indicators used to identify COPD. However, the characteristics of patients evaluated in our analysis were consistent with those of the previous reports.10,11
In our study, we have observed that the time-to-first exacerbation was shorter in late-diagnosed patients compared with early-diagnosed patients, which was expected. The overall rate per 100-person years within the 3-year period for late-diagnosed patients was higher versus early-diagnosed patients. A similar analysis using the same definitions for early and late diagnosis in the ARCTIC study also showed that earlier diagnosis and treatment of COPD could substantially improve overall health outcomes compared with late diagnosis in a Swedish population. The study reported that 69.5% of patients who were diagnosed late incurred higher annual direct costs than patients with an early diagnosis.11 In addition, late-diagnosed patients were more likely to be admitted as inpatients for COPD-related hospitalization, which was also comparable with the outcomes of the ARCTIC study.11 Results of the previous studies imply that late COPD diagnosis appears to be a common characteristic in primary care settings regardless of the country studied.
Overall, the treatment pattern observed in our study was comparable with the previous findings, with some differences. In our study, prescriptions in UK primary care were as per the GOLD strategy document and aligned with an earlier report.10 However, the use of ICS-LABA-LAMA combination therapy was slightly lower in our study compared with previous report.12
Although, the large sample size of COPD patients and the robust outcomes of this study provide data that are highly representative of the general population, there are acknowledged drawbacks of observational studies that use electronic health record data. The data used in this analysis were restricted to those of patients who were HES eligible and therefore limited to England-based patients only, and findings of this study may not be generalizable to the entire UK population and population of other countries. Further, lead-time bias may exist among the COPD patients included in our study and influence the results, as patients with early diagnosed COPD may demonstrate an apparent improvement in the time-to-first COPD exacerbation and may remain the same without changing the eventual disease course. Thus, we have included severity of COPD and adherence to medication as covariates to reduce the impact of this lead-time bias; however, it is not possible to completely rule this out.
Conclusion
This retrospective study demonstrated that about two-thirds of patients with COPD from UK primary care are diagnosed late despite presenting with respiratory symptoms or other potential disease manifestations. Late diagnosis was associated with a shorter time to and higher risk of first exacerbation as well as increased exacerbation rate, which was reflected in the increased rate of hospitalizations in late-diagnosed patients versus early-diagnosed patients with COPD.
Abbreviations
A&E, accident and emergency; BMI, body mass index; CCI, Charlson Comorbidity Index; COPD, chronic obstructive pulmonary disease; CPRD, Clinical Practice Research Datalink; GOLD, Global Initiative for Chronic Obstructive Lung Disease; HES, Hospital Episode Statistics; HCRU, healthcare resource utilization; ICD-10, International Classification of Diseases 10th Revision; ICS, inhaled corticosteroids; ISAC, Independent Scientific Advisory Committee; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist; OCS, oral corticosteroid; SABA, short-acting β2-agonist.
Data Sharing Statement
Novartis is committed to sharing with qualified external researchers’ access to patient-level data and supporting clinical documents from eligible studies. These requests are reviewed and approved by an independent review panel on the basis of scientific merit. All data provided are anonymized to respect the privacy of patients who have participated in the trial in line with applicable laws and regulations. This trial data availability is according to the criteria and process described on www.clinicalstudydatarequest.com.
Ethics Approvals
The study protocol was approved by the CPRD, Independent Scientific Advisory Committee for Medicines and Healthcare products Regulatory Agency database research, UK (ISAC 17_213), and the study was conducted in accordance with the principles of the Declaration of Helsinki.
Consent for Publication
All authors approved the final version and provided consent to publish this manuscript.
Acknowledgments
Editorial and writing support was provided by Santanu Bhadra and Harneet Kaur (Novartis), funded by Novartis AG, Basel, Switzerland, in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3). Data analysis was conducted by Pharmatelligence.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, analysis and interpretation of data, drafting the manuscript, revising the manuscript critically, read and approve the final draft of the manuscript for submission, gave final approval of the manuscript version to be published and agreed to be accountable for every step of the work.
Disclosure
RF, AC, HC and FSG are employees and shareholders of Novartis. EL is an employee of Novartis. KK reports grants and personal fees from AstraZeneca, grants and personal fees from Boehringer Ingelheim, grants and personal fees from Chiesi, grants and personal fees from ELPEN, grants and personal fees from GSK, grants and personal fees from Novartis, grants and personal fees from Menarini, personal fees from Sanofi, grants from NuvoAir, outside the submitted work; and was an employee of Novartis Pharma AG until October 31, 2018. DP reports board membership and consultancy agreements with Amgen, board membership, consultancy agreements, grants and unrestricted funding for investigator-initiated studies, payment for lectures/speaking engagements and payment for travel/accommodation/meeting expenses from AstraZeneca, board membership, consultancy agreements, grants and unrestricted funding for investigator-initiated studies (conducted through Observational and Pragmatic Research Institute Pte Ltd), payment for lectures/speaking engagements and payment for travel/accommodation/meeting expenses from Boehringer Ingelheim, board membership, consultancy agreements, grants and unrestricted funding for investigator-initiated studies (conducted through Observational and Pragmatic Research Institute Pte Ltd) and payment for lectures/speaking engagements from Chiesi, board membership and grants and unrestricted funding for investigator-initiated studies (conducted through Observational and Pragmatic Research Institute Pte Ltd) from Circassia, payment for lectures/speaking engagements from Cipla, consultancy agreements and payment for lectures/speaking engagements from GSK, payment for lectures/speaking engagements from Kyorin, grants and board membership, consultancy agreements, grants and unrestricted funding for investigator-initiated studies (conducted through Observational and Pragmatic Research Institute Pte Ltd), payment for lectures/speaking engagements and payment for travel/accommodation/meeting expenses from Mylan, board membership, consultancy agreements, grants and unrestricted funding for investigator-initiated studies (conducted through Observational and Pragmatic Research Institute Pte Ltd), payment for lectures/speaking engagements, payment for the development of educational materials and payment for travel/accommodation/meeting expenses from Mundipharma, board membership, consultancy agreements, grants and unrestricted funding for investigator-initiated studies (conducted through Observational and Pragmatic Research Institute Pte Ltd), payment for lectures/speaking engagements, payment for the development of educational materials, payment for travel/accommodation/meeting expenses and funding for patient enrolment and completion of research from Novartis, consultancy agreements and grants and unrestricted funding for investigator-initiated studies (conducted through Observational and Pragmatic Research Institute Pte Ltd) from Pfizer, board membership, grants and unrestricted funding for investigator-initiated studies (conducted through Observational and Pragmatic Research Institute Pte Ltd) and payment for lectures/speaking engagements from Regeneron Pharmaceuticals, grants and unrestricted funding for investigator-initiated studies (conducted through Observational and Pragmatic Research Institute Pte Ltd) from Respiratory Effectiveness Group, board membership, grants and unrestricted funding for investigator-initiated studies (conducted through Observational and Pragmatic Research Institute Pte Ltd) and payment for lectures/speaking engagements from Sanofi Genzyme, board membership, consultancy agreements, grants and unrestricted funding for investigator-initiated studies (conducted through Observational and Pragmatic Research Institute Pte Ltd), payment for lectures/speaking engagements from Teva, consultancy agreements and grants and unrestricted funding for investigator-initiated studies (conducted through Observational and Pragmatic Research Institute Pte Ltd) from Theravance, grants and unrestricted funding for investigator-initiated studies (conducted through Observational and Pragmatic Research Institute Pte Ltd) from UK National Health Service, non-financial support from Efficacy and Mechanism Evaluation programme, non-financial support from Health Technology Assessment, outside the submitted work; and stock/stock options from AKL Research and Development Ltd. which produces phytopharmaceuticals; and owns 74% of the social enterprise Optimum Patient Care Ltd. (Australia and UK) and 74% of Observational and Pragmatic Research Institute Pte Ltd. (Singapore). He also reports personal fees from ThermoFisher. RJ reports grants, personal fees and non-financial support from AstraZeneca, personal fees and non-financial support from Boehringer Ingelheim, personal fees from Chiesi, grants, personal fees and non-financial support from GSK, non-financial support from Novartis, non-financial support from Nutricia, personal fees and non-financial support from Observational and Pragmatic Research Institute Pte Ltd, personal fees from Pfizer, outside the submitted work. BJ is an employee of Pharmatelligence who received funding from Novartis to conduct analyses for this study. The authors report no other conflicts of interest in this work.
References
1. British Lung Foundation. Available from: https://statistics.blf.org.uk/copd#:~:text=In%20terms%20of%20diagnosed%20cases,suggests%20that%20prevalence%20is%20growing.
2. Snell N, Strachan D, Hubbard R, Gibson J, Gruffydd-Jones K, Jarrold I. S32 Epidemiology of chronic obstructive pulmonary disease (COPD) in the UK: findings from the British lung foundation’s ‘respiratory health of the nation’ project. Thorax. 2016;71(Suppl 3):A20. doi:10.1136/thoraxjnl-2015-207140
3. Jones RC, Price D, Ryan D, et al. Opportunities to diagnose chronic obstructive pulmonary disease in routine care in the UK: a retrospective study of a clinical cohort. Lancet Respir Med. 2014;2(4):267–276. doi:10.1016/S2213-2600(14)70008-6
4. Global Initiative for Chronic Obstructive Lung Disease; 2018. www.goldcopd.org.
5. Clinical Practice Research Datalink (CPRD). 2019. Available from: https://www.cprd.com/.
6. Quint JK, Mullerova H, DiSantostefano RL, et al. Validation of chronic obstructive pulmonary disease recording in the clinical practice research datalink (CPRD-GOLD). BMJ Open. 2014;4(7):e005540. doi:10.1136/bmjopen-2014-005540
7. NHS Digital. Hospital episode statistics (HES); 2019. Available from: https://digital.nhs.uk/data-and-information/data-tools-and-services/data-services/hospital-episode-statistics#summary.
8. Rothnie KJ, Müllerová H, Hurst JR, et al. Validation of the recording of acute exacerbations of COPD in UK primary care electronic healthcare records. PLoS One. 2016;11(3):e0151357. doi:10.1371/journal.pone.0151357
9. The National Institute for Health and Care Excellence (NICE). British national formulary; 2019. Available from: https://www.nice.org.uk/bnf-uk-only/.
10. Halpin DMG, de Jong HJI, Carter V, Skinner D, Price D. Distribution, temporal stability and appropriateness of therapy of patients with COPD in the UK in relation to GOLD 2019. EClinicalMedicine. 2019;14:32–41.
11. Larsson K, Janson C, Ställberg B, et al. Impact of COPD diagnosis timing on clinical and economic outcomes: the ARCTIC observational cohort study. Int J Chron Obstruct Pulmon Dis. 2019;14:995–1008. doi:10.2147/COPD.S195382
12. Brusselle G, Price D, Gruffydd-Jones K, et al. The inevitable drift to triple therapy in COPD: an analysis of prescribing pathways in the UK. Int J Chron Obstruct Pulmon Dis. 2015;10:2207–2217.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
