Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 16
Clinical Evolution and Quality of Life in Clinically Based COPD Chronic Bronchitic and Emphysematous Phenotypes: Results from the 1-Year Follow-Up of the STORICO Italian Observational Study
Authors Blasi F, Antonelli Incalzi R , Canonica GW, Schino P, Cuttitta G, Zullo A, Ori A, Scichilone N
Received 17 March 2021
Accepted for publication 30 June 2021
Published 21 July 2021 Volume 2021:16 Pages 2133—2148
DOI https://doi.org/10.2147/COPD.S310428
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Francesco Blasi, 1, 2 Raffaele Antonelli Incalzi, 3 Giorgio Walter Canonica, 4 Pietro Schino, 5 Giuseppina Cuttitta, 6 Alessandro Zullo, 7 Alessandra Ori, 7 Nicola Scichilone 8 On behalf of STORICO study group
1Internal Medicine Department, Respiratory Unit and Cystic Fibrosis Adult Center, Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico, Milan, 20122, Italy; 2Department of Pathophysiology and Transplantation, University of Milan, Milan, 20122, Italy; 3University Biomedical Campus of Rome, Rome, 00128, Italy; 4Department of Biomedical Sciences, Humanitas University, 20072 Pieve Emanuele, Milan, Italy; IRCCS Humanitas Research Hospital, Personalized Medicine, Asthma and Allergy, 20089, Rozzano, Milan, Italy; 5Miulli Hospital, Acquaviva delle Fonti, Bari, 70021, Italy; 6National Research Council, Palermo, 90146, Italy; 7Medineos Observational Research, Modena, 41123, Italy; 8DIBIMIS, University of Palermo, Palermo, 90127, Italy
Correspondence: Francesco Blasi
Internal Medicine Department, Respiratory Unit and Cystic Fibrosis Adult Center Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico, via Francesco Sforza, 35, Milan, 20122, Italy
Email [email protected]
Introduction: Understanding clinical evolution of chronic obstructive pulmonary disease (COPD) is crucial for improving disease management.
Materials and Methods: STORICO (NCT03105999), an Italian, multicenter, non-interventional, observational study conducted in 40 pulmonology centers, aimed to describe the 1-year clinical evolution and health status of clinicallbased phenotypes. Baseline and follow-up data of COPD subjects with a chronic bronchitis (CB) or emphysema (EM) phenotype were collected. The frequency of COPD symptoms during the 24 hours (gathered via the night-time, morning and day-time symptoms of COPD questionnaire) and the anxiety and depression levels (via the HADS Scale) were recorded at each visit.
Results: A total of 261 CB and 159 EM patients were analyzed. CB patients with ≥ 1 night-time symptom seemed to be more frequent (51.7%, 41.8% and 41.4% at baseline, 6-month and 12-month follow-up, respectively) than EM (37.7%, 32.1% and 30.2% at study visits) even if no statistical differences were observed at time points between phenotypes (chi-square test p-values presence/absence of night-time symptoms in CB vs EM at study visits > 0.0007). In the first 6 months, the frequency of patients with ≥ 1 night-time symptom decreased of 9.9% in CB and of 5.6% in EM. A clinically relevant decline of DLCO % predicted over 1 year in EM was observed, the mean (SD) being 61.5 (20.8) % at baseline and 59.1 (17.4) % at 12-month follow-up. EM had higher levels of anxiety and depression than CB (median (25th-75th percentile) HADS total score in CB: 7.0 (4.0– 13.0) and 7.0 (3.0– 12.0), in EM: 9.0 (3.0– 14.0) and 9.5 (3.0– 14.0) both at baseline and at 6-month follow-up, respectively), considering 1.17 as minimally clinical important difference (MCID) for the total score.
Conclusion: EM patients, evaluated in a real-world setting, seem to suffer from a worse clinical condition and health status compared to CB patients, appearing to have “more treatable” traits.
Keywords: COPD, clinical phenotype, clinical evolution, quality of life
Introduction
In patients affected by Chronic Obstructive Pulmonary Disease (COPD) cough and sputum production are associated with frequent exacerbations and hospitalizations1 and a recent publication confirms that exacerbations occur in less than half of COPD patients.2 Moreover, COPD subjects present a high prevalence of non-pulmonary comorbidities that can influence the natural history and the management of the disease.3 COPD is a very heterogeneous disease and understanding its clinical evolution is crucial for improving disease management and prognosis.
Notwithstanding the huge number of studies on the natural history of COPD, still a number of questions remain unsolved.4 Two important questions are still a matter of discussion in the literature. First, if different phenotypes have different natural history5 and, second, which clinical and functional variables are important in defining different subgroups of COPD patients and possibly in identifying patients with higher risk for disease progression, such as frequent exacerbators.6
Nowadays, the presence of chronic bronchitis seems to be associated with an increased risk of exacerbation1,7 and of COPD in 6–70% of patients in different series with a higher prevalence in more severe patients.8,9
The identification of treatable traits of the different COPD phenotypes will lead to a new precision medicine approach.10
The STORICO (NCT03105999) study (STudio Osservazionale sulla caratteRizzazione dei sIntomi delle 24 ore nei pazienti con broncopneumopatia cronica ostruttiva, observational study on characterization of 24-h symptoms in patients with COPD) offers the unique opportunity to prospectively analyze the clinical characteristics and quality of life evolution of subjects with COPD, with respect to their daily symptom variability in one-year follow-up. Aims of the present paper were (i) (primary objective) to describe the 12-month clinical evolution of patients (in terms of early-morning, day- and night-time COPD symptoms and lung function parameters) according to clinical phenotypes identified at enrolment and (ii) (secondary objective) to describe the quality of life, quality of sleep, level of anxiety and depression and physical activity during the 12-month follow-up in clinically based phenotypes.
Materials and Methods
Study Design
STORICO (NCT03105999) is an Italian, multicenter, non-interventional, observational study conducted in 40 pulmonology centers.
Consecutive recruitment of patients was conducted from February 2016 to April 2017, while the longitudinal phase of the study lasted 12 months, with scheduled visits after 6 and 12 months from baseline. Last patient last visit occurred in June 2018.
Study Population
All subjects aged ≥50, current or ex-smokers with a smoking history of at least 10 pack-years, with a diagnosis of COPD (defined as post-bronchodilator FEV1/FVC <0.7 with the presence of symptoms) and in stable conditions (ie with no major changes in the therapy) for at least 12 months according to the GOLD 2014 (stages A to D) 7 were enrolled.11–13 Patients participating in a clinical trial and those who had changed their COPD treatment regimen in the 3 months prior to enrolment were excluded, as were patients with exacerbations during the month prior to enrollment. Also, patients under continuous use of oxygen therapy or suffering from asthma, sleep apnea syndrome or other chronic diseases that reduced life expectancy to less than 3 years (Charlson index > 3) were excluded.11 Patients provided written informed consent before study participation.
We are here presenting data only for patients who completed baseline and follow-up visits within 6 (±2) and 12 (±3) months, respectively, and with available information on the frequency of COPD symptoms during each part of the 24-h day at baseline and both follow-up visits.
Clinical Assessment
At baseline visit, socio-demographic characteristics were recorded as well as clinical history, GOLD grouping (combined COPD assessment according to the GOLD document) and presence of relevant comorbidities. The GOLD grouping (stages A-D) was determined by the clinician at enrollment through the assessment of (i) symptoms (by means of the COPD Assessment Test (CAT)) or breathlessness (by means of the modified Medical Research Council (mMRC) dyspnea scale), (ii) GOLD classification of airflow limitation (GOLD 1-2-3-4), (iii) exacerbation history in the year before enrollment. GOLD 1 (mild airflow limitation) was attributed to patients with FEV1≥80% predicted, GOLD 2 (moderate airflow limitation) to patients with 50%≤FEV1<80% predicted, GOLD 3 (severe airflow limitation) to patients with 30%≤FEV1<50% predicted and GOLD 4 (very severe airflow limitation) to patients with FEV1<30% predicted.
The presence of anemia was assessed by hemoglobin <13.5 g/dl (for males) and <12 gr/dl (for females)14 and the estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2 was considered indicative of a chronic kidney disease.15
At each study visit, clinicians assigned a clinical COPD phenotype to subjects based on their judgment; chronic bronchitis (CB) was defined as the presence of productive cough for at least 3 months in two consecutive years, emphysema (EM) was chosen for patients who present with dyspnea and reduced tolerance to exercise as predominant symptoms and mixed COPD-asthma (MCA) for patients with a documented not completely reversible airflow obstruction, accompanied by symptoms or signs of obstruction reversibility.16,17
At study visits, spirometry was performed according to recommendations of American Thoracic Society (ATS) and European Respiratory Society (ERS): forced expiratory volume in the first second (FEV1) and forced vital capacity (FVC) were collected, and, if available, Residual Volume (RV), Total Lung Capacity (TLC) and diffusion capacity for carbon monoxide (DLCO) were recorded too.
For each patient, the number of exacerbations per patient (regardless of their severity) was collected at baseline visit (considering the last 5 years before enrollment). Exacerbations were defined as an acute event characterized by a worsening of the patient’s respiratory symptoms that was beyond day-to-day variations and led to a change in medication during follow-up; their occurrence and severity (mild/moderate/severe) were assessed.
Outcome Measures
The frequency of COPD symptoms (breathlessness, coughing, bringing up phlegm or mucus, chest tightness, chest congestion and wheezing) during each part of the day were assessed at study visits by means of the Night-time, Morning and Day-time Symptoms of COPD questionnaire.18 The level of perceived breathlessness and the extent to which it affected mobility were assessed by the modified Medical Research Council (mMRC) dyspnea scale ranging from 0 (breathless with strenuous exercise) to 4 (too breathless to leave the house or breathless when dressing or undressing).19
The patients completed at each visit the COPD Assessment Test (CAT) to measure health status on a single score ranging from 0 to 40 where higher scores represent worse health status.20
Health-related quality of life was evaluated by the St. George’s Respiratory Questionnaire (SGRQ), including the subject’s perception of his/her recent respiratory problems (Symptoms component), disturbances to daily physical activity (Activity component) and disturbances of psychosocial function (Impact component); a total score was also calculated. Scores range between 0 (no impairment) and 100 (highest impairment).21,22
Anxiety and depression states were investigated through the Hospital Anxiety and Depression Scale (HADS),23,25 with a total score (emotional distress) ranging between 0 and 42; anxiety and depression subscales scores (ranging 0–21) were also computed, with higher scores indicating more distress.
The impact of respiratory symptoms on sleep was assessed with the COPD and Asthma Sleep Impact Scale (CASIS),26 a self-administered, 7-item scale evaluating sleep impairment associated with COPD and asthma. The total score ranges 0–100, with higher scores indicating greater sleep deterioration in the previous week.
Physical activity was assessed with the International Physical Activity Questionnaire (IPAQ)27 and categorical score (low, medium, high physical activity) was calculated. All questionnaires and scale were administered in Italian language.
Sample Size
The sample size was not determined to test any a priori hypothesis as the STORICO study aim was descriptive. According to feasibility considerations, assuming the inclusion of 600 patients (10% of whom could have violated inclusion criteria), we simulated the achievable precision (ie the relative error – calculated as the ratio between 95% CI half-width and expected proportion) of the primary endpoint. The reached sample size at 12-month follow-up (n=446) allowed us to estimate the 12-month evolution of COPD symptoms with an acceptable level of precision (relative error < 30%).
Statistical Analysis
Data were analyzed in accordance with a pre-defined statistical analysis plan. The continuous, normally distributed variables were expressed as a mean ± SD and comparisons between independent groups (ie evaluable vs not evaluable patients) were performed with parametric Student’s t-test and comparisons of lung function parameters at baseline vs each follow-up visit or between follow-up visits at subject level were performed by means of the paired t-test. In case of not normally distributed parameters, median and interquartile range (IQR) were provided and not parametric tests (Mann–Whitney U for independent groups (ie comparisons of SGRQ scores at different time points in EM vs CB patients) and Wilcoxon signed-rank tests for correlated observations (ie comparisons at a subject level of lung function parameters or CASIS total score between time points)) were chosen.
Absolute and relative frequencies were calculated for qualitative data and differences between categorical variables were tested by chi-square test.
Alpha (with Bonferroni correction) was set to 0.0007 considering the total number of performed tests. The evolution of (night-time, early-morning and day-time) COPD symptoms during follow-up was described in CB and EM patients in terms of frequency of patients with symptoms: (i) present both at baseline and x-month follow-up (“always present”), (ii) absent both at baseline and x-month follow-up (“always absent”), (iii) present at x-month follow-up and not at baseline (“arising”) and (iv) present at baseline and not at x-month follow-up (“no more present”). The association between the evolution of COPD symptoms between baseline and 6-month follow-up (and between 6- and 12-month follow-up) vs clinical phenotypes was evaluated by means of chi-square tests.
Missing values were not replaced and did not contribute to the analysis of the variable. Frequency of missing data was given for all analyzed variables.
Statistical analyses were performed overall and within each phenotype.
Site monitoring, data management and statistical analysis were performed by MediNeos (Modena, Italy). Statistical analysis was performed using SAS v9.4 and Enterprise Guide v7.1.
Results
Subjects’ Characteristics
Among the 681 enrolled patients, 606 (89.0%) were evaluable at baseline (350 (57.8%), 216 (35.6%), 32 (5.3%) and 8 (1.3%) classified as CB, EM, MCA and EM+CB, respectively), while 446 patients (73.6% of the evaluable at baseline) were evaluable for the longitudinal phase and included in the present analysis. Disposition of patients is shown in Figure 1. Considering the 69 patients not attending to the 12-month follow-up visit, 12 died, 12 withdrew the informed consent to participate in the study, 44 were lost to follow-up and 1 discontinued for personal matters.
 |
Figure 1 Disposition of patients. *One patient could have more than one reason of exclusion. Abbreviations: CB, chronic bronchitis; MCA, mixed-COPD asthma. |
Patients evaluable for the longitudinal phase did not differ at baseline for gender, age, number of COPD exacerbations/year in the 5 years before baseline, FEV1, RV, TLC, SGRQ (symptoms, activity, impacts on daily life and total) scores compared to not evaluable patients (t-test or chi-square test p-values >0.05) (data not shown).
The clinical phenotype attributed by the clinician at baseline was stable as it was confirmed for 96% of patients (n= 427) at 6-month follow-up and for 94% of patients (n= 418) at 12-month follow-up.
Baseline socio-demographic and clinical characteristics are shown in Table 1.
 |
Table 1 Socio-Demographic and Clinical Characteristics of the Studied Subjects |
MCA group was not analysed due to the low number of patients; and so, from this moment onward, only results of CB and EM patients are described.
Circadian Rhythm of Symptoms During Follow-Up
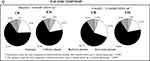
The frequency of patients with at least one night-time, early-morning and day-time symptom (in the week before visit) at the study visits is depicted in Figure 2.
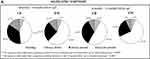 |
Figure 3 Continued. |
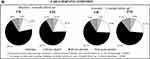 |
Figure 3 Continued. |
 |
Figure 4 Continued. |
At all study visits, CB patients with ≥1 night-time symptom seemed to be more frequent than EM even if no statistical differences were observed at time points between phenotypes (chi-square test p-values presence/absence of night-time symptoms in CB vs EM at study visits >0.0007). Moreover, in the first 6 months the frequency of patients with at least 1 night-time symptom decreased of 5.6% in EM and of 9.9% in CB (and then the frequency seemed stable in the 12-month assessment). As far as other symptoms, the distribution of patients with at least 1 early-morning (or day-time) symptom is similar between phenotypes (chi-square test p-values presence/absence of early-morning (or day-time) symptoms in CB vs EM at all study visits >0.0007).
As shown in Figure 3A, the evolution of night-time symptoms between study visits was not associated to clinical phenotype (chi-square test night-time symptoms evolution in CB vs EM p-values >0.0007). It can be noticed that 94 (36.0%) and 42 (26.4%) of CB and EM, respectively, have always present night-time symptoms, suggesting a higher presence of symptoms in the CB, and 41 CB (15.7%) and 18 EM (11.3%) have no more present night-time symptoms at 6-month follow-up. The proportion of CB and EM patients with arising night-time symptoms was similar (5.8% in CB and 5.7% in EM).
No relevant associations were found neither between clinical phenotype and evolution of early-morning and day-time COPD symptoms (see Figure 3B and C).
Exacerbations During Follow-Up
The median (IQR) number of exacerbation/year in the 5 years before enrollment was 1.00 (1.00–2.00) in the whole sample, 1.00 (1.00–2.00) and 1.00 (0.00–2.00) in CB and EM, respectively.
At least one exacerbation during the study occurred in 137 patients (22.6%) without relevant difference in frequency between phenotypes (82 (23.4%) CB and 44 (20.4%) EM). The exacerbations were prevalently of mild or moderate severity without differences between phenotypes again: in the whole sample 13.2% (n=80) and 11.4% (n=69) of patients had at least one exacerbation of mild or moderate severity, respectively.
Lung Function Parameters
As depicted in Figure 4A and B, lung function parameters were stable over time both in CB and in EM patients (paired t-test and Wilcoxon signed-rank test p-values of parameters at baseline vs 6-month follow-up, at baseline vs 12-month follow-up and at 6-month vs 12-month follow-up in CB and in EM patients >0.0007). In our opinion, however, the decline of DLCO % of the predicted over 1 year in EM, the mean (SD) being 61.5 (20.8) % at baseline and 59.1 (17.4) % at 12-month follow-up, could be considered clinically relevant.
Level of Dyspnea
The level of dyspnea, as per patient perception, was stable over time; at baseline, 6- and 12-month follow-up visits 104 (42.8%), 110 (45.3%) and 111 (45.7%) and 73 (49.3%), 74 (50.0%) and 71 (48.0%) CB and EM patients, respectively, had mMRC score ≥2.
Quality of Life, Quality of Sleep, Level of Anxiety and Depression and Physical Activity
As shown by SGRQ scores in Table 2, the quality of life did not show relevant changes during 12 months of observation; the overall median (IQR) change of SGRQ total score between baseline and 12-month follow-up was −0.2 (−5.5–2.7) points. Considering the SGRQ total score at the study visits, the differences between CB and EM did not reach statistical significance (p-values Mann–Whitney U-test total score CB vs EM at all study visits >0.0007).
 |
Table 2 Quality of Life, Quality of Sleep, Level of Anxiety and Depression and of Physical Activity by Clinical Phenotype (at Study Visits) |
In CB patients, the quality of sleep becomes better between baseline and follow-up visits; the median (25th-75th percentile) CASIS total score at the baseline was, in fact, 16.1 (7.1–39.3) vs 11.4 (2.9–25.7) at 6- and 12-month follow-up (p-values Wilcoxon signed-rank tests CASIS total score baseline vs 6-month follow-up and baseline vs 12-month follow-up <0.0001). Between baseline and follow-up visits also in the EM patients the quality of sleep improved significantly (p-values Wilcoxon signed-rank tests CASIS total score baseline vs 6-month follow-up and baseline vs 12-month follow-up <0.0001) but with lower increase respect to CB.
As shown in Table 2, at all study visits, levels of anxiety and depression were in general low. Considering as minimally clinical important difference (MCID) for the total score 1.17,28 EM compared to CB patients had higher levels of anxiety and depression (as assessed by the HADS total score) both at baseline and at 6-month follow-up. In CB, the median (25th-75th percentile) HADS total score was in fact 7.0 (4.0–13.0) at baseline and 7.0 (3.0–12.0) at 6-month follow-up, while in EM it was 9.0 (3.0–14.0) and 9.5 (3.0–14.0) at baseline and 6-month follow-up, respectively. As far as the variation over time, all the scores appeared stable without relevant differences between clinical phenotypes (the overall median (IQR) change of HADS total score between baseline and 12-month follow-up was −0.0 (−2.0–2.0) points).
Lastly, the levels of physical activity were mainly moderate at all the study visits both in CB and EM patients. The number of patients with low physical activity increased over 1 year more evidently in the EM patients (from 20.4% at baseline to 28.6% at 12-month follow-up) than in CB ones (from 32.9% at baseline to 35.6% at 12-month follow-up).
Therapies for COPD
The therapies for COPD ongoing at study visits are described in Figure 5.
Considering the whole sample, 99.0% (600 out of 606), 98.4% (439 out of 446) and 98.7% (440 out of 446) of patients were on therapy for COPD at baseline, 6-month and 12-month follow-up. Overall at all study visits, the most frequently assumed therapy was the triple (n=215 35.5% at baseline, n=159 35.7% at 6-month follow-up, n=160 35.9% at 12-month follow-up), followed by LABA+LAMA (n=175 28.9% at baseline, n=127 28.5% at 6-month follow-up, n=128 28.7% at 12-month follow-up), LAMA alone (n=98 16.2% at baseline, n=69 15.5% at 6-month follow-up, n=70 15.7% at 12-month follow-up) and ICS+LABA (n=87 14.4% at baseline, n=62 13.9% at 6-month follow-up, n=61 13.7% at 12-month follow-up).
It can be noticed that EM patients were more frequently prescribed with LABA+LAMA (n=88 40.7% at baseline, n=66 41.5% at 6-month follow-up, n=69 43.4% at 12-month follow-up), while CB patients with triple therapy (n=128 36.6% at baseline, n=98 37.5% at 6-month follow-up, n=101 38.7% at 12-month follow-up).
Discussion
COPD is a chronic respiratory disease characterized by chronic respiratory symptoms.
The STORICO study evaluated the evolution of symptoms in different COPD phenotypes in a 12-months follow-up. Considering the studied population, at all the study visits CB patients with ≥1 night-time symptom seem to be more frequent than EM (even if statistical significance was not reached) and in the first 6 months of follow-up there is a reduction in frequency of patients with ≥1 symptom higher in CB than in EM and, then, the frequency remained stable.
The interpretation of these changes is not easy, but they could be reasonably due to a change during follow-up of the therapy for COPD or of life habits (smoking in particular) or of the comorbidities profile; moreover, a trial effect could also have had a role in the observed variations.
During follow-up, a slight increase in frequency of EM patients assuming LABA+LAMA (from 40.7% at baseline to 43.4% at 12-month follow-up) and of CB patients assuming triple therapy (from 36.6% at baseline to 38.7% at 12-month follow-up) was observed. However, the analysis of these changes did not allow any final conclusion on a treatment effect on symptom changes.
On average, patients had 1 exacerbation/year in the 5 years before enrollment. During observation period 77% patients had no exacerbations with no difference between phenotypes.
This result is consistent with the observation of the reduction in the number of exacerbations registered in the last twenty years of clinical trials as assessed in the placebo arm of the studies29 and it is probably related to the improvement in the treatment of COPD. In fact, in a recent study on 273 Spanish COPD patients30 47.2% of patients were considered non-exacerbators (ie they visited the emergency department less than twice for a respiratory complaint but did not require admission for the complaint during the year before the enrollment). Another study31 found that 55.8% of patients managed in pulmonology centers was non-frequent exacerbators (defined as patients with ≤1 exacerbation in the year before enrollment). The lower rate observed in the STORICO study can potentially be also due to the better conditions of the enrolled population compared to the study of Calle Rubio; in fact, in this study the mean±SD number of exacerbations in the year before enrollment was 2.2 ±2.1 and the mean±SD CAT score was 16.9 (8.3), while in the STORICO, the median (25th-75th percentile) number of exacerbation/year in the 5 years before enrollment and CAT score at enrollment were 1.0 (1.0–2.0) and 13.0 (9.0–18.0), respectively.
Consistently with the low frequency of exacerbations observed in the study, we did not find a significant reduction of spirometric parameters that appear to be stable over the 12-month observation time. However, even if the overall lung function parameters were stable over time and in the different phenotypes, in our opinion the decline of DLCO % of the predicted over 1 year in EM, the median (25th-75th percentile) being 63.0 (46.0–76.0) at baseline and 56.0 (46.0–68.4) at 12-month follow-up, can be considered clinically relevant. This finding confirms that COPD functional abnormalities should be studied with a more comprehensive approach which should be more extensive than a simple spirometry. Moreover, these results underline the need for a lung function follow-up in EM phenotype, including diffusion testing, as suggested by the Italian recommendation.32
The substantial stability of quality of life over time was consistent with the results of a secondary analysis of a longitudinal observational study on 105 patients with advanced COPD in the Netherlands;33 in this study, the mean SGRQ total score did not change significantly during 1-year follow-up (mean ± SD change: +1.3 ± 14.9 points, p-value = 0.42) as in the STORICO study (median (25th-75th percentile) change of SGRQ total score between baseline and 12-month follow-up: −0.2 (−5.5–2.7) points). The result obtained in our study may be related to the low number of exacerbations recorded.
Considering the levels of anxiety and depression, results of a study on 831 COPD patients participating in the CHAIN cohort are available;33 according to this study, no statistically significant differences emerged in anxiety and depression at baseline between the exacerbator phenotype with chronic bronchitis and exacerbator phenotype with emphysema patients. Even if not statistically significant, the results show higher level of anxiety and depression in CB patients, consistently with the worse condition of these patients (lower FEV1% and higher CAT score and higher quote of patients with dyspnea) compared to EM. In the STORICO study, at study visits, levels of anxiety and depression are low and stable over time. Considering as MCID for the total score 1.1728 EM compared to CB, patients had higher levels of anxiety and depression (as assessed by the HADS total score) both at baseline and at 6-month follow-up.
Both in CB and EM, the quality of sleep improved between baseline and 12-month follow-up and the levels of physical activity were moderate. The number of patients with low activity increased over 1 year more evidently in the EM patients than in CB. This may be related to the worsening of lung function in the EM patients assessed with DLCO.
Our work has some limitations, the main of which is that the STORICO study was not specifically designed to investigate differences between clinical phenotypes, so no hypotheses on the proper sizes of the groups were made a priori. Not all patients had available complete data on frequency of COPD symptoms at all study timepoints. Nevertheless, the 12-month evolution of COPD symptoms was estimated with an acceptable level of precision.
Moreover, in order to minimize site and patient selection bias, every effort was made to select sites across a variety of geographic regions in Italy and sampling was based on consecutive enrolment. Nevertheless, it is not anticipated that the patients enrolled in this study were representative of the patient population in Italy, so results should be interpreted by keeping this into consideration.
To our knowledge, this is, however, one of unique real-world study aimed to describe in patients of different clinically based phenotypes the circadian rhythm of COPD symptoms during one-year of follow-up.
Conclusions
The STORICO study showed that, in a real-world setting, EM patients seem to suffer from a worse clinical condition and health status compared to CB patients, appearing to have “more treatable” traits. This is particularly true if exacerbations are taken into account, confirming the current focus on preventing exacerbations suggested by guideline and consensus documents. The small variations observed during 1 year of follow-up in clinical and patient reported outcomes could have been emphasized if a longer follow-up period would be considered.
Abbreviations
ATS, American Thoracic Society; BMI, body mass index; CASIS, COPD and Asthma Sleep Impact Scale; CAT, COPD Assessment Test; CB, chronic bronchitis; COPD, chronic obstructive pulmonary disease; DLCO, diffusion capacity for carbon monoxide; eGFR, estimated glomerular filtration rate; EM, emphysema; ERS, European Respiratory Society; FEV1, forced expiratory volume in the first second; FVC, forced vital capacity; GERD, gastroesophageal reflux disease; GOLD, Global Initiative for Chronic Obstructive Lung Disease; HADS, Hospital Anxiety and Depression Scale; ICS, inhaled corticosteroids; IPAQ, International Physical Activity Questionnaire; IQR, interquartile range; LABA, long-acting beta-agonist; LAMA, long-acting muscarinic antagonists; MCA, mixed COPD-asthma; mMRC, modified Medical Research Council; NK, unknown; PROs, patient reported outcomes; RV, residual volume; SD, standard deviation; SGRQ, St. George’s Respiratory Questionnaire; TLC, total lung capacity.
Data Sharing Statement
The data that support the findings of this study are available from authors and Laboratori Guidotti, Italy but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. There are legal and ethical restrictions since data contain potentially sensitive patient information. Data are, however, available from the authors and Laboratori Guidotti upon reasonable request. Data request may be sent to the first author ([email protected]) and to Stefania Barsanti (Laboratori Guidotti, [email protected]).
Ethics Approval and Consent to Participate
The study was approved by the Ethics Committee of the coordinating center (Fondazione Toscana G. Monasterio Pisa, Italy) and was conducted in accordance with the Declaration of Helsinki and the Good Clinical Practices guidelines for observational studies, complying with all requirements of local regulations. Patients provided written informed consent before study participation.
Acknowledgments
We are grateful to Sara Rizzoli for her help in drafting the manuscript. We are also grateful to the participating centers, involved in data collection and to Giovannetti Clara, Barsanti Stefania, Briguglio Chiara, for their contribution in the interpretation of the work and in critical revision of the article.
Members of the STORICO Study Group – Participating Centers
Pietro Schino (Acquaviva delle Fonti); Giuseppina Cuttitta (Palermo); Maria Pia Foschino (Foggia); Renato Prediletto (Pisa); Carmelindo Mario Enrico Tranfa (Napoli); Maria Cristina Zappa (Roma); Pasquale Patriciello (Pollena Trocchia); Luciana Labate (Bari); Salvatore Mariotta (Roma); Stefano Nava (Bologna); Alessandro Vatrella (Salerno); Michele Mastroberardino (Avellino); Riccardo Sarzani (Ancona); Antonio Iuliano (Milano); Lamberto Maggi (Bergamo); Anna Zedda (Casoria); Alberto Pesci (Monza); Giuseppe Sera (Torino); Antonello Nicolini (Sestri Levante); Salvatore Walter Di Donato (Mondragone); Silvia Forte (Roma); Mario Del Donno (Benevento); Federica Rivolta (Abbiategrasso); Mauro Ferliga (Chiari); Antonio Filippo Raco (Menaggio); Luigi Di Re (Teramo); Gaetano Cabibbo (Modica); Rosario Maselli (Catanzaro); Carlo Gulotta (Orbassano); Stefano Nardini (Vittorio Veneto); Enrico Eugenio Guffanti (Casatenovo); Walter Castellani (Firenze); Luca Triolo (Roma); Giovanni Passalacqua (Messina); Bianca Beghè (Modena); Salvatore Lo Cicero (Milano); Enzo Faccini (Dolo); Elena Atzeni (Nuoro); Roberto Tazza (Terni); Piercarlo Giamesio (Asti).
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
Funding
Laboratori Guidotti and Malesci, Italy (https://www.labguidotti.it/; http://www.malesci.it) provided unconditional financial support to the study.
Disclosure
F.B. reports grants and personal fees from ASTRAZENECA, grants and personal fees from BAYER, personal fees from CHIESI, personal fees from GSK, personal fees from GUIDOTTI, grants and personal fees from GRIFOLS, grants from INSMED, personal fees from MENARINI, personal fees from NOVARTIS, grants and personal fees from PFIZER, personal fees from TEVA, personal fees from VERTEX, personal fees from ZAMBON, outside the submitted work. G.C. reports personal fees for speaker/advisory board fees from AstraZeneca, Chiesi, Menarini, Guidotti-Malesci, Erbazeta, during the conduct of the study; personal fees from AstraZeneca, Chiesi, Menarini, Erbazeta, Guidotti-Malesci, and Lusofarmaco, outside the submitted work. A.Z. and A.O. are employees of Medineos Observational Research. A.Z. reports personal fees from Laboratori Guidotti, during the conduct of the study; personal fees from Chiesi, Novartis, AstraZeneca, and Novonordisk, outside the submitted work. The other authors declare that they have no competing interests.
References
1. Burgel PR, Nesme-Meyer P, Chanez P, et al. Cough and sputum production are associated with frequent exacerbations and hospitalizations in COPD subjects. Chest. 2009;135(4):975–982. doi:10.1378/chest.08-2062
2. Han MK, Quibrera PM, Carretta EE, et al. Frequency of exacerbations in patients with chronic obstructive pulmonary disease: an analysis of the SPIROMICS cohort. Lancet Respir Med. 2017;5(8):619–626. doi:10.1016/S2213-2600(17)30207-2
3. Divo M, Cote C, de Torres JP, et al. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186(2):155–161. doi:10.1164/rccm.201201-0034OC
4. Celli BR, Decramer M, Wedzicha JA, et al. An official American thoracic society/European respiratory society statement: research questions in COPD. Eur Respir Rev. 2015;24(136):159–172. doi:10.1183/16000617.00000315
5. Han MK, Agusti A, Calverley PM, et al. Chronic obstructive pulmonary disease phenotypes: the future of COPD. Am J Respir Crit Care Med. 2010;182(5):598–604. doi:10.1164/rccm.200912-1843CC
6. Segal LN, Martinez FJ. Chronic obstructive pulmonary disease subpopulations and phenotyping. J Allergy Clin Immunol. 2018;141(6):1961–1971. doi:10.1016/j.jaci.2018.02.035
7. Lahousse L, Seys LJM, Joos GF, et al. Epidemiology and impact of chronic bronchitis in chronic obstructive pulmonary disease. Eur Respir J. 2017;50(2):1602470. doi:10.1183/13993003.02470-2016
8. Landt E, Çolak Y, Lange P, et al. Chronic Cough in individuals with COPD: a Population-Based Cohort Study. Chest. 2020;157(6):1446–1454. doi:10.1016/j.chest.2019.12.038
9. Vestbo J, Edwards LD, Scanlon PD, et al. Changes in forced expiratory volume in 1 second over time in COPD. N Engl J Med. 2011;365(13):1184–1192. doi:10.1056/NEJMoa1105482
10. Agusti A, Bel E, Thomas M, et al. Treatable traits: toward precision medicine of chronic airway diseases. Eur Respir J. 2016;47(2):410–419. doi:10.1183/13993003.01359-2015
11. Canonica GW, Blasi F, Scichilone N, et al. On behalf of STORICO study group characterization of circadian COPD symptoms by phenotype: methodology of the STORICO observational study. Eur J Intern Med. 2017;43:62–68. doi:10.1016/j.ejim.2017.05.021
12. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management and prevention of COPD; 2014. Available from: http://goldcopd.org.
13. Scichilone N, Antonelli Incalzi R, Blasi F, et al.; STORICO study group. Circadian rhythm of COPD symptoms in clinically based phenotypes. Results from the STORICO Italian observational study. BMC Pulm Med. 2019;19(1):171. doi:10.1186/s12890-019-0935-2
14. Mayo Clinic. Low Hemoglobin count. Available from: https://www.mayoclinic.org/symptoms/low-hemoglobin/basics/definition/sym-20050760.
15. National Kidney Foundation. KDOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–266.
16. Miravitlles M, Soler-Cataluña JJ, Calle M, et al. Spanish COPD guidelines (GesEPOC): pharmacological treatment of stable COPD. Arch Bronconeumol. 2012;48(7):247–257. doi:10.1016/j.arbres.2012.04.001
17. Lange P, Halpin DM, O’Donnell DE, MacNee W. Diagnosis, assessment, and phenotyping of COPD: beyond FEV1. Int J Chron Obstruct Pulmon Dis. 2016;11:3–12. doi:10.2147/COPD.S85976
18. Miravitlles M, Worth H, Soler Catalauña JJ, et al. Observational study to characterize 24-hour COPD symptoms and their relationship with patient-reported outcomes; results from the ASSESS study. Respir Res. 2014;15(1):122. doi:10.1186/s12931-014-0122-1
19. Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54(7):581–586. doi:10.1136/thx.54.7.581
20. Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34(3):648–654. doi:10.1183/09031936.00102509
21. Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure for chronic airflow limitation. The St George’s respiratory questionnaire. Am Rev Respir Dis. 1992;145(6):1321–1327. doi:10.1164/ajrccm/145.6.1321
22. Meguro M, Barley EA, Spencer S, Jones PW. Development and validation of an improved COPD-specific version of the St George’s respiratory questionnaire. Chest. 2007;132(2):456–463. doi:10.1378/chest.06-0702
23. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi:10.1111/j.1600-0447.1983.tb09716.x
24. Barth J, Martin CR. Factor structure of the hospital anxiety and depression scale (HADS) in German coronary heart disease patients. Health Qual Life Outcomes. 2005;3(1):15. doi:10.1186/1477-7525-3-15
25. Pokrzywinski RF, Meads DM, McKenna SP, Glendenning GA, Revicki DA. Development and psychometric assessment of the COPD and Asthma Sleep Impact Scale (CASIS). Health Qual Life Outcomes. 2009;7(1):98. doi:10.1186/1477-7525-7-98
26. Ainsworth BE, Bassett DR
27. Puhan MA, Frey M, Büchi S, Schünemann HJ. The minimal important difference of the hospital anxiety and depression scale in patients with chronic obstructive pulmonary disease. Health Qual Life Outcomes. 2008;6(1):46. doi:10.1186/1477-7525-6-46
28. Andreas S, Röver C, Heinz J, Straube S, Watz H, Friede T. Decline of COPD exacerbations in clinical trials over two decades - a systematic review and meta-regression. Respir Res. 2019;20(1):186. doi:10.1186/s12931-019-1163-2
29. Hernández Vázquez J, Ali García I, Jiménez-García R, et al. COPD phenotypes: differences in survival. Int J Chron Obstruct Pulmon Dis. 2018;20(13):2245–2251. doi:10.2147/COPD.S166163
30. Calle Rubio M, Casamor R, Miravitlles M. Identification and distribution of COPD phenotypes in clinical practice according to Spanish COPD Guidelines: the FENEPOC study. Int J Chron Obstruct Pulmon Dis. 2017;12:2373–2383. doi:10.2147/COPD.S137872
31. Bettoncelli G, Blasi F, Brusasco V, et al. The clinical and integrated management of COPD. An official document of AIMAR (Interdisciplinary Association for Research in Lung Disease), AIPO (Italian Association of Hospital Pulmonologists), SIMER (Italian Society of Respiratory Medicine), SIMG (Italian Society of General Medicine). Multidiscip Respir Med. 2014;9(1):25. doi:10.1186/2049-6958-9-25
32. Wilke S, Spruit MA, Wouters EF, Schols JM, Franssen FM, Janssen DJ. Determinants of 1-year changes in disease-specific health status in patients with advanced chronic obstructive pulmonary disease: a 1-year observational study. Int J Nurs Pract. 2015;21(3):239–248. doi:10.1111/ijn.12265.
33. Cosio BG, Soriano JB, López-Campos JL, et al. CHAIN study. distribution and outcomes of a phenotype-based approach to guide COPD management: results from the CHAIN Cohort. PLoS One. 2016;11(9):e0160770. doi:10.1371/journal.pone.0160770
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.