Back to Journals » Clinical Ophthalmology » Volume 17
Clinical Evaluation of a Hydrophobic Intraocular Lens Using a Preloaded Automated Injector in a Korean Population
Authors Kim HK, Seo KY , Yoon KC, Choi CY, Chung TY, Hyon JY, Rendon A, Kim HS
Received 16 June 2023
Accepted for publication 9 October 2023
Published 3 November 2023 Volume 2023:17 Pages 3353—3363
DOI https://doi.org/10.2147/OPTH.S421864
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Hong Kyun Kim,1 Kyoung Yul Seo,2 Kyung Chul Yoon,3 Chul Young Choi,4 Tae-Young Chung,5 Joon Young Hyon,6 Alexis Rendon,7 Hyun Seung Kim8
1Department of Ophthalmology, School of Medicine, Kyungpook National University, Daegu, Republic of Korea; 2Institute of Vision Research, Department of Ophthalmology, Yonsei University College of Medicine; Cornea Dystrophy Research Institute, Seoul, Republic of Korea; 3Department of Ophthalmology, Chonnam National University Medical School and Hospital, Gwangju, Republic of Korea; 4Department of Ophthalmology, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Republic of Korea; 5Department of Ophthalmology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Republic of Korea; 6Department of Ophthalmology, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Republic of Korea; 7Alcon Vision LLC, Fort Worth, TX, USA; 8Department of Ophthalmology and Visual Science, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Republic of Korea
Correspondence: Hyun Seung Kim, Department of Ophthalmology and Visual Science, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Republic of Korea, Email [email protected]
Purpose: This study assessed post-market clinical outcomes of the Clareon monofocal intraocular lens (IOL) preloaded in the AutonoMe Delivery System in a real-world setting of Korean patients.
Methods: This prospective, multicenter, single-arm study in Korea was conducted from July 2020 to December 2021. Patients were ≥ 20 years old with unilateral or bilateral cataracts who received Clareon IOLs (CNA0T0) preloaded in an automated injector system. Best corrected distance visual acuity (BCDVA) and uncorrected distance visual acuity (UCDVA) were evaluated under photopic conditions. Surgeon delivery system preference was assessed using a survey questionnaire. Glistenings, surface haze, adverse events, posterior capsule opacification (PCO), and Nd:YAG capsulotomy rates were also assessed during the 12-month postoperative follow-up.
Results: Mean ± SD monocular BCDVA was 0.02 ± 0.11 and 0.00 ± 0.10 logMAR at 1 month and 12 months, respectively. BCDVA of 0.2 logMAR or better was achieved by 94.4% and 99.1% of eyes at 1 month and 12 months after implantation, respectively. Mean monocular UCDVA was 0.11 ± 0.14 and 0.07 ± 0.13 logMAR at 1 month and 12 months, respectively. UCDVA of 0.3 logMAR or better was achieved by 97.4% of eyes at 12 months after implantation. Preparation of the automated injector system was rated as “very easy” or “easy” and CNA0T0 IOL delivery was rated as “very controllable” or “controllable” by all surgeons. Only grade 0 glistenings and no surface haze were observed during the 12-month follow-up. No clinically significant PCO or Nd:YAG capsulotomy were reported throughout the study; clinically nonsignificant PCO was reported in 23% of eyes.
Conclusion: This 12-month real-world study of the CNA0T0 IOL and the automated injector system demonstrated excellent visual outcomes and high surgeon satisfaction.
Keywords: visual acuity, intraocular lens delivery, glistenings, surface haze, surgeon’s preference questionnaire
Introduction
Cataract surgery using phacoemulsification followed by intraocular lens (IOL) implantation is the most common surgical procedure worldwide.1 Monofocal IOLs are frequently implanted lenses because of their relatively low cost, optimal distance visual acuity outcomes, and fewer associated photic phenomena, including halos and glare.2,3
The Clareon® Aspheric Hydrophobic Acrylic Monofocal IOL (Alcon Vision LLC, Fort Worth, TX, USA; CNA0T0 IOL) was launched in Korea in 2020. The CNA0T0 IOLs are foldable, 1-piece IOLs that use flexible acrylic material (PEA/HEMA copolymer) with 1.5% water content. The CNA0T0 IOL optics and architecture were based on the AcrySof® IOL platform (Alcon), and the material and manufacturing were modified to improve lens clarity, minimize edge glare, and reduce the risk of posterior capsule opacification (PCO).4–6 Recent studies with Clareon IOLs in the United States, Europe, India, and Japan demonstrated good visual outcomes and improved lens clarity characteristics, including low levels of surface haze and grade 0 glistenings (according to a 4-grade scale adapted from Miyata et al, grade 0 glistenings were defined as 0–25 microvacuoles/mm2 on the glistenings assessment subjective scale).4–8
In additional to advancements in IOL technology, there have been innovations in IOL delivery systems. The CNA0T0 IOL is available preloaded in the AutonoMe® Automated Preloaded Delivery System (Alcon; automated injector system). The use of preloaded delivery systems may improve cataract surgery outcomes by eliminating the IOL loading phase. In contrast with manually loaded IOLs, preloaded delivery systems allow for reduced device preparation time,9,10 which can significantly shorten total surgical case times and potentially increase efficiency of surgical centers and help meet the increased demand for cataract surgery.9,11–13 Preloaded delivery systems can also improve the consistency of IOL haptic folding and reduce the risk of IOL damage9,10,14,15 by eliminating loading variability or user error and reducing the risk of contamination by eliminating the need to handle the IOL.12,16–18 A prospective, randomized, comparative study reported that the AutonoMe Delivery System provided safe and easy IOL delivery; no haptic misconfigurations were observed.19 Furthermore, preloaded delivery systems are pre-packaged sterile single-use devices that have demonstrated increased microbiologic safety and reduced risk of patient inflammatory reactions (eg, toxic anterior segment syndrome and endophthalmitis).14,18
The AutonoMe Delivery System was designed to protect the corneal incision and provide a safer IOL delivery (Figure 1).6,20,21 It has a proprietary 3-mm nozzle tip and depth guard designed to minimize corneal tissue damage and corneal incision stretch.21,22 Compared with a manual delivery system, the automated injector system demonstrated a smaller wound enlargement and less damage to the device nozzle.22,23 The CO2-powered mechanism of the automated injector system allows for a single-handed surgical technique, improving surgeon’s control over the IOL advancement speed compared with the mechanism of action of other delivery systems (ie, push-type [syringe-style] or screw-type [twist-style] advancement).15,22 Additionally, the use of single-handed IOL delivery allows the surgeon’s second hand to stabilize the eye or manipulate the IOL as needed.22
 |
Figure 1 The automated injector system components and features. Abbreviation: IOL, intraocular lens. |
The AutonoMe Delivery System was reported as safe and effective in studies in Japan, India, and Europe;4,5,8,24 however, at the time of this report, there were no published outcomes from Korean patients implanted with Clareon IOLs or feedback on the use of the AutonoMe Delivery System from Korean surgeons. As this device was recently launched in Korea, there is a need to evaluate clinical outcomes specific to the Korean population. The purpose of this postmarket clinical study was to describe real-world efficacy and safety outcomes in Korean patients implanted with CNA0T0 IOLs preloaded in the automated injector system through 1 year after implantation and to assess IOL delivery performance and real-world experience from surgeons in Korea.
Methods
Study Design
This was a prospective, multicenter, single-arm study that assessed the clinical outcomes, safety, and IOL delivery performance of the CNA0T0 IOL preloaded in the automated injector system at 7 sites in Korea. Similar methods were used as described by Titiyal et al.8 The study was conducted from July 2020 to December 2021, with the final database lock on 19 January 2022. The study was registered with the Clinical Research Information Service on 26 December 2019 (KCT0004634). Patients attended up to 10 visits over 1 year. Study visits included a screening visit, an operative visit, and postoperative visits on day 1, week 1, and months 1, 6, and 12.
Patients were 20 years or older with age-related unilateral or bilateral cataracts requiring an IOL implantation with calculated lens power between +15.0 and +30.0 D and preoperative regular corneal astigmatism of <1.00 D. Patients with clinically significant corneal abnormalities, ocular trauma, previous refractive surgery, previous corneal transplant, history of retinal conditions or degenerative eye disorders, or amblyopia were excluded from the study. Patients who were pregnant or breastfeeding were also excluded from the study.
The study was conducted in compliance with Good Clinical Practice and with international and national regulations, laws, and guidelines such as ISO 14155:2011 and in accordance with the ethical medical research principles outlined in the Declaration of Helsinki. This study was approved by the Chonnam National University Hospital Institutional Review Board (IRB), Yonsei University Health System Severance Hospital IRB, Seoul National University Bundang Hospital IRB, Samsung Medical Center IRB, Kangbuk Samsung Hospital IRB, The Catholic University of Korea Seoul St. Mary’s Hospital IRB, and Kyungpook National University Hospital IRB. All patients included in this study signed informed consent.
Surgical Procedure
Standard phacoemulsification procedure was performed for cataract removal using surgeon’s preferred methodology (including femtosecond laser) per local standard of practice. The AutonoMe Delivery System was used to implant the Clareon monofocal IOL (model CNA0T0) into the capsular bag in the posterior chamber of the eye. IOL power was calculated targeting emmetropia (±0.50 D). Personalized A-constants were not allowed in this study; all surgeons used the manufacturer’s recommended A-constant of 119.1. Surgeons selected the most suitable IOL power using the same IOL power calculation formula at each site for all cases. For patients implanted bilaterally, an IOL power adjustment based on the refractive outcomes of the first operated eye was allowable for the second eye.
Surgeons used their own discretion for the initial incision size. Ophthalmic viscosurgical devices allowed during the surgery included Viscoat® (Alcon), DisCoVisc® (Alcon), or Provisc® (Alcon).
Efficacy Assessments
Best corrected distance visual acuity (BCDVA) and uncorrected distance visual acuity (UCDVA) were evaluated at 4 m using an Early Treatment of Diabetic Retinopathy Study chart under photopic conditions at 1 week, 1 month, 6 months, and 1 year after the implantation. Absolute prediction error was calculated as the absolute difference between the spherical equivalent of the preoperative predicted refraction and the postoperative manifest refraction.
The primary effectiveness endpoint was the percentage of eyes achieving monocular BCDVA of 0.2 logMAR or better at 1 month post-implantation. Secondary effectiveness endpoints included monocular BCDVA, monocular UCDVA, and absolute prediction error at 1 month after surgery.
Exploratory assessments included IOL clarity characteristics, such as glistenings and surface haze. Glistenings were graded according to a 4-grade scale adapted from Miyata et al, and grade 0 glistenings were defined as 0–25 microvacuoles/mm2 on the glistenings assessment subjective scale.7 The presence or absence of IOL surface haze was assessed using slit-lamp examination. PCO and neodymium-doped yttrium aluminium garnet (Nd:YAG) capsulotomy rates were also evaluated.
Surgeon’s Preference of New Delivery System
Surgeon preference regarding the AutonoMe Delivery System was assessed with a survey questionnaire conducted after the completion of all surgeries at each site. A survey response was obtained from a single surgeon at sites where a minimum of 14 implantations had been performed. The questionnaire assessed the surgeon’s current method of cataract surgery (ie, preferred delivery system, typical incision size and architecture, and insertion technique) and asked the surgeons to rate their overall experience with the performance of the AutonoMe Delivery System (ease of preparation, ease of insertion, control of the IOL, ergonomics, intuitiveness/ease of use, and device preference).
Safety
Safety endpoints included ocular and nonocular adverse events (AEs), PCO and Nd:YAG capsulotomy, device deficiencies, IOL tilt and decentration, intraocular pressure, and surgical problems. AEs were reported from the time patients signed informed consent to study exit and were summarized using descriptive statistics.
Results
Patients
Of the 98 patients enrolled in the study, 12 discontinued because of screening failures before implantation. Of the 86 patients with attempted implantation, 125 eyes of 85 patients were successfully implanted with CNA0T0 IOLs. Of these patients, 40 were bilaterally implanted. Baseline demographic data are summarized in Table 1. Mean age ± SD was 69.1 ± 6.7 years, 46/85 patients (54%) were female, and all patients were Korean.
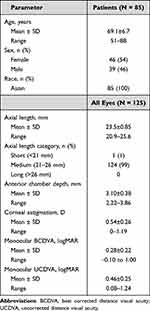 |
Table 1 Patient Demographics and Baseline Characteristics (All-Implanted Analysis Set) |
There were 7 patients who discontinued from the study (due to AEs [n = 4], loss to follow up [n = 1], or withdrawal by the patient [n = 2]); 79 patients completed the study and were included in the analysis. There were 116/125 eyes (93%) that completed the 1-year postoperative visit.
Surgical Procedure
The mean final corneal incision size after implantation of the CNA0T0 preloaded in the automated injector was 2.39 ± 0.26 mm (range, 2.2–2.8 mm). Most final incisions (87/125; 70%) were within the 2.2–2.4 mm range. The implanted IOLs ranged in power from +15.0 to +27.0, and the most frequently implanted IOL powers were +21.5 (16/125; 13%) and +22.0 (12/125; 10%).
Visual Acuity Outcomes
Mean ± SD monocular BCDVA for all eyes was 0.02 ± 0.11 logMAR at 1 month and 0.00 ± 0.10 logMAR at 12 months after implantation (Figure 2A). The primary effectiveness endpoint, BCDVA of 0.2 logMAR or better, was achieved by 94.4% of eyes at 1 month after implantation; at 12 months, 99.1% of eyes had BCDVA of 0.2 logMAR or better. Most eyes (83.1% and 87.8%) achieved BCDVA of 0.1 logMAR or better at 1 month and 12 months after implantation, respectively (Figure 2B).
Mean ± SD monocular UCDVA for all eyes was 0.11 ± 0.14 logMAR at 1 month and 0.07 ± 0.13 logMAR at 12 months after implantation (Figure 3A). More than half of all eyes (60.8% and 68.7%) achieved UCDVA of 0.1 logMAR or better at 1 and 12 months, respectively. Most eyes (97.4%) achieved UCDVA of 0.3 logMAR or better at 12 months after implantation (Figure 3B).
Mean absolute prediction error ± SD for all eyes was 0.33 ± 0.27 D at 1 month and 0.31 ± 0.29 D at 12 months after implantation (Figure 4).
Surgeon-Reported Preferences
One site was not included in the questionnaire analysis because the minimum number of study eye implants (14) was not met. Most surgeons indicated they were currently using the manually loaded Monarch Delivery System (Alcon) in their practice (4/6; 67%), with a 2.0–2.4 mm incision size (4/6; 67%), 2-plane incision architecture (4/6; 67%), and a wound-assisted insertion technique (4/6; 67%).
All surgeons (100%) rated both the preparation of the automated injector system before implantation and incision site insertion as “very easy” or “easy” and reported that the CNA0T0 IOL was “very controllable” or “controllable” during delivery (Supplementary Table 1). All surgeons (100%) rated the comfort of the hand posture when using the automated injector system during IOL delivery as “very comfortable” or “comfortable.” Most surgeons found the automated injector system to be “more intuitive” or “much more intuitive”, easier to use compared with their current delivery systems, and preferred the automated injector system to their current delivery system (Supplementary Table 1).
Safety Outcomes
There were no glistenings and no surface haze observed at any time throughout the 12 months of the study. There were no reports of clinically significant PCO or Nd:YAG capsulotomy for the duration of the study (Table 2).
 |
Table 2 Eyes with Subjective Posterior Capsule Opacification |
One ocular serious AE (cystoid macular oedema) was reported in 1 eye (0.8%; Supplementary Table 2). Ocular AEs occurred in 11/126 eyes (9%). Four of 85 patients who received the CNA0T0 IOL (4.7%) discontinued due to a serious AE (fatal cardiac arrest, fatal tuberculosis, fatal malignant lung neoplasm, and fatal recurrent lung cancer). There were device deficiencies because of a plunger issue (ie, under or overriding IOL) in 2/126 eyes (1.6%); in 1 of these instances, the study IOL did not advance in the injector, and the eye was implanted with a nonstudy IOL and included in the safety analysis only. There were no eyes with IOL position change; no IOL tilt ≥1° or decentration ≥0.5 mm was reported at any visit.
Discussion
This was the first clinical evaluation of the performance and safety of the CNA0T0 IOL preloaded in the automated injector system in a Korean population in a real-world setting. The results from a Korean population provide valuable insights into the clinical effectiveness and safety of CNA0T0 IOLs, particularly in the context of a specific country and a distinct group of surgeons. This emphasis on a Korean population helps contextualize the real-world applicability and performance of the CNA0T0 IOL in this specific demographic group, contributing to the broader understanding of its clinical utility. The CNA0T0 IOL demonstrated good visual outcomes during the 12-month follow-up. There were no findings of clinically significant PCO or Nd:YAG capsulotomies at 12 months after implantation. Furthermore, there were no reports of surface haze, and only grade 0 glistenings were reported throughout the 12-month follow-up. The use of the CNA0T0 IOL preloaded in the automated injector system resulted in high surgeon satisfaction and positive feedback on ease of use.
In the current study, mean monocular BCDVA improved from 0.28 logMAR preoperative to −0.002 logMAR 12 months after implantation, and all eyes achieved BCDVA of 0.3 logMAR or better. Mean monocular UCDVA for all eyes improved from 0.46 logMAR preoperative to 0.07 logMAR at 12 months after implantation, and 97.4% of eyes achieved UCDVA of 0.3 logMAR or better at 12 months. The visual acuity outcomes in the Korean population were consistent with outcomes from several 1-year studies conducted in other regions. In a multicenter Clareon IOL clinical trial in the United States (n = 350), mean monocular BCDVA at 12 months after implantation was −0.05 logMAR, and BCDVA of 0.3 logMAR was achieved by 99.7% of patients.5 There were similar visual acuities reported in 1-year studies conducted in Japan (n = 384) and India (n = 111) with the Clareon IOL preloaded in the AutonoMe Delivery System.4,8
Short-term results for this study were also consistent with findings from other studies. At 1 month post-implantation, the current study reported mean BCDVA of 0.02 logMAR. Similarly, in a study of 144 eyes implanted with the Clareon IOL preloaded in the AutonoMe Delivery System, mean BCDVA was 0.03 logMAR at 4 to 6 weeks after implantation in patients without ocular comorbidities.24 Another study (n = 50) reported that BCDVA of −0.09 logMAR was achieved at 3 months after implantation with Clareon IOLs preloaded with the AutonoMe Delivery System.20
The Clareon IOLs were manufactured with the goal to improve clarity (ie, reduce surface haze, sub-surface nano-glistenings [SSNGs], and glistenings). The smoother surface of the Clareon IOLs was associated with lower surface haze, fewer SSNGs, and fewer glistenings compared with other IOLs in vitro.6 A clinical study reported that at 12 months after Clareon IOL implantation, glistenings were rarely observed and were graded 0 on the Miyata scale.25 The current study observed no surface haze and only grade 0 glistenings during 12 months of follow-up. The square-edge design and fibronectin binding of the Clareon IOL were intended to reduce PCO. In this study, there were no reports of clinically significant PCO or Nd:YAG capsulotomy (Table 2). There were 29/125 eyes (23%) with clinically nonsignificant PCO. Overall, 96/125 eyes (77%) had no observed PCO at 12 months postoperatively. This was consistent with previous findings from 2 studies in Japan that reported a 1% to 5% rate for Nd:YAG capsulotomy,4,26 as well as Clareon IOL studies in other populations.5,8
Advancements in the design of IOL delivery systems have improved efficiency and outcomes of cataract surgery, including shorter total surgical case time and reduced risk of loading variability and device contamination.11–13 The automated injector system used in this study is the first preloaded delivery system to use an automated mechanism of action. It provides intuitive ergonomic single-handed control of IOL delivery designed to benefit the surgeon, such that the second hand can be used to stabilize the globe during IOL delivery. Overall, the automated injector system received high satisfaction feedback from surgeons in Korea. Most surgeons found the automated injector system to be “more intuitive” or “much more intuitive” and reported that it was easier to use compared with their current delivery systems. These findings were consistent with surgeon responses from previous studies that assessed surgeon satisfaction in Japan and India.4,8
Based on the 2018 and 2020 national surveys of surgical practice in Korea, the majority of surgeons used phacoemulsification, and 64% to 66% used an incision size of 2.8 mm when performing surgery.27,28 The automated injector system allows for IOL delivery through initial incisions as small as 2.2 mm; in this study, most final incisions (87/125; 70%) were within the 2.2–2.4 mm range (mean, 2.39 ± 0.26 mm), indicating minimal wound stretch. Previous studies have shown that a smaller incision would result in smaller postoperative surgically induced astigmatism.29,30 Although data on incision wound architecture were not collected as part of this study, other studies have assessed effects of the automated injector system on wound healing. A clinical study (n = 40) that assessed corneal morphology with different IOL delivery systems reported less trauma to the wound, reduced wound retraction, and a more regular architecture of the corneal incision with the automated injector system compared with other delivery systems.22 Additionally, the automated injector system demonstrated a smaller enlargement of the corneal wound during implantation and smaller corneal thickness increase compared with another delivery system.15,31 In a study that evaluated corneal tissue trauma in patients with grade 4 cataract (n = 40), the use of the automated injector system resulted in less corneal thickness increase at the incision site and less endothelial cell loss 1 hour and 1 day after surgery.15 The results of these studies suggest that the automated injector system is able to deliver IOLs through a small incision with minimal wound enlargement.
This study has a number of limitations, including the single-arm study design without a control group. Six eyes implanted with the Clareon IOL were not targeted for emmetropic postoperative refraction with the predicted residual refraction outside of ±0.50 D. Additionally, 3 eyes included in the analyses had preoperative regular astigmatism >1.00 D (Table 1). However, these deviations did not affect the results of the primary endpoints, and these eyes were included in the analysis. These deviations could have affected the secondary effectiveness endpoint of monocular UCDVA.
Because of the real-world setting, some patients in this study had ocular comorbidities, such as diabetic retinopathy, dry eye syndrome, meibomian gland dysfunction, and epiretinal membrane. Investigators judged that the outcomes of the study would not be confounded by the presence of these comorbidities. The inclusion of patients with ocular comorbidities increased the generalizability of these results to the larger “real-world” population. Restrictions related to COVID-19 (ie, travel limitations, quarantine, and social distancing) affected visit schedules and assessments; some visits may have been conducted late. However, 79 of the 85 patients with successful implantation completed the current study despite COVID-19–related disruptions. Other strengths of this study included the real-world setting with Korean patients and high patient retention through the 12-month follow-up period.
In conclusion, this 12-month real-world study of the CNA0T0 IOL preloaded in the automated injector system in Korea demonstrated visual outcomes consistent with studies in other regions; 100% of eyes experienced no surface haze and 100% of eyes had grade 0 glistenings. High surgeon satisfaction was reported for the automated preloaded injector based on a questionnaire that assessed device ease of use, control of the IOL delivery, and ergonomics. Overall, good visual outcomes were achieved with the CNA0T0 IOL preloaded in the automated injector system in a Korean population.
Abbreviations
AE, adverse event; BCDVA, best corrected distance visual acuity; IOL, intraocular lens; Nd:YAG, neodymium-doped yttrium aluminium garnet; PCO, posterior capsule opacification; SSNG, sub-surface nano-glistenings; UCDVA, uncorrected distance visual acuity.
Data Sharing Statement
The data used to support the primary findings of this study are available upon reasonable request from the study sponsor, Alcon Research LLC.
Acknowledgments
Medical writing assistance was provided by Lisa Denny, PhD, of ICON plc (Blue Bell, PA), and was funded by Alcon.
Author Contributions
All authors made a significant contribution to the work reported, whether that was in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; agreed on the journal to which the article has been submitted; and agreed to be accountable for all aspects of the work.
Funding
This study was funded by Alcon Research LLC. Alcon assisted with the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript.
Disclosure
Hong Kyun Kim, Kyoung Yul Seo, Joon Young Hyon, and Hyun Seung Kim were consultants to Alcon. Kyung Chul Yoon and Chul Young Choi were consultants and clinical investigators for Alcon. Tae-Young Chung was a consultant/speaker and a clinical investigator for Alcon. Alexis Rendon is an employee of Alcon Research LLC. The authors report no other conflicts of interest in this work.
References
1. Grzybowski A. Recent developments in cataract surgery. Ann Transl Med. 2020;8(22):1540. doi:10.21037/atm-2020-rcs-16
2. Mencucci R, Cennamo M, Venturi D, Vignapiano R, Favuzza E. Visual outcome, optical quality, and patient satisfaction with a new monofocal IOL, enhanced for intermediate vision: preliminary results. J Cataract Refract Surg. 2020;46(3):378–387. doi:10.1097/j.jcrs.0000000000000061
3. de Silva SR, Evans JR, Kirthi V, Ziaei M, Leyland M. Multifocal versus monofocal intraocular lenses after cataract extraction. Cochrane Database Syst Rev. 2016;12:CD003169. doi:10.1002/14651858.CD003169.pub4
4. Oshika T, Sasaki N, Clinical Study Group on New Intraocular Lens and Delivery System. One-year multicenter evaluation of a new hydrophobic acrylic intraocular lens with hydroxyethyl methacrylate in an automated preloaded delivery system. J Cataract Refract Surg. 2022;48(3):275–279. doi:10.1097/j.jcrs.0000000000000746
5. Lehmann R, Maxwell A, Lubeck DM, Fong R, Walters TR, Fakadej A. Effectiveness and safety of the Clareon monofocal intraocular lens: outcomes from a 12-month single-arm clinical study in a large sample. Clin Ophthalmol. 2021;15:1647–1657. doi:10.2147/OPTH.S295008
6. Werner L, Thatthamla I, Ong M, et al. Evaluation of clarity characteristics in a new hydrophobic acrylic IOL in comparison to commercially available IOLs. J Cataract Refract Surg. 2019;45(10):1490–1497. doi:10.1016/j.jcrs.2019.05.017
7. Miyata A, Uchida N, Nakajima K, Yaguchi S. Clinical and experimental observation of glistening in acrylic intraocular lenses. Jpn J Ophthalmol. 2001;45(6):564–569. doi:10.1016/s0021-5155(01)00429-4
8. Titiyal JS, Basak SK, Shetty N, et al. Twelve-months follow-up postmarket study of a hydrophobic intraocular lens using a preloaded automated injector in an Indian population. Clin Ophthalmol. 2022;16:4215–4225. doi:10.2147/OPTH.S379054
9. Mendicute J, Bascaran L, Pablo L, et al. Multicenter evaluation of time, operational, and economic efficiencies of a new preloaded intraocular lens delivery system versus manual intraocular lens delivery. Clin Ophthalmol. 2021;15:591–599. doi:10.2147/OPTH.S263658
10. Wang L, Wolfe P, Chernosky A, Paliwal S, Tjia K, Lane S. In vitro delivery performance assessment of a new preloaded intraocular lens delivery system. J Cataract Refract Surg. 2016;42(12):1814–1820. doi:10.1016/j.jcrs.2016.10.014
11. Jones JJ, Chu J, Graham J, Zaluski S, Rocha G. The impact of a preloaded intraocular lens delivery system on operating room efficiency in routine cataract surgery. Clin Ophthalmol. 2016;10:1123–1129. doi:10.2147/OPTH.S107726
12. Black D, Corbett D, Roberts TV, et al. Clinical evaluation of a novel preloaded intraocular lens delivery system during routine cataract surgery. Clin Ophthalmol. 2020;14:2291–2300. doi:10.2147/OPTH.S260925
13. Wu Y, Yan H, Yan W. Preloaded vs manually loaded IOL delivery systems in cataract surgery in the largest ambulatory surgery center of northwestern China: an efficiency analysis. BMC Ophthalmol. 2020;20(1):469. doi:10.1186/s12886-020-01721-5
14. Nanavaty MA, Kubrak-Kisza M. Evaluation of preloaded intraocular lens injection systems: ex vivo study. J Cataract Refract Surg. 2017;43(4):558–563. doi:10.1016/j.jcrs.2017.02.019
15. Mastropasqua L, Toto L, D’Ugo E, et al. In vivo and in vitro results of an automated preloaded delivery system for IOL implantation in cataract surgery. Int Ophthalmol. 2020;40(1):125–134. doi:10.1007/s10792-019-01154-0
16. Dada T, Muralidhar R. Insertion of the Sensar IOL via the Monarch II delivery system. J Cataract Refract Surg. 2005;31(9):1684. doi:10.1016/j.jcrs.2005.09.008
17. Weston K, Nicholson R, Bunce C, Yang YF. An 8-year retrospective study of cataract surgery and postoperative endophthalmitis: injectable intraocular lenses may reduce the incidence of postoperative endophthalmitis. Br J Ophthalmol. 2015;99(10):1377–1380. doi:10.1136/bjophthalmol-2014-306372
18. Chung B, Lee H, Choi M, Seo KY, Kim EK, Kim TI. Preloaded and non-preloaded intraocular lens delivery system and characteristics: human and porcine eyes trial. Int J Ophthalmol. 2018;11(1):6–11. doi:10.18240/ijo.2018.01.02
19. Khoramnia R, Yildirim TM, Weindler J, Naujokaitis T, Dzhambazova M, Auffarth GU. Preloaded injectors used in a clinical study: videographic assessment and laboratory analysis of injector nozzle damage. J Cataract Refract Surg. 2021;47(10):1338–1344. doi:10.1097/j.jcrs.0000000000000587
20. Negishi K, Masui S, Torii H, Nishi Y, Tsubota K. Refractive stability of a new single-piece hydrophobic acrylic intraocular lens and corneal wound repair after implantation using a new automated intraocular lens delivery system. PLoS One. 2020;15(9):e0238366. doi:10.1371/journal.pone.0238366
21. Liu J, Wolfe P, Hernandez V, Kohnen T. Comparative assessment of the corneal incision enlargement of 4 preloaded IOL delivery systems. J Cataract Refract Surg. 2020;46(7):1041–1046. doi:10.1097/j.jcrs.0000000000000214
22. Cennamo M, Favuzza E, Salvatici MC, Giuranno G, Buzzi M, Mencucci R. Effect of manual, preloaded, and automated preloaded injectors on corneal incision architecture after IOL implantation. J Cataract Refract Surg. 2020;46(10):1374–1380. doi:10.1097/j.jcrs.0000000000000295
23. Fang H, Zhang L, Schickhardt S, et al. A laboratory evaluation of nozzle tip damage in four generations of intraocular lens injector systems using a self-developed damage scale. Sci Rep. 2022;12(1):2723. doi:10.1038/s41598-022-06696-5
24. Bedar MS, Kellner U. Clinical experience with the Clareon® IOL and the AutonoMe® implantation system. Ophthalmologe. 2020;117(11):1100–1104. doi:10.1007/s00347-020-01075-9
25. Stanojcic N, O’Brart D, Hull C, et al. Visual and refractive outcomes and glistenings occurrence after implantation of 2 monofocal, aspheric, hydrophobic acrylic IOLs. J Cataract Refract Surg. 2020;46(7):986–994. doi:10.1097/j.jcrs.0000000000000201
26. Oshika T, Fujita Y, Inamura M, Miyata K. Mid-term and long-term clinical assessments of a new 1-piece hydrophobic acrylic IOL with hydroxyethyl methacrylate. J Cataract Refract Surg. 2020;46(5):682–687. doi:10.1097/j.jcrs.0000000000000142
27. Chung JK, Lee HK, Kim MK, et al. Cataract surgery practices in the Republic of Korea: a survey of the Korean Society of Cataract and Refractive Surgery 2018. Korean J Ophthalmol. 2019;33(5):451–457. doi:10.3341/kjo.2019.0064
28. Rho CR, Kim JH, Chung IK, et al. Cataract surgery practice in the Republic of Korea: a survey of the Korean Society of Cataract and Refractive Surgery 2020. Korean J Ophthalmol. 2021;35(4):272–279. doi:10.3341/kjo.2020.0001
29. Masket S, Wang L, Belani S. Induced astigmatism with 2.2- and 3.0-mm coaxial phacoemulsification incisions. J Refract Surg. 2009;25(1):21–24. doi:10.3928/1081597X-20090101-04
30. Hayashi K, Yoshida M, Hayashi H. Postoperative corneal shape changes: microincision versus small-incision coaxial cataract surgery. J Cataract Refract Surg. 2009;35(2):233–239. doi:10.1016/j.jcrs.2008.10.031
31. Yildirim TM, Labuz G, Baur ID, et al. Corneal incision enlargement in two preloaded intraocular lens injectors: an intraindividual in vivo study. J Refract Surg. 2021;37(5):331–336. doi:10.3928/1081597X-20210204-01
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



