Back to Journals » International Journal of General Medicine » Volume 15
Clinical-Epidemiological Characteristics and Mortality in Patients with Sickle Cell Anemia: A Retrospective Cohort Study of 1980 at 2018
Authors Pompeo CM , Ferreira Júnior MA, Cardoso AIDQ, Souza MDC, Frota OP, Mota FM, Ivo ML
Received 6 October 2021
Accepted for publication 7 December 2021
Published 2 February 2022 Volume 2022:15 Pages 1057—1074
DOI https://doi.org/10.2147/IJGM.S342971
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Carolina Mariano Pompeo,1 Marcos Antonio Ferreira Júnior,1 Andreia Insabralde de Queiroz Cardoso,1 Mercy da Costa Souza,2 Oleci Pereira Frota,1 Felipe Machado Mota,1 Maria Lúcia Ivo2
1Integrated Institute of Health, Federal University of Mato Grosso do Sul, Campo Grande, Mato Grosso do Sul, Brazil; 2Graduate Program in Health and Development in the Midwest Region, Federal University of Mato Grosso do Sul, Campo Grande, Mato Grosso do Sul, Brazil
Correspondence: Carolina Mariano Pompeo
Integrated Institute of Health, Federal University of Mato Grosso do Sul, Campo Grande, Mato Grosso do Sul, Brazil
, Tel +55 67 99984-7048
, Email [email protected]
Purpose: To analyze the clinical-epidemiological characteristics and mortality in patients with sickle-cell anemia (SCA).
Patients and Methods: A cohort study with retrospective data, conducted in two reference hospitals for SCA treatment from January 1980 to December 2018, recorded in two reference services. With a 5% significance level, the Chi-Square and Student’s t-tests were employed in the inferential statistical analysis.
Results: A total of 128 patients with SCA were studied. Diagnosis up to the fifth day of life was made in 10 patients. There were 19 deaths, of which 12 (63.2%) were female, and the average age at death was 27.05 (± 14.78) years. The leading causes of death were septic shock and cardiogenic shock. The use of invasive medical devices was considered a risk factor for death (RR=2.63; 95% CI=1.16– 5.96; p=0.018), and monitoring time up to 20 years presented a 31% reduction in the risk of dying (RR=0.31; 95% CI=0.12– 0.82; p=0.011) when compared to the monitoring of more than 20 years.
Conclusion: These findings are to be considered in the treatment of patients with SCA, mainly regarding early diagnosis and access to the treatment immediately afterward, since they are fundamental in improving survival and reducing severe complications.
Keywords: hematologic diseases, early diagnosis, neonatal screening, survival, epidemiology
Introduction
Sickle-cell disease (SCD) consists of a group of hereditary hemoglobinopathies that result from a single genetic mutation in the β globin chain.1 Different changes in the structure or synthesis of the globin chains determine the disease’s various genotypes and clinical characteristics, among which sickle cell anemia (SCA) stands out, defined as the homozygous state of SCD and considered the most serious among the others.2
Its pathophysiology is considered the result of a cycle with the polymerization of hemoglobin S, altered rheology, and increased vasoclusion-mediated adhesion, with hemolysis-mediated endothelial dysfunction and combined activation of sterile inflammation.3,4
Furthermore, recent studies have demonstrated the association of increased levels of microparticles derived from cell membranes and activated after vascular damage, a common condition in SCA, and reduced levels of nitric oxide, protein S, and protein C, with an increase in vasoclusive crises. The alteration of these markers was related to the activation of prothrombotic elements that favor such events and can trigger a vicious circle of vascular damage, release of microparticles, and new vasoclusions.5
In this way, various clinical manifestations can occur in which vasoclusion and inflammation episodes cause damage to the organs, a more apparent clinical condition with increased age of the patients.6,7
SCA is considered a world public health problem, which must be prioritized in the health systems for adequate prevention, diagnosis, and treatment.8 Approximately 64.4% of the births of individuals with sickle-cell trait and 75.5% of the homozygous cases occur in sub-Saharan Africa.9
In the African (AFRO) and American region (AMRO) of the World Health Organizations regions, the median of children born with SCA is nearly 237,381 cases (IQ 191,067–295,354) and 11,143 cases (IQ 6305–19,823), which corresponds to 3.6% and 77.6% of the total births until the year 2010, respectively. In addition, the projected increase in cases by the year 2050 for both regions is 11,697,397 (IQ 8,461,417–16,020,136) and 417,065 (IQ 195,281–862,232) median 28 times higher for the African region when compared to the region of the Americas.10
In Brazil, the estimation of SCD cases is also considered high11 and can be associated with the large miscegenation. An incident of newborns with SCD surpasses an incident in other countries in North America and Latin America. The sheet in the United States of America is 1:16,305 live births, in Costa Rica 1:14,189 live births, and in Venezuela is 1: 5627 while in Brazil, the incidence found was 1:1000 live births.11 A study in Jamaica found a higher prevalence of disease than in Brazil, with 3.15: 1000 births.12
In the state of Mato Grosso do Sul (MS), Midwest region of the country, the estimated prevalence of SCD is 1: 4837 live births and 1: 4145 tracked by neonatal screening in the period from 2011 to 2015, for SCA the prevalence is 1.0127: 1000 births13 with the predominance of the CAR haplotype (69.1%), whose clinical condition is more severe.14
Given the high number of cases and in order to early identify the positives for early intervention, treatment and monitoring, the examination that detects SCD in Brazil was included in the National Neonatal Screening Program (Programa Nacional de Triagem Neonatal, PNTN), established by GM Ordinance No. 822/01 of the Brazilian Ministry of Health.15
In MS, the diagnosis of the hemoglobinopathies, among them SCD, is made mainly by the Institute of Research, Teaching and Diagnosis of the Association of Parents and Friends of the Different (Instituto de Pesquisa, Ensino e Diagnóstico da Associação de Pais e Amigos dos Excepcionais, IPED/APAE).16
Despite the advances for the organization of a health care network aimed at people with SCD, there is still no visibility to the treatment to generate the necessary support for the patients and their families, intending to reduce mortality and improve quality of life.17
Thus, this research is justified by the possibility of analyzing the health level of people affected by the disease in the state of MS, Brazil, especially the most severe cases. Furthermore, the state of MS was chosen because it is a dry border region with Paraguay and Bolivia, which favors greater miscegenation. In addition, the Brazilian health system often welcomes patients from these foreign regions.
In addition to that, there are few studies published on Mortality due to SCD and SCA in the Brazilian regions. Thus, research studies that can guide strategies for health assistance aimed at the existing reality are required.18
Given the need for robust evidence, this study was conducted with the objective of analyzing the clinical-epidemiological characteristics and mortality in patients with SCA.
Materials and Methods
Participants and Data Collection
An epidemiological study of a single-group cohort, with retrospective data, carried out in the databases of the medical chart file services of two public health institutions, which are a reference for the treatment of SCA in the municipality of Campo Grande, capital of the state of MS, Brazilian Midwest region.
The data were collected from June 2019 to March 2020, with the inclusion of patients of all ages diagnosed with a homozygous form disease SCA with crisis (D57.0) and SCA without crisis (D57.1), according to the International Disease Code (ICD-10).19
Patients resided in the state capital and cities in all four health macroregions. The state regulation system coordinated the forward to reference services. These patients were registered and monitored in both outpatient and hospital admissions between January 1980 to December 2018. For better structuring, this observational study, the guidelines of the STROBE (STrengthening the Reporting OBservational studies in Epidemiology) initiative were followed.20
A total of 25 patients with incomplete records were excluded. Data on care and admissions, as well as some medical records, were not found.
The variables researched were related to mortality and the epidemiological and clinical-laboratory characteristics and those of the treatment used.
Statistical Analysis
The databases were structured using the Microsoft Excel® software, version 2017, and the statistical analysis was performed with the Statistical Package for the Social Sciences - SPSS® software, version 25.0. To calculate the mortality coefficients per 100,000 inhabitants and lethality per 100, the Brazilian Institute of Geography and Statistics (Instituto Brasileiro de Geografia e Estatística, IBGE) was used.21 The standardized mortality rate by gender and age group was calculated by the direct method using the population of the United Nations (UN).22 Finally, the prevalence map of cases per 100,000 inhabitants was plotted in the Qgis® public domain software, version 3.12.
The Lost Potential Years (LPY) were calculated based on what is proposed by Romender and McWhinnie:23 where the deaths were grouped by age group and the age means were calculated for each group to subsequently subtract the age limit of 76.3 years old, which is the life expectancy of the Brazilian population.24 Then, the number of deaths in each group was multiplied by the remaining number of years for the limit age to be achieved, and the sum of these values represents the estimated value of LPY.
For the qualitative variables, a descriptive analysis was performed with distributions of absolute and relative frequencies. In contrast, for the quantitative variables, descriptive statistics of measures of central tendency and dispersion (standard deviation – SD) and median (interquartile range – IQR) were performed. The values of the laboratory tests were compiled throughout the monitoring period and presented by means of central tendency and dispersion measures. Non-normal data distribution was determined using the Kolmogorov–Smirnov test.
In comparing the groups under study with the general and clinical characteristics of the patients, the Chi-Square test was used for the categorical variables. For the quantitative variables referring to the previous and subsequent periods to the drug treatment with hydroxyurea (HU) in patients with SCA, the Student’s t-test was used.
Subsequently, the logistic regression analysis was performed for the final model’s fit. Relative Risk (RR), with a 95% Confidence Interval (CI), was used to estimate the magnitude of the associations. Finally, the explanatory variables with p-value<0.20 in the bivariate analysis were subjected to classic multiple logistic regression analysis.
In the modeling process, the variables were arranged in accordance with the lowest p-value and analyzed using the Backward Wald Stepwise method. The model’s fit was verified employing the Hosmer-Lemeshow test. The adjusted model was adopted as null hypothesis (H0), and the non-adjusted model as the alternative hypothesis (H1). The model’s fit was accepted when p<0.05. For all the statistical tests, a 5% significance level was considered.
Ethical Aspects
This study was conducted in accordance with the Declaration of Helsinki. Therefore, data confidentiality is guaranteed, and a secrecy statement for the use of the database and medical records was sent to the ethics committee for approval.
Was approved by the Committee of Ethics in Research with Human Beings of the Federal University of Mato Grosso do Sul, under Opinion No. 3,226,971, and obtained an informed consent waiver based on the observational nature of the study, its retrospective design, and the non-identification and confidentiality of the data used to be published, collected or analyzed.
Results
A total of 128 patients with confirmed diagnoses of HbSS were studied, of which 69 (53.91%) were female. The age group of up to 30 years old represented 60% of the population under study, with 87 cases. The mean age of the cohort was 23.45 (±15.84) years. For males, the mean age was 20.90 (± 15.56) years, and 25.81 (± 15.81) years for females.
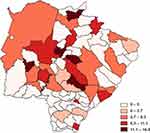
The prevalence of cases for the entire state of MS was 4.61 per 100,000 inhabitants. Among the state health macro-regions, the estimated prevalence per 100,000 inhabitants showed that the Campo Grande region, where the state capital is located, had the highest prevalence with 6.51 (n=99; 77.34%), followed by Corumbá with 5.19 (n=07; 5.47%), Três Lagoas with 3.53 (n=10; 7.8%) and Dourados with 1.07 cases per 100,000 inhabitants (n=09; 7.03%). Figure 1 illustrates the prevalence of cases in the state of Mato Grosso do Sul, Brazil.
Concerning the decades when the patients with SCA were born, it was observed that there was a progressive increase from the 1940s, the highest incidence being verified in the 1970s with 4.13 per 100,000 inhabitants (n=19) and the highest frequency of number of cases in the 2010s (n=35) (Figure 2).
 |
Figure 2 Frequency and incidence (100,000 inhabitants) of the sickle-cell anemia cases stratified in decade of birth, monitored in Mato Grosso do Sul, Brazil (n=128). |
Diagnosis after the fifth day of life occurred in 118 (91.53%) of the 128 patients, and 87 (67.97%) were not diagnosed by the Brazilian National Neonatal Screening Program (PNTN).
In relation to the clinical and hospital data, 115 (89.84%) patients presented up to two associated comorbidities, the most frequent being hepatopathy and aseptic osteonecrosis in 16 (30.19%) cases each and heart disease in 14 (26.42%) subjects. In addition to that, 109 (85.16%) patients presented at least one hospitalization, and 121 (94.53%) had some complication during the follow-up period, among which pain crisis stood out in 99 (81.82%) cases, as well respiratory tract infections such as pneumonia, which occurred in 80 (66.12%) patients, and upper airway infections in 62 (51.24%) cases.
Almost all patients in the cohort (85.93%) were hospitalized at least once. In addition to that, 29 (22.66%) patients needed hospitalization in the Intensive Care Center (ICC). ICC admission numbers and days of hospital stay in the ward or ICC are shown in Figure 3
Almost all clinical cases had levels of laboratory markers outside normal parameters, except for creatinine and mean corpuscular volume (Figure 4). The distribution of values is shown in Figure 5.
A total of 89 (69.50%) patients used hydroxyurea (HU); of these, 66 (74.16%) with regular use, according to the records. The median age for treatment initiation was 15.00 (IQR 18.00) years old, and the median use period was 16.50 (IQR 17.50) years. Hemotherapy was used in 111 (86.72%) patients, with a median of 20.00 (IQR 33.00) transfusions for the entire cohort.
Concerning the follow-up period, the total of patients monitored for less than 20 years was discretely higher than the total of those monitored for more than 20 years, with 68 (53.13%) and 60 (46.87%) cases, respectively. The median survival of the cohort was 18.88 (IQR 25.67) years.
When the patients’ clinical conditions before and after the use of HU were analyzed, a reduction in clinical complications such as pneumonia (p=0.025), bronchopneumonia (p=0.023) and anemia (p=0.048) was observed. In their turn, pain conditions presented increased frequency after treatment initiation (p=0.035), when all the patients who used HU developed pain crises (Table 1).
 |
Table 1 Main Clinical Complications of Patients with Sickle-Cell Anemia, Before and After the Use of Hydroxyurea, Monitored Between 1980 and 2018. Mato Grosso do Sul, Brazil, 2020 (n=128) |
The minimum initial HU dose was 15 mg, and the maximum was 1500 mg, with a median dose used of 500.00 mg (IQR 740.00). The last dose values prescribed were 110 mg and 2000 mg for the minimum and maximum doses, respectively, with a median of 1000.00 (IQR 750.00). There was a reduction in the number of hemotransfusions (p=0.027) and the use of analgesic medications after initiation of the HU therapy, even with a discrete increase in pain events. In relation to hospitalizations, there was a reduction in their frequency (p=0.037) and the median of hospitalization days and an increase in the median interval between hospitalizations after initiation of the HU therapy, although without statistical significance. Parallel to this, there was a reduction in the median frequency of complications after therapy with the drug. Figure 6 shows the clinical and hospital data in relation to the use of HU.
Regarding the use of antimicrobials for the patients with SCA before and after the use of HU, it presented a discrete reduction in frequency (50.56% vs 48.31%, respectively) throughout the follow-up period. The median use frequency values were up to 11.00 (IQR 12.00) and 7.00 (IQR 10.00) antimicrobials before and after initiation of therapy with the drug, respectively. Concerning the classes used, there was a statistically significant difference in the use of penicillins, 1st generation cephalosporins, and aminoglycosides, which presented a reduction in use. At the same time, there was an increase in the use of 3rd generation cephalosporins and glycopeptides (Table 2).
 |
Table 2 Use of Antimicrobials in the Periods Before and After the Use of Hydroxyurea in Patients with Sickle-Cell Anemia, Monitored Between 1980 and 2018. Mato Grosso do Sul, Brazil, 2020 (n=128) |
A total of 19 deaths were reported, with a global mortality rate of 0.78 per 100,000 inhabitants and a lethality rate of 14.84 per 100 inhabitants. When the LPY as a result of SCA was analyzed, the result was 934.2 years with a rate of 33.62 per 100,000 inhabitants.
Most of the deaths were among the women (n=12), with mortality rates of 0.96 and 0.56 per 100,000 inhabitants for the female and male genders, respectively. The standardized mortality rates by gender, based on the UN population, were 11.18 and 6.75 per 100,000 inhabitants, in the same order.
In relation to the age group, the highest frequencies and mortality rates were found among individuals aged from 30 to 35 years old, with 5 cases and 0.04 deaths per 100,000 inhabitants, respectively. The average age at death was 27.05 (± 14.78) years. Regarding the direct standardization based on the UN population, the highest mortality rate was recorded among individuals aged 18 years old or more, with 0.99 per 100,000 inhabitants.
The leading causes of death recorded in official documents were a septic shock in 6 (35.29%) and cardiogenic shock in 4 (23.53%) of the 19 cases. In 12 (66.67%) and 3 (16.67%) cases, the main complaints prior to death were pain and dyspnea, respectively. Table 3 shows the causes of death by the age of the 19 cases.
 |
Table 3 Causes of Death Stratification by Age at the Time of Death. Mato Grosso do Sul, Brazil, 2020 (n=19) |
Monitoring time up to 20 years was related to a 31% reduction in the risk of dying (RR: 0.31; 95% CI=0.12–0.82; p=0.011) compared to the patients with longer follow-ups (Table 4).
 |
Table 4 Number of Deaths According to the Demographic and Clinical Data of Patients with Sickle-Cell Anemia, Monitored Between 1980 and 2018. Mato Grosso do Sul, Brazil, 2020 (n=128) |
The use of analgesic medications was associated with higher mortality (p=0.023), especially in the case of regular analgesics (p=0.012). In addition to that, the use of invasive devices was associated with a higher risk of dying (RR: 2.63; p=0.018) (Table 5). Among the devices used by the patients, the most frequent ones were central venous catheter and invasive mechanical ventilation.
 |
Table 5 Number of Deaths According to Medication Use and Procedures Performed in Patients with Sickle-Cell Anemia, Mato Grosso do Sul, Brazil, 2020 (n=128) |
Among the antimicrobials used, the most recurrent among the death cases were those of the aminoglycoside class (34.38%), with a 4.12 times higher risk of dying when compared to the group that did not use drugs from that class (95% IC=1.82–9.35; p<0.001). Of this class, amikacin was the most used.
In relation to the comorbidities, the most recurrent was heart disease in 28.57% of the death cases. Among the laboratory tests, almost all the cases presented altered values. Creatinine was increased in 35.29% of the death cases and 64.71% of the survivors and was associated with a higher risk of dying (RR: 0.28; p=0.014). Figure 7 shows the distribution of values for laboratory markers between cases that died and those that survived.
In addition to that, after analysis of the multiple logistic regression model, it was possible to observe statistical evidence of the association of the death outcome with the use of invasive devices and changes in the creatinine laboratory variable; where the probability of the patients who used invasive devices dying was 7.2 times greater in relation to those who did not use the devices mentioned above (95% CI=1.24–41.64; p=0.028). For normal creatinine levels, the probability of the patients dying decreases 96% about those with altered mean creatinine values (95% CI=0.01–0.34; p=0.002).
Discussion
This study showed that SCA remains a disease of major clinical importance due to its severity and early mortality. In the 1980–2018 period, a total of 128 patients were monitored in the reference services, with a discrete predominance of the female gender and age group below 30 years old. Furthermore, the patients’ follow-up period was less than 20 years in a little less than half of the cases. Other Brazilian states such as Amazonas25 - North region - and Paraíba26 - Northeast region - also present similar data concerning gender and age group, follow-up period, and age at diagnosis.
The progressive increase in the number of cases diagnosed since the 1940s, with the highest increment in the 2010s, coincides with the implementation of the PNTN, which occurred in 2001 and was consolidated with the screening of more than 85% of the total live births, already in the second half of this decade.13
The diagnosis was considered ideal in a small portion of the cohort; in nearly 90% of the cases, it was made after the fifth day of life, and the total of patients who had no diagnosis made by the PNTN was above 60%. This data points to a worrying reality since treatment for SCA must also be implemented in the first weeks of life to reduce the number of vasoclusive events and the common complications of the disease.27,28
Some comorbidities are common in patients with SCA. In this study, there was a higher frequency of hepatopathy, aseptic osteonecrosis, and heart disease cases. In addition, several research studies point to the systemic effects of vasoclusion and for lesions in many organs, which lead to the development of SCA-related comorbidities.3,6
The hepatic system is prone to the effects of the countless blood transfusions that predispose to iron overload and possible contaminations by other diseases such as viral hepatitis. In addition to that, sickle-cell crises, cholestasis, and hepatic sequestration are important conditions and characterize the main SCD hepatic diseases.29,30
Osteonecrosis is considered one of the comorbidities that most impact the quality of life of the patient with SCA and is mainly related to the repeated sickle-cell crises and tissue ischemia evolving to bone necrosis31 as well as in the cardiovascular system, where sickle-cell crises can also lead to cardiac ischemia, change in cardiac output due to the frequent hemolyses and cardiac remodeling, as well as the consequences of these events in the heart.32,33
Almost all the patients presented at least one hospitalization, with frequency and length of stay in days during the entire monitoring period similar to those observed in another study.34 These figures can be associated both with the number of comorbidities and to the complications developed by the patients during the follow-up period, especially pain crisis.26
Despite the expressive number of hospitalizations observed, this data can be underestimated since dozens of patients lived in the state’s inland and may have sought medical care in their municipalities of residence. This is due to the territorial distance separating them from the specialized centers for treatment and the logistical difficulties the patients face.25,28,35
In addition to the vasoclusive conditions, the number of hemotransfusions and the hemoglobin levels report an important event in the course of the disease: anemia. A common condition observed in patients with SCA, anemia, occurs mainly due to the reduced half-life of the red blood cells. In this way, hemotransfusion therapy becomes important to correct the levels of red blood cells and prevent stroke.36
Parallel to this, serum ferritin, an important finding in polytransfunded patients, also presented increased levels concerning the cohort mean. This biochemical marker is important for evaluating hepatic iron overload, a situation that can evolve to severe hepatic complications.37,38
Hemolysis-related laboratory changes, such as indirect bilirubin levels, reticulocytes, lactic dehydrogenase (LDH), and C-reactive protein (CRP), presented altered values in a large percentage of the patients studied. These markers correlate with various dysfunctions resulting from anemia and tissue hypoxia, which can culminate in cardiovascular damage reflected in diastolic dysfunction and pulmonary hypertension.39
When the periods before and after the use of HU by the patients with SCA were analyzed, there was a reduction in the frequency of hemotransfusions and use of analgesics and the number of hospitalizations. These facts are also reported by several other studies that pointed to the reduction in the number of hemotransfusions and hospitalizations after initiation of HU use.17,40–42
In addition to that, there was a significant reduction in complications such as pneumonia, bronchopneumonia and anemia,43 which can be justified by the evidence that the use of HU is beneficial to the cardiopulmonary systems with improvements in the pulmonary function and mean oxygen saturation tests,44 in addition to preventing damage to the target organs, reducing the number of acute complications and improving survival.45,46
Although in this cohort, the analysis of the pain conditions has presented an increase in frequency after initiation of HU, this data differs from the one found in other studies, which showed a significant reduction in pain crises in adults and children with SCA after the introduction of this medication.17,47,48
As it was not possible to analyze the degree of adherence to treatment by the cohort patients, even with constant regular-use information in the medical files in more than 70% of the patients studied, it is not possible to infer if all of them were actually taking the medication or if they had regular access to it. For this, it becomes necessary to understand that the effective use of HU, mainly in countries with few financial resources, results from aspects such as guidelines, training, adequate infrastructure, and accessibility to drug treatment.49
The lethality rate found was similar to that observed by another Brazilian study,50 and the higher specific mortality observed among women is also not different from already conducted studies;51,52 such as the death age range of 30 to 35 years old,50 where it is possible to evidence that the survival of the patients with SCA is considered lower than that of the Brazilian population.50
Although there is no difference in mortality between men and women in worldwide surveys, higher mortality among women may be associated with complications related to pregnancy and the puerperium since pregnancy can be considered an important risk factor for death among women.53 Furthermore, using oral contraceptive methods by women of childbearing age may increase the risk of thromboembolic events and vasoclusive complications such as ischemic stroke.54 However, pregnancy and contraception data could not be evaluated in this research.
Furthermore, the mortality rate increases significantly after adulthood. For example, a study carried out in Rio de Janeiro, Brazil, found a mortality rate of 10.48% for children under 18 years of age and 16.77% for cases aged 18 years and over.50 Another study55 found a mean age of death of 29.4 (± 19.5) years for SCD as the primary cause of death and 36.0 (± 20.6) as a secondary cause according to official death records, age data similar to that found in this research.
These data report the need for improvement in care, access to health services, and a comprehensive and continuous treatment model to provide better survival, lower mortality, and better quality of life of the population in the various regions of the country.28,56
An important tool in reducing mortality rates is neonatal screening, where early diagnosis and treatment in SCA play a fundamental role. Its benefit in decreasing mortality is old. A study published in 1988 already demonstrated a decrease in the mortality rate among children screened by the neonatal screening program compared to non-screened ones (1.8% vs 8%).57 Recent studies corroborate the assertion, with reduced mortality and improved overall survival with the evolution of neonatal screening programs.58,59
Septic shock was observed as one of the main causes of death, just as several other studies evidenced that infection is known to be the main cause of death in patients with SCA who, in their most serious conditions, can evolve to septic shock and culminate in the aforementioned outcome.50,52,60 In addition to that, heart disease was also observed as the leading cause of death in several other studies.61,62 Among the main complaints referred to in the period prior to death, pain stood out, a common manifestation in SCA due to vasoclusive episodes and damage to organs and tissues.63
The use of analgesic medications was also associated with a higher risk of death, especially in the case of regular analgesics. Although without statistical association, opioid analgesics were used by 16 of the 19 patients who died. This increase in the use frequency of opioids was observed in a study carried out with 3368 patients with SCA, and an association was found between the increase in the use of regular analgesics and complications associated with the disease.64
Likewise, antimicrobials were also associated with a higher risk of death, among which the most frequently found in the death cases were those of the aminoglycoside class. A recent study found an association between higher mortality and antimicrobial resistance,65 a fact that can corroborate this research’s data.
In addition, almost all of the cases presented altered laboratory test values concerning the normality parameters; however, creatinine was associated with higher mortality, as observed in other studies.66,67
The use of invasive devices is associated with a higher risk of death, just as the probability of the patients who made use of invasive devices dying was greater in relation to those who did not use them, as observed in a study conducted by Allareddy et al, where the patients who made use of mechanical ventilation had a greater chance of dying when compared to those who did not use it.68
Although this study is one of the first to study mortality from SCA in the state of MS, Brazil, it has limitations arising from secondary records that prevent the collection of many data, such as HbF levels not available sequentially in medical records. Furthermore, it is not possible to say whether the cases were followed up and used appropriate medications or not. The absence of some information prevented important analyzes such as the effect of HbF level and regularity of follow-up on cohort mortality.
Despite these limitations, this study constitutes an essential source of epidemiological and mortality data from SCA in Brazil, where the real condition of the disease is still unknown.
Conclusion
The SCA cases analyzed in this study present a discrete increase in the female patients, whose predominant age range was between 30 and 39 years old, with a life expectancy below the general population, predominant age group of the patients who died between 30 and 35 years old and specific mortality more accentuated in women.
SCA diagnosis had a progressive increase over the years, mainly since 2010. However, diagnosis after the fifth day of life was observed in nearly 90% of the cases, when more than 60% of the diagnoses were not made by the PNTN.
There was the predominance of comorbidities such as hepatopathies, aseptic osteonecrosis, and heart disease. The main clinical changes were related to the vasoclusive conditions; in addition to that, the laboratory tests evidenced that a large percentage of the patients had low hemoglobin levels, increased serum ferritin, and biochemical conditions compatible with hemolysis.
After initiation of HU, there was a reduction in the number of hemotransfusions and hospital admissions, in addition to the decrease in complications of the respiratory tract and anemia. However, there has been an increase in pain conditions after initiation of HU, which is dependent on the use and adherence to the medication, which was not possible to assess in this study.
The death cases are mainly due to septic shock and the risks are increased for patients with more than 20 years of follow-up, who, when hospitalized, make greater use of analgesics, antimicrobials and with laboratory test results outside the normal range, with particular attention to creatinine; in addition to the use of invasive devices. These points denote the need for greater attention targeting the treatment provided to the patients, mainly concerning access to the services, medications, tests, and therapies required for increased survival and reduced number of clinical problems.
In addition, the data found on the risk factors and causes of death among the patients with SCA point to the need for prospective longitudinal studies that can ratify these findings and investigate in detail each of the risk factors pointed out by this research.
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001, and by Federal University of Mato Grosso do Sul.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Piel FB, Steinberg MH, Rees DC. Sickle cell disease. N Engl J Med. 2017;376(16):1561–1573. doi:10.1056/NEJMra1510865
2. Aleluia MM, Fonseca TCC, Souza RQ, et al. Comparative study of sickle cell anemia and hemoglobin SC disease: clinical characterization, laboratory biomarkers and genetic profiles. BMC Hematol. 2017;17(1):15. doi:10.1186/s12878-017-0087-7
3. Sundd P, Gladwin MT, Novelli EM. Pathophysiology of sickle cell disease. Annu Rev Pathol. 2019;14:263–292. doi:10.1146/annurev-pathmechdis-012418-012838
4. Sedrak A, Kondamudi NP. Sickle Cell Disease. StatPearls; 2019.
5. Piccin A, Murphy C, Eakins E, et al. Circulating microparticles, protein C, free protein S and endothelial vascular markers in children with sickle cell anaemia. J Extracell Vesicles. 2015;4(1):28414. doi:10.3402/jev.v4.28414
6. Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet. 2010;376(9757):2018–2031. doi:10.1016/S0140-6736(10)61029-X
7. Kato GJ, Hebbel RP, Steinberg MH, Gladwin MT. Vasculopathy in sickle cell disease: biology, pathophysiology, genetics, translational medicine, and new research directions. Am J Hematol. 2009;84(9):618–625. doi:10.1002/ajh.21475
8. World Health Organization. Sickle-cell anaemia: report by the secretariat. World Health Organization; 2006. Available from: https://apps.who.int/iris/handle/10665/20890.
9. Piel FB, Patil AP, Howes RE, et al. Global epidemiology of sickle haemoglobin in neonates: a contemporary geostatistical model-based map and population estimates. Lancet. 2013;381(9861):142–151. doi:10.1016/S0140-6736(12)61229-X
10. Piel FB, Hay SI, Gupta S, Weatherall DJ, Williams TN. Global burden of sickle cell anaemia in children under five, 2010–2050: modelling based on demographics, excess mortality, and interventions. PLoS Med. 2013;10(7):e1001484. doi:10.1371/journal.pmed.1001484
11. Huttle A, Maestre GE, Lantigua R, Green NS. Sickle cell in sickle cell disease in Latin America and the United States. Pediatr Blood Cancer. 2015;62(7):1131–1136. doi:10.1002/pbc.25450
12. Serjeant GR, Chin N, Asnani MR, et al. Causes of death and early life determinants of survival in homozygous sickle cell disease: the Jamaican cohort study from birth. PLoS One. 2018;13(3):e0192710. doi:10.1371/journal.pone.0192710
13. Kikuchi BA, Ivo ML, Barbieri AR, Camargo Filho R, Amargo Filho R, Nascimento V. Evaluation of the implantation of the national neonatal screening program regarding coverage index, disease prevalence and sickle cell trait in Mato Grosso do Sul-Brazil: 2001–2015. IJDR. 2018;8(3):19279–19283.
14. Salles R, Ivo M, Sakamoto T, Cavalcanti M, Brum M, Pontes E. Identification of BS gene haplotypes in individuals with falciform anemia in Mato Grosso do Sul. Int J Dev Res. 2018;8(3):15955–15958.
15. Ramalho AS, Magna LA, Paiva-e-Silva R. Government Directive MS # 822/01: unique aspects of hemoglobinopathies for public health in Brazil. Cad Saúde Pública. 2003;19:1195–1199. doi:10.1590/S0102-311X2003000400040
16. Institute of Research, Teaching and Diagnosis of the Association of Parents and Friends of the Different. Neonatal Screening [homepage on the Internet]; 2020 [cited September 6, 2020]Available from:http://www.ipedapae.org.br/triagem-neonatal. Accessed January 6, 2022
17. Silva-Pinto AC, Angulo IL, Brunetta DM, et al. Clinical and hematological effects of hydroxyurea therapy in sickle cell patients: a single-center experience in Brazil. São Paulo Med J. 2013;131(4):238–243. doi:10.1590/1516-3180.2013.1314467
18. Arduini GA, Rodrigues LP, Marqui ABT. Mortality by sickle cell disease in Brazil. Rev Bras Hematol Hemoter. 2017;39(1):52–56. doi:10.1016/j.bjhh.2016.09.008
19. World Health Organization. International Statistical Classification of Diseases and Related Health Problems 10th Revision; 2010 [
20. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–349. doi:10.1016/j.jclinepi.2007.11.008
21. Brazilian Institute of Geography and Statistics. Resident population of Federation Units and Major Regions sent to the Court of Auditors of Unity – 2001–2020; 2020 [
22. Nations United. Total population (both sexes combined) by broad age group, region, subregion and country, 1950–2100 (thousands); 2020. Available from: https://population.un.org/wpp/Download/Standard/Population/.
23. Romeder J, McWhinnie J. Potential years of life lost between ages 1 and 70: an indicator of premature mortality for health planning. Int J Epidemiol. 1977;6(2):143. doi:10.1093/ije/6.2.143
24. Brazilian Institute of Geography and Statistics. Complete mortality table for Brazil – 2018; 2019 [
25. Cesar P, Dhyani A, Schwade LA, et al. Epidemiological, clinical, and severity characterization of sickle cell disease in a population from the Brazilian Amazon. Hematol Oncol Stem Cell Ther. 2019;12(4):204–210. doi:10.1016/j.hemonc.2019.04.002
26. Marques T, Vidal SA, Braz AF, Teixeira M. Clinical and care profiles of children and adolescents with sickle cell disease in the Brazilian Northeast region. Rev Bras Saude Mater Infant. 2019;19(4):881–888. doi:10.1590/1806-93042019000400008
27. de Montalembert M, Tshilolo L, Allali S. Sickle cell disease: a comprehensive program of care from birth. Hematology. 2019;2019(1):490–495. doi:10.1182/hematology.2019000053
28. Sarat CNF, Ferraz MB, Júnior MAF, Souza A, Cardoso A, Ivo ML. Prevalence of sickle cell disease in adults with delayed diagnosis. Acta Paul Enferm. 2019;32(2):202–209. doi:10.1590/1982-0194201900028
29. Theocharidou E, Suddle AR. The liver in sickle cell disease. Clin Liver Dis. 2019;23(2):177–189. doi:10.1016/j.cld.2018.12.002
30. Pecker LH, Patel N, Creary S, et al. Diverse manifestations of acute sickle cell hepatopathy in pediatric patients with sickle cell disease: a case series. Pediatr Blood Cancer. 2018;65(8):e27060. doi:10.1002/pbc.27060
31. Naseer ZA, Bachabi M, Jones LC, Sterling RS, Khanuja HS. Osteonecrosis in sickle cell disease. South Med J. 2016;109(9):525–530. doi:10.14423/SMJ.0000000000000516
32. Gladwin MT. Cardiovascular complications and risk of death in sickle-cell disease. Lancet. 2016;387(10037):2565–2574. doi:10.1016/S0140-6736(16)00647-4
33. Hammoudi N, Lionnet F, Redheuil A, Montalescot G. Cardiovascular manifestations of sickle cell disease. Eur Heart J. 2020;41(13):1365–1373. doi:10.1093/eurheartj/ehz217
34. Paulukonis ST, Feuchtbaum LB, Coates TD, et al. Emergency department utilization by Californians with sickle cell disease, 2005–2014. Pediatr Blood Cancer. 2017;64(6):e26390. doi:10.1002/pbc.26390
35. Lee L, Smith-Whitley K, Banks S, Puckrein G. Reducing health care disparities in sickle cell disease: a review. Public Health Rep. 2019;134(6):599–607. doi:10.1177/0033354919881438
36. Abboud MR. Standard management of sickle cell disease complications. Hematol Oncol Stem Cell Ther. 2020;13:85–90. doi:10.1016/j.hemonc.2019.12.007
37. Ajebo G, Mangaonkar AA, Ahmad I, et al. Correlation of serum ferritin levels with liver iron concentration by MRI Measurement in sickle cell patients with transfusional iron overload. Blood. 2018;132(Supplement1):4926. doi:10.1182/blood-2018-99-119597
38. Shrestha A, Grover P, Ahmed Z, et al. Clinical impact of iron overload on morbidity in patients with sickle cell disease. Blood. 2017;130(Supplement1):4787.
39. Zhang X, Shah BN, Machado R, Saraf SL, Gordeuk VR. Biomarkers of cardiopulmonary, renal, and liver dysfunction in an adult sickle cell disease cohort. Blood. 2019;134:3574. doi:10.1182/blood-2019-131813
40. Inusa BP, Hsu LL, Kohli N, et al. Sickle cell disease—genetics, pathophysiology, clinical presentation and treatment. Tela Neonatal Int J. 2019;5(2):20. doi:10.3390/ijns5020020
41. de Araújo OMR, Ivo ML, De souza AS, et al. Acute events in hospitalized patients with sickle cell anemia before and after the use of hydroxyurea. Int Arch Med. 2016:9. Available from: http://imed.pub/ojs/index.php/iam/article/view/2066.
42. Wang WC, Ware RE, Miller ST, et al. A multicenter randomised controlled trial of hydroxyurea (hydroxycarbamide) in very young children with sickle cell anaemia. Lancet. 2011;377(9778):1663. doi:10.1016/S0140-6736(11)60355-3
43. Maharaj K, Bodkyn C, Greene C, Bahadursingh S. The effect of hydroxyurea therapy on adverse clinical events and haematological indices in paediatric patients with sickle cell anaemia. West Indian Med J. 2019;68(2). Available from: https://www.mona.uwi.edu/fms/wimj/system/files/article_pdfs/wimj-iss2-2019_80_85_2.pdf.
44. Narang I, Kadmon G, Lai D, et al. Higher nocturnal and awake oxygen saturations in children with sickle cell disease receiving hydroxyurea therapy. Ann Am Thorac Soc. 2015;12(7):1044–1049. doi:10.1513/AnnalsATS.201410-473OC
45. Qureshi A, Kaya B, Pancham S, et al. Guidelines for the use of hydroxycarbamide in children and adults with sickle cell disease. Br J Haematol. 2018;181(4):460–475. doi:10.1111/bjh.15235
46. Strouse JJ, Heeney MM. Hydroxyurea for the treatment of sickle cell disease: efficacy, barriers, toxicity, and management in children. Pediatr Blood Cancer. 2012;59(2):365–371. doi:10.1002/pbc.24178
47. Steinberg MH, Barton F, Castro O, et al. Effect of hydroxyurea on mortality and morbidity in adult sickle cell anemia: risks and benefits up to 9 years of treatment. JAMA. 2003;289(13):1645–1651. doi:10.1001/jama.289.13.1645
48. Mvalo T, Topazian HM, Kamthunzi P, et al. Real‐world experience using hydroxyurea in children with sickle cell disease in Lilongwe, Malawi. Pediatr Blood Cancer. 2019;66(11):e27954. doi:10.1002/pbc.27954
49. Power-Hays A, Ware RE. Effective use of hydroxyurea for sickle cell anemia in low-resource countries. Curr Opin Hematol. 2020;27(3):172–180. doi:10.1097/MOH.0000000000000582
50. Lobo C, Nascimento E, Jesus L, Freitas T, Lugon JR, Ballas SK. Mortality in children, adolescents and adults with sickle cell anemia in Rio de Janeiro, Brazil. Rev Bras Hematol Hemoter. 2018;40(1):37–42. doi:10.1016/j.bjhh.2017.09.006
51. Araujo OMR, Ivo ML, Ferreira júnior MA, Pontes ERJC, Bispo IMGP, Oliveira E. Survival and mortality among users and non-users of hydroxyurea with sickle cell disease. Rev Lat Am Enfermagem. 2015;23:67–73. doi:10.1590/0104-1169.3385.2526
52. Ramos JT, de Amorim FS, Pedroso FKF, Nunes ACC, Rios MA Mortalidade por doença falciforme em estado do nordeste brasileiro. R Enferm Cent O Min; 2015. Available from: http://www.seer.ufsj.edu.br/index.php/recom/article/view/859. Accessed January 6, 2022.
53. Smith-Whitley K. Complications in pregnant women with sickle cell disease. Hematology Am Soc Hematol Educ Program. 2019;2019(1):359–366. doi:10.1182/hematology.2019000039
54. Qureshi AI, Malik AA, Adil MM, Suri MFK. Oral contraceptive use and incident stroke in women with sickle cell disease. Thromb Res. 2015;136(2):315–318. doi:10.1016/j.thromres.2015.04.013
55. Santo AH. Sickle cell disease related mortality in Brazil, 2000–2018. Hematol Transfus Cell Ther. 2020. doi:10.1016/j.htct.2020.09.154
56. Kanter J, Smith WR, Desai PC, et al. Building access to care in adult sickle cell disease: defining models of care, essential components, and economic aspects. Blood Adv. 2020;4(16):3804–3813. doi:10.1182/bloodadvances.2020001743
57. Vichinsky E, Hurst D, Earles A, Kleman K, Lubin B. Newborn screening for sickle cell disease: effect on mortality. Pediatrics. 1988;81(6):749–755. doi:10.1542/peds.81.6.749
58. Pompeo CM, Ferreira Júnior MA, Cardoso A, Souza M, Mota FM, Ivo ML. Survival of sickle cell disease patients diagnosed during newborn screening: systematic review. Res Soc Dev. 2021;10(11):e95101119329. doi:10.33448/rsd-v10i11.19329
59. Runkel B, Klüppelholz B, Rummer A, et al. Screening for sickle cell disease in newborns: a systematic review. Syst Rev. 2020;9(1):1–9. doi:10.1186/s13643-020-01504-5
60. Desselas E, Thuret I, Kaguelidou F, et al. Mortality in children with sickle cell disease in mainland France from 2000 to 2015. haematologica. 2020;105(9):e440–3. doi:10.3324/haematol.2019.237602
61. Shah P, Suriany S, Kato R, et al. Tricuspid regurgitant jet velocity and myocardial tissue Doppler parameters predict mortality in a cohort of patients with sickle cell disease spanning from pediatric to adult age groups - revisiting this controversial concept after 16 years of additional evidence. Am J Hematol. 2021;96:31–39.
62. Chaturvedi S, Labib Ghafuri D, Kassim A, Rodeghier M, DeBaun MR. Elevated tricuspid regurgitant jet velocity, reduced forced expiratory volume in 1 second, and mortality in adults with sickle cell disease. Am J Hematol. 2017;92(2):125–130. doi:10.1002/ajh.24598
63. Ogu UO, Billett HH. Comorbidities in sickle cell disease: adult providers needed. Indian J Med Res. 2018;147(6):527. doi:10.4103/ijmr.IJMR_1019_18
64. Kang HA, Barner JC, Richards KM, Bhor M, Paulose J, Kutlar A. Association between vaso-occlusive crises and opioid prescriptions among patients with sickle cell disease: a retrospective claims-based study. J Health Econ Outcomes Res. 2020;7(1):94. doi:10.36469/jheor.2020.13348
65. Srisuwananukorn A, Han J, Raslan R, et al. Antimicrobial resistance is a risk factor for mortality in adults with sickle cell disease. haematologica. 2020;106:1745–1748. doi:10.3324/haematol.2020.267872
66. Curtis SA, Danda N, Etzion Z, Cohen HW, Billett HH. Longitudinal analysis of patient specific predictors for mortality in sickle cell disease. PLoS One. 2016;11(10):e0164743. doi:10.1371/journal.pone.0164743
67. Maitra P, Caughey M, Robinson L, et al. Risk factors for mortality in adult patients with sickle cell disease: a meta-analysis of studies in North America and Europe. haematologica. 2017;102(4):626–636. doi:10.3324/haematol.2016.153791
68. Allareddy V, Roy A, Lee MK, et al. Outcomes of acute chest syndrome in adult patients with sickle cell disease: predictors of mortality. PLoS One. 2014;9(4):e94387. doi:10.1371/journal.pone.0094387
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.