Back to Journals » Neuropsychiatric Disease and Treatment » Volume 16
Clinical, Electrophysiological and Radiological Features of Nitrous Oxide-Induced Neurological Disorders
Authors Bao L , Li Q, Li Q, Chen H, Zhang R, Shi H, Cui G
Received 1 November 2019
Accepted for publication 13 March 2020
Published 15 April 2020 Volume 2020:16 Pages 977—984
DOI https://doi.org/10.2147/NDT.S236939
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jun Chen
Lei Bao,* Qing Li,* Qingjie Li, Hao Chen, Ruixue Zhang, Hongjuan Shi, Guiyun Cui
Department of Neurology, The Affiliated Hospital of Xuzhou Medical University, Xuzhou, Jiangsu 221004, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Lei Bao
Department of Neurology, The Affiliated Hospital of Xuzhou Medical University, Xuzhou, Jiangsu 221004, People’s Republic of China
Tel/Fax +86 516 85802129
Email [email protected]
Purpose: We summarized the clinical manifestations, laboratory and electrodiagnostic characteristics and magnetic resonance imaging (MRI) findings of nitrous oxide (N2O) abuse-induced neurological disorders.
Patients and Methods: We retrospectively reviewed 33 patients with N2O abuse-induced neurological disorders and reported their demographic data, clinical manifestations, laboratory examinations, nerve conduction studies, together with spinal and brain MRI.
Results: The most frequent clinical manifestations included numbness and weakness in the extremities and unspecified gait disturbance. Low serum vitamin B12 levels were found in 9 patients, and high homocysteine levels were noted in 27 patients. Nerve conduction studies showed a sensory-motor neuropathy. Sixteen patients showed bilateral high-intensity T2 signal within the posterior column on spinal MRI, and four patients showed cerebral white matter lesions on brain MRI.
Conclusion: N2O abuse has become a significant public health problem because of the severe neurological disorders related to chronic abuse. Clinical physicians should be aware of the toxic effects of N2O.
Keywords: nitrous oxide, neurological disorders, subacute combined degeneration, neuropathy, vitamin B12
Introduction
Nitrous oxide (N2O), commonly known as laughing gas, is one of the most widely used anesthetic, to relief pain and anxiety in both dental and medical applications.1,2 However, N2O is also recreationally used by young people for its euphoric and hallucinogenic effects.3,4 With the benefits of its low cost, legal status and quick effect, N2O abuse has become a common problem in many parts of the world. In fact, N2O has been reported to be the fourth most prevalent inhalant among adolescents in the USA and the second most popular recreational drug in the UK.5 In recent years, the recreational use of nitrous oxide gas is becoming increasingly popular in China. Despite its widespread abuse, the risk of adverse effects of N2O exposure is not sufficiently recognized yet.
Previous studies suggest that chronic abuse of N2O exponentially increases the risk of permanent neurological deficits and that stopping exposure to the toxin is the most critical first step for treatment.6 Multiple case studies have reported that the neurological and psychiatrical disorders, such as subacute combined degeneration, myelopathy, demyelinating polyneuropathy and emotional disorders, are related to N2O abuse.7–10 There have even been deaths related to N2O inhalation.11
Considering about the lack of awareness of N2O-abusing toxicity, we conducted a clinical study, which included a comprehensive and updated review of N2O abuse-induced neurological disorders. In this study, we also summarized the clinical manifestations, laboratory and electrodiagnostic characteristics, and magnetic resonance findings of 33 patients having N2O abuse-induced neurological disorders.
Patients and Methods
Ethical Statements
The Clinical Research Ethics Committee of the Affiliated Hospital of Xuzhou Medical University approved this study protocol. The protocols were in accordance with the Declaration of Helsinki. Written informed consents were acquired from all participants or their legal guardians.
Participants
Thirty-three patients diagnosed with N2O-induced neurological disorders at our institution from 2015 to 2019 were recruited in our study after ethical approval from the Clinical Research Ethics Committee of the Affiliated Hospital of Xuzhou Medical University. All patients had developed neurological symptoms such as acute ascending paresthesia, symmetrical distal limb weakness, cognitive decline or vision decline after exposure to N2O. All patients had undergone laboratory panel for a full range of laboratory tests, including complete blood count test, vitamin B12, folate, homocysteine, glycohemoglobin, thyroid function, syphilis, HIV, viral antibodies, rheumatic indicators, and paraneoplastic antibodies. The diagnosis of N2O related neurological disorders was comprehensively considered based on clinical examination, laboratory data, and radiological findings. For comparison, we selected 40 age- and sex-matched healthy volunteers were recruited for controls.
Clinical Manifestations and Electrophysiological Findings
The charts of these patients were reviewed, and information regarding age, gender, height and weight and neurological manifestations (such as muscle weakness, loss of sensory, or cognitive decline) were recorded. Nerve conduction studies of all participants were performed on median, ulnar, peroneal, tibial, and sural nerves depending on the clinical manifestation. The data of compound muscle action potential (CMAP) amplitude, distal latency, sensory nerve action potential (SNAP) amplitude and conduction velocity were extracted.
Magnetic Resonance Imaging (MRI) Features
All patients underwent MR scanning of the cervical, thoracic, lumbar spine and brain. T1-weighted MRI sequences with and without gadolinium were obtained. T2-weighted MRI sequences were used to obtain sagittal and axial images. The involved spinal cord segments (the number of vertebral segments), and their locations in the sagittal image (cervical, thoracic, or lumbar) were collected.
Statistical Analysis
Data on the clinical presentation, laboratory tests, and outcomes were presented as mean ± standard error. Single factors were analyzed using Student’s two-tailed t-test. All analyses were performed using GraphPad Prism software (La Jolla, CA), statistical significance was set at p < 0.05.
Results
Patient Characteristics
Among all the recruited 33 patients included in the systematic review, N2O abuse-related neurological disorders occurred most frequently among youth (mean age: 22.4 ± 2.7 years). The median N2O exposure period was 18 months and the mean time from the last N2O exposure to the onset of symptoms was 14.2 ± 2.2 days. The average BMI was 28.27 ± 1.95 kg/m2, which was above the normal cutoff values set by WHO guideline (25 kg/m2). The demographic data of the study population are summarized in Table 1.
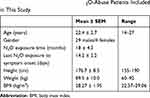 |
Table 1 Demographic Data of 33 N2O-Abuse Patients Included in This Study |
Clinical Manifestations
Neurological symptoms were summarized as below: numbness in the extremities (30 cases, 90.9%), weakness (27 cases, 81.8%), unspecified gait disturbance (15 cases, 45.5%), cognitive decline (8 cases, 24.2%) and vertigo and vomiting (4 cases, 12.1%). Three patients complained about tremor of both hands, 2 patients had bladder dysfunction and 1 patient initially presented with the only symptom of vision decline.
Reported signs included symmetric paresthesia (90.9%), quadriparesis (81.8%), ataxia (60.6%), Lhermitte’s sign (18.2%) and Babinski’s sign (9.1%). Sensory deficit in a stocking glove distribution pattern and sharp spinal level were both presented in this study. Besides, vibration and position sense impairment was also found on a considerable number of patients (72.7%). Tendon reflex abnormalities were ubiquitous and ranged from generalized hyperreflexia with clonus to absent reflexes. The findings are specified in Table 2.
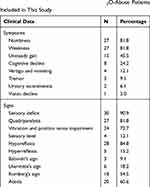 |
Table 2 Clinical Manifestations of the 33 N2O-Abuse Patients Included in This Study |
Laboratory Data
Nine patients presented low serum vitamin B12 levels, while high homocysteine levels were found in 27 patients. Other patients’ vitamin B12 and homocysteine levels were within the normal range. The serum ferritin and folate levels were in the normal range in all 33 patients. In our study, there are only 2 patients showing mild decreased red blood cells (RBCs) count and hemoglobin levels with increased mean corpuscular volume (MCV) at the time of admission. The laboratory data are summarized in Table 3.
 |
Table 3 Laboratory Data of the 33 N2O-Abuse Patients Included in This Study |
Electrophysiological Characteristics
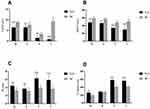
As the majority of patients have typical symptoms and signs of peripheral neuropathy, all patients in the present study underwent nerve conduction studies. Figure 1 contains the motor nerve conduction studies results. The mean compound muscle action potentials (CMAPs) amplitudes of N2O-abuse patients were significantly reduced in the peroneal nerve and tibial nerve. A significant prolonged distal latency and F-wave latency, and conduction velocity slowing was also observed. In contrast, CMAPs in the median and ulnar nerves were only moderately decreased among patients. Figure 2 shows the sensory nerve conduction studies results. Severe reduction of sensory nerve action potentials and conduction velocity of N2O-abuse patients in the sural nerves were observed. Sensory nerve action potentials in the median and ulnar nerves were relatively preserved. Overall, our results indicated a sensory-motor neuropathy with the involvement occurred mainly in the lower limbs.
MRI Findings
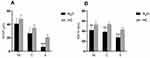
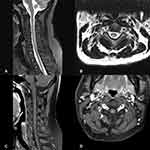
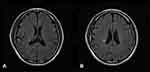
16 N2O-abused patients (48%) had increased T2-weighted signal intensities in the posterior column on axial and sagittal series (Figure 3). There existed large variations in lesion size; however, they most commonly extended for 3–4 spinal segments. The lesion distribution of vertebral level is shown in Figure 4. The cervical spinal cord was more frequently impaired than other areas of spinal cord. The most commonly impaired vertebral levels were C3, C4 and C5 (50.6% each), followed by C2 (41.6%) and C6 (37.2%). In spite of lesions to the spinal cord, cerebral white matter lesions were also founded in bilateral frontal, periventricular, and centrum semiovale regions on brain MRI of our 4 patients (Figure 5).
Discussion
N2O has been regarded as an effective and safe anesthetic for more than a century and applied on two billion patients.12,13 However, N2O is also used as an inhalant drug among recreational users for euphoric and hysterical effects and can cause vitamin B12 deficiency, and severe neural and psychiatric symptoms. The adverse effects of N2O exposure are insufficiently recognized despite its widespread use and the increasing cases reported on N2O-induced neurological disorders. Therefore, we performed a comprehensive retrospective review at our institution in patients with neurological disorders related to N2O abuse.
Neurological disorders caused by N2O use have long been noticed and studied. N2O abuse could cause subacute combined degeneration, myelopathy, demyelinating polyneuropathy, emotional disorders and even death.6,9-11 In our present study, the most common clinical manifestation was the classic presentation of peripheral neuropathy with superficial sensory deficit and distal limb paresis. The symptoms and signs of ataxia, vibration and position sense impairment and unsteady gait were also founded on a considerable number of patients, suggesting the posterior column was frequently affected. The presence of lower limb weakness, sharp spinal level, hyperreflexia and positive Babinski sign in a few of our patients indicated N2O-induced myelopathy. As for the symptom of urinary incontinence in two of our patients could either be due to autonomic neuropathy or due to myelopathy. One patient in our study initially presented with the only symptom of vision decline, which has not been described in former reports, suggesting optic nerve can also be damaged by N2O abuse.
There is a growing number of clinical supports for using N2O as a treatment adjuvant in refractory depression. In a double-blind proof-of-concept trial, 20 patients with treatment-resistant depression were randomized to 50% N2O/50% O2 or placebo (50% O2) for 1 h in a cross-over design with sessions separated by 1 week. Symptoms of depression improved significantly at 2 and 24 h after receiving N2O compared with placebo. Three patients (15%) had a full remission after N2O compared with none after placebo. N2O is thought to exert its antidepressant action via inhibition of N-methyl-D-aspartate (NMDA)-type glutamate receptors.1,14 The current evidence for the use of N2O as a treatment in mood disorders is small, there is substantial interest in further exploring the potential utility of this novel approach in major psychiatric disorders. However, still, we should not ignore its psychiatric complications with chronic use. It has been reported that nitrous oxide abuse can produce psychiatric symptoms, showing as personality changes, emotional disorders (eg, anxiety, depression, mania), impulsive and aggressive behaviors, hallucination and delusions.15
The main mechanism of neurological disorders caused by N2O abuse is its interference with the function of vitamin B12. Vitamin B12 is crucial for the synthesis and maintenance of myelin. N2O irreversibly oxidizes cobalt in vitamin B12, resulting in demyelination and other pathological changes in the nervous system.16,17 However, in our present study, only one-third of the patients were associated with low serum vitamin B12, while a total of 24 patients had normal or even elevated levels of serum vitamin B12. The proportion of patients with low vitamin B12 levels in our study was significantly less than previous reports. It has been reported that a normal or increased serum vitamin B12 level does not accurately reflect the underlying tissue availability of vitamin B12, and should not exclude vitamin B12 deficiency. The phenomenon that patients with vitamin B12 deficiency have normal or elevated serum B12 concentrations is termed “functional vitamin B12 deficiency”. In this case, serum methylmalonic acid and homocysteine levels are more accurate to reflect vitamin B12 functional statuses.18,19 In our study, 27 of the 33 patients presented with high homocysteine levels, which was consistent with previous reports. Therefore, our results suggested that the level of homocysteine is a better biomarker for diagnosis of N2O-induced neurological disorders and a normal serum vitamin B12 level cannot be used to exclude genuine vitamin B12 deficiency and damage in this condition.
Previous study has described detailed electrodiagnostic features of N2O-induced neuropathy. N2O abuse could induce sensory-motor neuropathy with axonal and myelin damage (decreased CMAP and SNAP, slowing of MCV and SCV as well as prolongation of DL), and with predominant lower-limb involvement.20 These findings were corroborated in our present study. Besides that, we also found electrophysiological abnormalities of 3 patients who came to our hospital for memory loss or vision decline without symptoms of peripheral neuropathy.
Histopathological studies have revealed that N2O intoxication can result in brain atrophy, demyelination in the spinal cord and subcortical white matter in the brain. High-intensity signals in the posterior columns of cervical or thoracic spinal cord on T2-weighted MR scans in N2O-abuse patients have already been described before. However, few studies have reported changes on their brain MRI. Our present study further refined the neuroimaging characteristics of N2O-abuse patients. First, we found that the cervical spinal cord was more frequently impaired than other areas of the spinal cord, with the most common affected vertebral levels being C3, C4 and C5. The length of the spinal cord lesions was usually longer than three spinal segments. The typical MRI change in the spinal cord can be summarized as longitudinally extensive myelopathy (LEM), with predominant posterior columns of cervical cord involvement. Secondly, 4 of 33 patients showed white matter lesions in frontal, periventricular, or centrum semiovale regions on brain MRI, suggesting demyelination of cerebral white matter, which is the first report and a novel finding of N2O abuse causing cerebral white matter injuries.
Conclusion
N2O abuse could induce severe neurological disorders, especially among young abusers. Nerve conduction studies showed a symmetrical sensory-motor neuropathy, with prominent lower limb involvement. The typical spinal MRI change of N2O abuse is longitudinally extensive myelopathy (LEM), with predominant posterior columns of cervical cord involvement. Cerebral white matter lesions could also be found on brain MRI.
Funding
This study was supported by National Natural Science Foundation of China (NSFC) NO. 81771282.
Disclosure
The authors declare no competing financial interests.
References
1. Nagele P, Duma A, Kopec M, et al. Nitrous oxide for treatment-resistant major depression: a proof-of-concept trIal. Biol Psychiatry. 2015;78(1):10–18. doi:10.1016/j.biopsych.2014.11.016
2. Sanders RD, Weimann J, Maze M. Biologic effects of nitrous oxide: a mechanistic and toxicologic review. Anesthesiology. 2008;109(4):707–722. doi:10.1097/ALN.0b013e3181870a17
3. van Amsterdam J, Nabben T, van den Brink W. Recreational nitrous oxide use: prevalence and risks. Regul Toxicol Pharmacol. 2015;73(3):790–796. doi:10.1016/j.yrtph.2015.10.017
4. Lan SY, Kuo CY, Chou CC, et al. Recreational nitrous oxide abuse related subacute combined degeneration of the spinal cord in adolescents - A case series and literature review. Brain Dev. 2019;41(5):428–435. doi:10.1016/j.braindev.2018.12.003
5. Oussalah A, Julien M, Levy J, et al. Global burden related to nitrous oxide exposure in medical and recreational settings: a systematic review and individual patient data meta-analysis. J Clin Med. 2019;8:4. doi:10.3390/jcm8040551
6. Cheng HM, Park JH, Hernstadt D. Subacute combined degeneration of the spinal cord following recreational nitrous oxide use. BMJ Case Rep. 2013;2013. doi:10.1136/bcr-2012-008509
7. Choi C, Kim T, Park KD, Lim OK, Lee JK. Subacute combined degeneration caused by nitrous oxide intoxication: a report of two cases. Ann Rehabil Med. 2019;43(4):530–534. doi:10.5535/arm.2019.43.4.530
8. Neveu J, Perelman S, Suisse G, Monpoux F. Severe hyperhomocysteinemia and peripheral neuropathy as side effects of nitrous oxide in two patients with sickle cell disease. Arch Pediatr. 2019;26(7):419–421. doi:10.1016/j.arcped.2019.09.006
9. Chen T, Zhong N, Jiang H, Zhao M, Chen Z, Sun H. Neuropsychiatric symptoms induced by large doses of nitrous oxide inhalation: a case report. Shanghai Arch Psychiatry. 2018;30(1):56–59. doi:10.11919/j.issn.1002-0829.217084
10. Edigin E, Ajiboye O, Nathani A. Nitrous oxide-induced B12 deficiency presenting with myeloneuropathy. Cureus. 2019;11(8):e5331.
11. Backstrom B, Johansson B, Eriksson A. Death from nitrous oxide. J Forensic Sci. 2015;60(6):1662–1665. doi:10.1111/1556-4029.12879
12. Sund Kristensen H, Berthelsen PG. Risus sardonicus and laughing gas–when nitrous oxide lost its innocence. Acta Anaesthesiol Scand. 1994;38(8):751–752. doi:10.1111/j.1399-6576.1994.tb03995.x
13. Sessler DI. Nitrous oxide is an effective and safe anesthetic. Turk J Anaesthesiol Reanim. 2017;45(1):1–2. doi:10.5152/TJAR.2017.23011
14. Buhre W, Disma N, Hendrickx J, et al. European society of anaesthesiology task force on nitrous oxide: a narrative review of its role in clinical practice. Br J Anaesth. 2019;122(5):587–604. doi:10.1016/j.bja.2019.01.023
15. Cousaert C, Heylens G, Audenaert K. Laughing gas abuse is no joke. An overview of the implications for psychiatric practice. Clin Neurol Neurosurg. 2013;115(7):859–862. doi:10.1016/j.clineuro.2013.04.004
16. Reynolds E. Vitamin B12, folic acid, and the nervous system. Lancet Neurol. 2006;5(11):949–960. doi:10.1016/S1474-4422(06)70598-1
17. Girdwood RH. Abnormalities of vitamin B12 and folic acid metabolism–their influence on the nervous system. Proc Nutr Soc. 1968;27(1):101–107. doi:10.1079/PNS19680021
18. Li J, Ren M, Dong A, et al. A retrospective study of 23 cases with subacute combined degeneration. Int J Neurosci. 2016;126(10):872–877. doi:10.3109/00207454.2015.1077331
19. Maamar M, Mezalek ZT, Harmouche H, Adnaoui M, Aouni M, Maaouni A. Contribution of spinal MRI for unsuspected cobalamin deficiency in isolated sub-acute combined degeneration. Eur J Intern Med. 2008;19(2):143–145. doi:10.1016/j.ejim.2007.03.017
20. Li HT, Chu CC, Chang KH, et al. Clinical and electrodiagnostic characteristics of nitrous oxide-induced neuropathy in Taiwan. Clin Neurophysiol. 2016;127(10):3288–3293. doi:10.1016/j.clinph.2016.08.005
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.