Back to Journals » Infection and Drug Resistance » Volume 16
Clinical Efficacy of Ulinastatin Combined with Azithromycin in the Treatment of Severe Pneumonia in Children and the Effects on Inflammatory Cytokines and Oxidative Stress: A Retrospective Cohort Study
Authors Dian D, Zhang W, Lu M, Zhong Y, Huang Y, Chen G, Chen Z, Yu L, Sun J
Received 4 July 2023
Accepted for publication 17 October 2023
Published 8 November 2023 Volume 2023:16 Pages 7165—7174
DOI https://doi.org/10.2147/IDR.S428900
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Dongchun Dian,1,* Weilong Zhang,1,* Minjun Lu,1 Yong Zhong,1 Yurong Huang,1 Guiling Chen,1 Zhangquan Chen,2 Luxin Yu,2 Jianbo Sun1
1The First Dongguan Affiliated Hospital, Guangdong Medical University, Guangdong, 523000, People’s Republic of China; 2Guangdong Medical University, Guangdong, 510000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jianbo Sun, Email [email protected]
Purpose: This retrospective cohort study aimed to evaluate the clinical efficacy of ulinastatin (UTI) and azithromycin (AZM) combination therapy in treating severe pneumonia in children and its impact on inflammatory cytokines and oxidative stress.
Patients and Methods: This retrospective cohort study was conducted from January 1, 2019, to January 1, 2021, involving pediatric patients diagnosed with severe mycoplasma pneumonia (SMPP). The pediatric patients were divided into two groups: those receiving UTI and AZM combination therapy (treatment group) and those receiving azithromycin alone (control group). We compared the two groups regarding clinical data, disease outcomes, inflammatory cytokines, and oxidative stress levels.
Results: Baseline characteristics did not significantly differ between the two groups. UTI, in combination with AZM, significantly improved blood oxygen levels, inflammatory infection markers, and relevant clinical symptoms in patients with SMPP on the 3rd day of treatment. Additionally, it significantly reduced the levels of inflammatory cytokines TNF-a, IL-6, IL-1β, and IL-10, as well as oxidative stress markers GSH and SOD.
Conclusion: Combining UTI and AZM can rapidly alleviate clinical symptoms and effectively control the progression of patients with SMPP. Therefore, this treatment approach deserves consideration for clinical promotion and utilization.
Keywords: severe mycoplasma pneumonia, ulinastatin, azithromycin, pediatrics
Introduction
Mycoplasma pneumoniae pneumonia (MPP) is a common, self-limited disease associated with Mycoplasma pneumoniae (M. pneumoniae), which tends to have higher incidence in preschool children. However, a small proportion of children with MPP may progress to severe mycoplasma pneumoniae pneumonia (SMPP), which can involve other complications that pose a life-threatening risk. SMPP is characterized by persistent fever, respiratory dysfunction, and pulmonary inflammatory infiltration accompanied by systemic inflammation.1 Once the children develop into SMPP, the duration of disease is significantly prolonged, making it challenging to cure and increasing the likelihood of recurrence.
The primary treatment strategies for SMPP include symptomatic management and antibacterial treatment.2 AZM is the mainstay antibacterial drug; however, its effectiveness in children with SMPP is diminishing due to the increasing drug resistance caused by prolonged antibiotic use.3 Furthermore, while AZM has been demonstrated to possess anti-inflammatory effects in certain diseases,4,5 its primary mechanism of action against SMPP remains the targeting of the ribosomes of the bacterium, disrupting bacterial protein synthesis, and thereby achieving its antibacterial effects.6 As a result, children with SMPP often exhibit suboptimal response to AZM. Recent evidence7,8 has shown that combining AZM with other drugs or traditional Chinese medicine compounds can improve clinical efficacy.
UTI, a human protease inhibitor, inhibits multiple proteases activities and releases inflammatory factors.9,10 It has been shown to have protective effects against acute lung injury11 and is currently utilized in treating ARDS, multi-organ failure, and sepsis. Numerous clinical trials have demonstrated the effectiveness of UTI in treating ARDS, with outcomes including reduce mortality, rates of ventilator associated pneumonia, duration of mechanical ventilation, length of ICU stay, and length of hospital stay.12
However, whether the combination of UTI and AZM effectively improves treatment outcomes in children with SMPP remain unknown. Therefore, this study aims to evaluate the efficacy of UTI combined with AZM in children with SMPP and its impact on inflammatory cytokines and oxidative stress.
Materials and Methods
Trial Design and Participants
This study was conducted at the Pediatric Department of Southeast Central Hospital in Dongguan City from January 2019 to January 2021. A total of 137 children diagnosed with SMPP were enrolled in the study. These diagnostic criteria for SMPP followed as the Guidelines for Diagnosis and Treatment of Community-Acquired Pneumonia in Children (2019 Edition).13 Among them, 53 children received combined UTI with AZM treatment, while 84 received AZM treatment alone. Based on the inclusion and exclusion criteria, we analyzed clinic data from 80 eligible children, 40 children in the UTI+AZM group and 40 children in the AZM group. The details are presented in Figure 1.
 |
Figure 1 Flowchart for case grouping. |
The inclusion criteria were follows:
- Extremely high fever or persistent high fever lasting more than 5 days. (The physical temperature of more than 37.5°C was defined as fever, more than 40.0°C was defined as extremely fever).
- Significantly increased respiratory rate, with rate ≥ 50 breaths per minute (for individuals over one year old) or requiring assistance with breathing (indicated by moaning, nasal flaring, or the presence of the three-concave sign).
- Cyanosis or oxygen saturation < 92%, PaO2 < 80mmHg, PaCO2 > 50mmHg. (1mmHg = 0.133kPa).
- Chest X-ray imaging revealing two-thirds unilateral lung infiltration, multi-lobar lung infiltration, pleural effusion, pneumothorax, atelectasis, lung necrosis, lung abscess, or other pulmonary complications.
- Diagnosis of SMPP based on a positive Mycoplasma pneumoniae antibody titer of 1:160, confirmation through throat sputum culture, or positive retesting of M. pneumoniae DNA by PCR.
The exclusion criteria were:
- Age below 1 year and weight below 6 kg.
- Presence of systemic diseases other than MPP, congenital diseases, prior history of drug therapy or immunosuppressive therapy, contraindications to UTI therapy, or refusal to receive UTI treatment.
Written informed consent was obtained from the legal guardians of all participants in accordance with the principles stated in the Declaration of Helsinki. The trial protocol was approved by the institutional review board of Dongguan Southeast Central Hospital.
Therapeutic Method and Outcome Judgment
In the AZM treatment group (AG), patients were administered AZM (10mg/kg) mixed with a 30mL 5% glucose solution intravenously once daily for one week. In the Combined Treatment Group (CG), patients received UTI (5000U/kg, NMPN H20040476; Guangdong Techpool Biochemical Pharmaceutical Co., Ltd., Guangdong, China) and AZM (10mg/kg) mixed with a 30mL 5% glucose solution intravenously twice daily for a duration of one week. Both groups received standard conventional treatment as per the “Guidelines for Diagnosis and Treatment of Community-Acquired Pneumonia in Children (2019 edition).”11 It included appropriate antimicrobial therapy, symptomatic treatment, maintenance of fluid and electrolyte balance, and supportive care.
The outcomes were assessed according to the Pan Jin lan14 method as follows:
- Cure: Resolution of clinical symptoms and signs, with the imaging indicates the resolution of lung inflammation.
- Significant improvement: Resolution of clinical symptoms and signs, with normal some laboratory infection indicators: WBC, CRP, LDH, and normalization or significant reduction of infection indicators to the level of healthy individuals is defined as normal. The imaging suggests the disappearance or a 2/3 reduction in lung inflammation.
- Effective: Improvement in some clinical symptoms and signs, with a slight improvement in objective indicators.
- Ineffective: No improvement or worsening of main symptoms and signs after 72 hours of medication, minimal or aggravated changes in objective indicators, or some cases, mortality was observed.
Detection of Inflammatory Factors and Oxidative Free Radicals
Serum inflammatory cytokines, including IL-6 (JL14113), IL-10 (JL19246), IL-1β (JL28060), TNF-a (JL28792), and SAA (JL10489), were measured using enzyme-linked immunosorbent assay (ELISA) method. The EILSA procedure was performed following manufacturer’s instructions. Additionally, the oxidative free radical indicators GSH and SOP were assessed ELISA kits. All ELISA kit obtained from Jianglai Biology, Shanghai.
Statistic Analysis
Data analysis was performed using SPSS 17.0 statistical software and GraphPad Prism 8.0 software. The chi-square test was used to compare categorical variables. For normally distributed data, a two-tailed ttest was conducted. Non-parametric tests, such as the Mann–Whitney U-test or Wilcoxon rank-sum test, were used for data with non-normal distribution. The results were expressed as mean ± standard deviation (mean ± SD) for normally distributed data. A p-value less than 0.05 was considered statistically significant.
Results
Participants’ Characteristics
The study collected cases as shown in Figure 1. Before admission, a comparison was made between the baseline characteristics of patients in the AG group and the UG group (Table 1). These characteristics primarily included age, number of individuals, gender, symptoms, signs, and other comorbidities. The results presented in e 1 indicated that cough, fever, and dyspnea were common clinical symptoms observed in SMPP patients. A few patients may have had both viral and microbial infections simultaneous. Furthermore, no notable variations were found in in these baseline characteristics between the two patient groups.
 |
Table 1 Baseline Clinical Characteristics of Patients with Severe Mycoplasma Pneumonia (χ2) |
UTI Can Significantly Improve the Clinical Efficacy of SMPP
When evaluating the blood oxygen indicators of both patient groups on the 7th day of treatment, it was observed that significant improvement in blood oxygen respiratory indicators was evident in both groups (Figure S1A–C), both CRP and WBC in two groups have shown varying degrees of reduction (Figure S1D–E). Surprisingly, the UG group exhibited a better efficacy in reducing the inflammatory infection marker LDH (Figure S1F), there was no significance difference between C However, upon further analysis of data for other treatment days, it was discovered that on the 3rd day of treatment, the combination of UTI and AZM significantly improved in patients’ blood oxygen saturation (SaO2), whereas the group treated with AZM alone did not yield a significant improvement in SaO2 (Figure 2A), In contrast, AZM monotherapy only partially improved arterial oxygen (PaO2) levels and arterial carbon dioxide (PaCO2) (Figure 2B and C). Moreover, on the 3rd day of treatment, the UG group showed significant improvement in inflammatory maker, while AZM monotherapy did not improve the inflammatory infection indicator (Figure 2D–F). Further analysis of clinical symptom improvement on the 3rd day of treatment revealed that the combination of UTI and AZM significantly improved relevant clinical symptoms associated with SMPP (Table 2). The clinical efficacy indicators for both the 7th day and the 3rd day also show the same results (Figure S2A–C). In other words, the combination therapy of UTI and AZM exhibited rapid control of severe pneumonia, significantly shortening the course of the disease.
 |
Table 2 Clinical Symptom Outcomes When the 3rd Day of Treatment (χ2) |
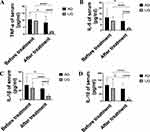
UTI Improved the Expression of Inflammatory Factors
SMPP is accompanied by excessive inflammation [1], thus we examined how UTI affects the expression of inflammatory factors. Our findings indicated that UTI significantly decrease TNF-α, IL-6, IL1, and IL-10 expression (Figure 3A–D and Figure S3A–D). Therefore, UTI is pivotal in ameliorating systemic inflammation, which could contribute to the therapeutic mechanism underlying the recovery of SMPP.
UTI Protected Against Oxidative Damage
Previous studies have shown that UTI possessed anti-oxidative properties and attenuate free radical damage via the ROS/MAPK/Nrf2 signaling pathway15 and JNK/c-Jun pathway16 signaling pathways in both in vitro and in vivo models. In this study, we investigated the biological role of UTI in counteracting oxidative stress by evaluating the levels of GSH and SOD (Figure 4A and B and Figure S4A and B). Our results revealed that the combined treatment of UTI and AZM partially mitigated oxidative damage caused by free radicals in SMPP, These findings suggest that this synergistic effect of UTI and AZM could be attributed, at least in part, to the modulation of oxidative stress. Thereby promoting the recovery of SMPP.
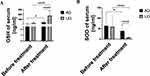 |
Figure 4 UTI alleviated oxidative stress. Notes: (A and B): GSH and SOD of serum were assayed using EILSA. (A) GSH. (B) SOD. *P<0.05.**** P<0.0001. |
Discussion
In the initial stages, M. pneumoniae typically colonizes ciliated epithelium and does not infiltrate lung parenchymal cells. It attaches to respiratory epithelial cells through key amino acid sites, disrupting the normal structure of cilia. This disruption can result in pneumonia and bronchitis, often in conjunction with other respiratory pathogenic microorganisms infections,17 such as Respiratory syncytial virus and Streptococcus pneumoniae.18 Additionally, early stages MPP shares similarities with other upper respiratory diseases, making it necessary to differentiate it from alternative pathogens. Performing clinical bacterial culture to identify M. pneumoniae can be expensive and time-consuming. Moreover, specific IgM antibodies against M. pneumoniae may not be detectable within the first 7 days after symptom onset and can persist in the serum for several months after infection.19 Furthermore, the prevalence of macrolide resistance among MPP patients has significantly increased in recent years, rendering conventional antibiotics ineffective, a small-scale cohort study has revealed that macrolide resistance has been spreading globally for 15 years. Currently, its prevalence is reported to be 0–15% in Europe and the United States, approximately 30% in Israel, and as high as 90–100% in Asia.20 These complex factors contribute to misdiagnosis, missed diagnosis, and the progression to SMPP. The exact pathogenic mechanisms underlying SMPP remain unclear, although studies have indicated that systemic inflammation,21,22 endoplasmic reticulum stress23,24 and immune dysregulation25,26 are significant factors influencing its development and progression. Furthermore, this study confirmed the effective alleviation of the inflammatory response in SMPP patients through the use of UTI AZM belongs to the class of macrolide antibiotics, which, in addition to exerting antibacterial effects, also demonstrate a certain degree of anti-inflammatory activity. The primary mechanisms of its anti inflammatory action include the inhibition of pro-inflammatory factors such as IL-1, IL-8, and TNF-α synthesis and release,27 reduction in the infiltration of inflammatory cells28,29 and decreased release of superoxide radicals.30 Furthermore, related research has indicated that AZM can participate in immune regulation in conditions such as pneumonia and inflammatory bowel disease by modulating macrophage phenotype transformation, it can transform pro-inflammatory M1 macrophages into anti-inflammatory M2 macrophages, thereby exerting anti-inflammatory effects.31 However, many of these anti-inflammatory mechanisms of AZM rely on the pathogen-induced immune regulation pathways, most of its anti inflammatory effects are achieved by affecting pathogen-mediated and released immune-activating substances or the biological activities of pathogens, subsequently influencing overall systemic immune regulation functions.32,33 Therefore, its anti-inflammatory action may have certain limitations in drug resistant pathogens. The results of this study confirm that in the early and later stages of SMPP, while AZM does possess some anti-inflammatory properties, its anti-inflammatory effects when used as a standalone treatment are weaker compared to when used in conjunction with UTI. We hypothesize that in the context of SMPP, AZM primarily exerts its anti-inflammatory effects by influencing pathogen proliferation and affecting the host’s immune regulation function. This phenomenon may account for the delayed onset of AZM’s anti-inflammatory action. When used in combination with UTI, which is a critical drug affecting multiple inflammatory pathways, it may lead to faster control of systemic inflammation and alleviation of the patient’s disease burden, resulting in a rapid improvement in the patient’s condition.
UTI, a protease inhibitor derived from fresh human urine, is commonly used to treat pancreatitis, nephritis, sepsis, and other diseases.34 Its main pharmacological effect lies in inhibiting protease activity, including trypsin, α-chymotrypsin, granulocyte elastase, hyaluronidase, plasmin, and others.35 In this study, we demonstrated that the combination of UTI and AZM significantly reduced the duration of SMPP, alleviated clinical symptoms, and rapidly controlled disease progression in the short term. Our findings establish UTI as a beneficial adjunctive treatment for children with SMPP. Compared to AZM alone, the combination of UTI and AZM resulted in a notable reduction in the levels of inflammatory cytokines, including TNF-α, IL-6, IL-10, and IL-1β. Previous studies36–40 have indicated that these inflammatory cytokines are typically produced through the NF-κB/MAPKs/JNK signaling pathways. It has been reported that UTI can attenuate the release of inflammatory cytokines by inhibiting the phosphorylation of p65 in the NF-κB signaling pathway, as well as inhibiting the phosphorylation of ERK and JNK within the MAPKs family.41 In an in vitro experiment, UTI has been demonstrated to inhibit LPS-mediated macrophage inflammation and oxidative stress levels. The primary mechanism involves the regulation of Thr183 phosphorylation on JNK and the degradation of IκB-Α within the JNK/NF-κB pathway, as well as the promotion of p62-mediated Keap1 degradation within the PI3K/Akt/Nrf2 pathway, leading to the induction of Nrf nuclear translocation.33 Additionally, UTI can also influence the expression levels of ERK/MER, affecting macrophage apoptosis, thereby impacting immune-inflammatory responses.42 It is worth noting that oxidative stress is closely associated with inflammatory responses. Studies have demonstrated that oxidative stress leads to an excessive generation of ROS and a decrease in the levels and activity of antioxidant enzymes, resulting in an imbalance between the oxidative and antioxidant systems.43 Furthermore, excessive accumulation of ROS can trigger and mediate the inflammatory response44,45 Our study confirmed that UTI effectively increased the levels of glutathione (GSH) and superoxide dismutase (SOD) in the antioxidant system, which could inhibit oxidative stress and alleviate inflammation. Notably, lysosomal membrane permeabilization contributes to oxidative stress and mediates cell death.46 Fortunately, one of the pharmacological mechanisms of UTI involves the stabilization of lysosomal membranes. By enhancing lysosomal stability, UTI improves oxidative stress and alleviates cellular damage to a certain extent. The destruction of cells can result in the release of damage-associated molecular patterns (DAMPs), such as HMGB1 and HSP,47,48 which are recognized by pattern recognition receptors (PRRs) on macrophages and subsequently trigger inflammatory responses.49 Therefore, UTI mitigates oxidative stress and cellular damage by maintaining lysosomal stability, likely serving as one of the underlying mechanisms by which UTI improves SMPP. Furthermore, UTI has been confirmed to affect neutrophil infiltration, regulate Th17/Treg cell balance, and modulate the release of immune-related cytokines in acute kidney injury induced by crush syndrome.50 Based on the aforementioned evidence, we have elucidated that the primary mechanism of action of UTI involves the regulation of key protein expression and degradation within inflammatory signaling pathways, phosphorylation levels of crucial amino acids, immune cell infiltration, and the balance of cytokine secretion. Through these mechanisms, UTI modulates the systemic inflammation levels and oxidative stress levels in the body.
Conclusion
This trial provides evidence that the combination of UTI and AZM effectively and promptly alleviates the systemic inflammatory response, effectively controls the progression of SMPP, and improves the condition of affected children. Importantly, UTI demonstrates no significant toxic side effects, suggesting its potential for clinical use. However, this trial has certain limitations, as it did not further investigate the specific pharmacological mechanisms underlying UTI’s anti-inflammatory effects. Additionally, future studies should consider expanding the sample size to provide a more comprehensive evaluation of the efficacy of this treatment approach for SMPP.
Acknowledgments
We thank the participants for their cooperation. This study was supported in part by grants from Key projects of Dongguan City (202050715032212).
Disclosure
The authors declare that they have no competing interests.
References
1. Lee YC, Chang CH, Lee WJ, et al. Altered chemokine profile in refractory Mycoplasma pneumoniae pneumonia infected children. J Microbiol Immunol Infect. 2021;54(4):673–679. doi:10.1016/j.jmii.2020.03.030
2. Jones BE, Herman DD, Dela Cruz CS, et al. Summary for clinicians: clinical practice guideline for the diagnosis and treatment of community-acquired Pneumonia. Ann Am Thorac Soc. 2020;17(2):133–138. doi:10.1513/AnnalsATS.201909-704CME
3. Kamel AM, Monem MSA, Sharaf NA, Magdy N, Farid SF. Efficacy and safety of azithromycin in Covid-19 patients: a systematic review and meta-analysis of randomized clinical trials. Rev Med Virol. 2022;32(1):e2258. doi:10.1002/rmv.2258
4. Zhang R, Ma J, Zheng P, Zheng R, Meng X, Wang Y. Ulinastatin plus biapenem for severe pneumonia in the elderly and its influence on pulmonary function and inflammatory cytokines. Am J Transl Res. 2021;13(5):5027–5034.
5. Patel A, Joseph J, Periasamy H, Mokale S. Azithromycin in combination with ceftriaxone reduces systemic inflammation and provides survival benefit in a murine model of polymicrobial sepsis. Antimicrob Agents Chemother. 2018;62(9). doi:10.1128/AAC.00752-18
6. Parnham MJ, Erakovic Haber V, Giamarellos-Bourboulis EJ, Perletti G, Verleden GM, Vos R. Azithromycin: mechanisms of action and their relevance for clinical applications. Pharmacol Ther. 2014;143(2):225–245.
7. Wang H, Zhao M, Liu S, Wang X. Efficacy and safety of reduning injection combined with azithromycin in the treatment of mycoplasma pneumonia among children: a systematic review and meta-analysis. Phytomedicine. 2022;106:154402. doi:10.1016/j.phymed.2022.154402
8. Xiao Z, Jiang Y, Gao X, et al. Comparison of the ameliorative effects of Qingfei Tongluo formula and azithromycin on Mycoplasma pneumoniae pneumonia. J Nat Med. 2017;71(4):685–692. doi:10.1007/s11418-017-1098-1
9. Inoue K, Takano H. Urinary trypsin inhibitor as a therapeutic option for endotoxin-related inflammatory disorders. Expert Opin Investig Drugs. 2010;19(4):513–520. doi:10.1517/13543781003649533
10. Luo X, Huan L, Lin F, et al. Ulinastatin ameliorates IL-1β-induced cell dysfunction in human nucleus pulposus cells via Nrf2/NF-κB pathway. Oxid Med Cell Longev. 2021;2021:5558687. doi:10.1155/2021/5558687
11. Cao C, Yin C, Shou S, et al. Ulinastatin protects against LPS-induced acute lung injury by attenuating TLR4/NF-κB pathway activation and reducing inflammatory mediators. Shock (Augusta, Ga). 2018;50(5):595–605. doi:10.1097/SHK.0000000000001104
12. Zhang X, Zhu Z, Jiao W, Liu W, Liu F, Zhu X. Ulinastatin treatment for acute respiratory distress syndrome in China: a meta-analysis of randomized controlled trials. BMC Pulm Med. 2019;19(1):196. doi:10.1186/s12890-019-0968-6
13. Ni X. Guideline for diagnosis and treatment of community-acquired pneumonia in children (2019 edition). Clin Med Res Pract. 2019;4(06):201.
14. Jinlan P. Efficacy observation and follow-up of two administration methods for Mycoplasma pneumonia. Primary Med Forum. 2013;17(23):3003–3004.
15. Wu X, Jiao W, Chen J, Tao Y, Zhang J, Wang Y. Ulinastatin alleviates early brain injury after intracerebral hemorrhage by inhibiting oxidative stress and neuroinflammation via ROS/MAPK/Nrf2 signaling pathway. Acta Cir Bras. 2022;37(6):e370606. doi:10.1590/acb370606
16. Li C, Ma D, Chen M, et al. Ulinastatin attenuates LPS-induced human endothelial cells oxidative damage through suppressing JNK/c-Jun signaling pathway. Biochem Biophys Res Commun. 2016;474(3):572–578. doi:10.1016/j.bbrc.2016.04.104
17. Tsolia MN, Psarras S, Bossios A, et al. Etiology of community-acquired pneumonia in hospitalized school-age children: evidence for high prevalence of viral infections. Clin Infect Dis. 2004;39(5):681–686. doi:10.1086/422996
18. Toikka P, Juvén T, Virkki R, Leinonen M, Mertsola J, Ruuskanen O. Streptococcus pneumoniae and Mycoplasma pneumoniae coinfection in community acquired pneumonia. Arch Dis Child. 2000;83(5):413–414. doi:10.1136/adc.83.5.413
19. Tsai TA, Tsai CK, Kuo KC, Yu HR. Rational stepwise approach for Mycoplasma pneumoniae pneumonia in children. J Microbiol Immunol Infect. 2021;54(4):557–565. doi:10.1016/j.jmii.2020.10.002
20. Pereyre S, Goret J, Bébéar C. Mycoplasma pneumoniae: current knowledge on macrolide resistance and treatment. Front Microbiol. 2016;7:974. doi:10.3389/fmicb.2016.00974
21. Liu X, Lin Z, Yin X. Pellino2 accelerate inflammation and pyroptosis via the ubiquitination and activation of NLRP3 inflammation in model of pediatric pneumonia. Int Immunopharmacol. 2022;110:108993. doi:10.1016/j.intimp.2022.108993
22. Shimizu T. Inflammation-inducing factors of mycoplasma pneumoniae. Front Microbiol. 2016;7:414. doi:10.3389/fmicb.2016.00414
23. Pritchard KA Jr, Jing X, Teng M, et al. Role of endoplasmic reticulum stress in impaired neonatal lung growth and bronchopulmonary dysplasia. PLoS One. 2022;17(8):e0269564. doi:10.1371/journal.pone.0269564
24. Walenna NF, Kurihara Y, Chou B, Ishii K, Soejima T, Hiromatsu K. Chlamydia pneumoniae infection-induced endoplasmic reticulum stress causes fatty acid-binding protein 4 secretion in murine adipocytes. J Biol Chem. 2020;295(9):2713–2723. doi:10.1074/jbc.RA119.010683
25. Zhang Z, Dou H, Tu P, et al. Serum cytokine profiling reveals different immune response patterns during general and severe Mycoplasma pneumoniae pneumonia. Front Immunol. 2022;13:1088725. doi:10.3389/fimmu.2022.1088725
26. Li J, Luu LDW, Wang X, et al. Metabolomic analysis reveals potential biomarkers and the underlying pathogenesis involved in Mycoplasma pneumoniae pneumonia. Emerg Microbes Infect. 2022;11(1):593–605. doi:10.1080/22221751.2022.2036582
27. Gualdoni GA, Lingscheid T, Schmetterer KG, Hennig A, Steinberger P, Zlabinger GJ. Azithromycin inhibits IL-1 secretion and non-canonical inflammasome activation. Sci Rep. 2015;5:12016. doi:10.1038/srep12016
28. Piacentini GL, Peroni DG, Bodini A, et al. Azithromycin reduces bronchial hyperresponsiveness and neutrophilic airway inflammation in asthmatic children: a preliminary report. Allergy Asthma Proc. 2007;28(2):194–198. doi:10.2500/aap.2007.28.2958
29. Andrada AC, Azuma MM, Furusho H, et al. Immunomodulation mediated by azithromycin in experimental periapical inflammation. J Endod. 2020;46(11):1648–1654. doi:10.1016/j.joen.2020.07.028
30. Jain S, Durugkar S, Saha P, Gokhale SB, Naidu VGM, Sharma P. Effects of intranasal azithromycin on features of cigarette smoke-induced lung inflammation. Eur J Pharmacol. 2022;915:174467. doi:10.1016/j.ejphar.2021.174467
31. Murphy BS, Sundareshan V, Cory TJ, Hayes D, Anstead MI, Feola DJ. Azithromycin alters macrophage phenotype. J Antimicrob Chemother. 2008;61(3):554–560. doi:10.1093/jac/dkn007
32. Varenyiova Z, Rojas-Hernandez LS, Spano J, et al. Azithromycin promotes proliferation, and inhibits inflammation in nasal epithelial cells in primary ciliary dyskinesia. Sci Rep. 2023;13(1):14453. doi:10.1038/s41598-023-41577-5
33. Hall IH, Schwab UE, Ward ES, Butts JD, Wolford ET, Ives TJ. Disposition and intracellular activity of azithromycin in human THP-1 acute monocytes. Int J Antimicrob Agents. 2002;20(5):348–360. doi:10.1016/S0924-8579(02)00187-5
34. Liu S, Xu J, Gao Y, et al. Multi-organ protection of ulinastatin in traumatic cardiac arrest model. World J Emerg Surg. 2018;13:51. doi:10.1186/s13017-018-0212-3
35. Umeadi C, Kandeel F, Al-Abdullah IH. Ulinastatin is a novel protease inhibitor and neutral protease activator. Transplant Proc. 2008;40(2):387–389. doi:10.1016/j.transproceed.2008.01.034
36. Yu H, Lin L, Zhang Z, Zhang H, Hu H. Targeting NF-κB pathway for the therapy of diseases: mechanism and clinical study. Signal Transduct Target Ther. 2020;5(1):209. doi:10.1038/s41392-020-00312-6
37. Tan YY, Zhou HQ, Lin YJ, et al. FGF2 is overexpressed in asthma and promotes airway inflammation through the FGFR/MAPK/NF-κB pathway in airway epithelial cells. Milit Med Res. 2022;9(1):7. doi:10.1186/s40779-022-00366-3
38. Behl T, Rana T, Alotaibi GH, et al. Polyphenols inhibiting MAPK signalling pathway mediated oxidative stress and inflammation in depression. Biomed Pharmacother. 2022;146:112545. doi:10.1016/j.biopha.2021.112545
39. Chen X, Li X, Zhang W, et al. Activation of AMPK inhibits inflammatory response during hypoxia and reoxygenation through modulating JNK-mediated NF-κB pathway. Metabolism. 2018;83:256–270. doi:10.1016/j.metabol.2018.03.004
40. Xiao A, Hongwei L, Yan C, Wang Y, Hailong LI. Research progress of traditional Chinese medicine compound and active ingredients regulating NFkB/MAPKs/JNK signaling pathway to mediate inflammatory response and antiatherosclerosis. J Trad Chin Med. 2019;47(06):109–114.
41. Ju M, He H, Chen S, et al. Ulinastatin ameliorates LPS‑induced pulmonary inflammation and injury by blocking the MAPK/NF‑κB signaling pathways in rats. Mol Med Rep. 2019;20(4):3347–3354. doi:10.3892/mmr.2019.10561
42. Li J, Shao R, Xie Q, et al. Ulinastatin promotes macrophage efferocytosis and ameliorates lung inflammation via the ERK5/Mer signaling pathway. FEBS Open Bio. 2022;12(8):1498–1508. doi:10.1002/2211-5463.13461
43. van der Pol A, van Gilst WH, Voors AA, van der Meer P. Treating oxidative stress in heart failure: past, present and future. Eur J Heart Fail. 2019;21(4):425–435. doi:10.1002/ejhf.1320
44. Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 2010;49(11):1603–1616.
45. Hussain T, Tan B, Yin Y, Blachier F, Tossou MC, Rahu N. Oxidative stress and inflammation: what polyphenols can do for us? Oxid Med Cell Longev. 2016;2016:7432797. doi:10.1155/2016/7432797
46. Wang M, Li J, Dong S, et al. Silica nanoparticles induce lung inflammation in mice via ROS/PARP/TRPM2 signaling-mediated lysosome impairment and autophagy dysfunction. Part Fibre Toxicol. 2020;17(1):23. doi:10.1186/s12989-020-00353-3
47. Chen R, Kang R, Tang D. The mechanism of HMGB1 secretion and release. Exp Mol Med. 2022;54(2):91–102. doi:10.1038/s12276-022-00736-w
48. Hasenauer A, Bédat B, Parapanov R, et al. Effects of cold or warm ischemia and ex-vivo lung perfusion on the release of damage associated molecular patterns and inflammatory cytokines in experimental lung transplantation. J Heart Lung Transpl. 2021;40(9):905–916. doi:10.1016/j.healun.2021.05.015
49. Franklin TC, Xu C, Duman RS. Depression and sterile inflammation: essential role of danger associated molecular patterns. Brain Behav Immun. 2018;72:2–13. doi:10.1016/j.bbi.2017.10.025
50. Yang X-Y, Song J, Hou S-K, et al. Ulinastatin ameliorates acute kidney injury induced by crush syndrome inflammation by modulating Th17/Treg cells. Int Immunopharmacol. 2020;81:106265. doi:10.1016/j.intimp.2020.106265
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.