Back to Journals » Drug Design, Development and Therapy » Volume 8
Clinical efficacy of Spasmofen® suppository in the emergency treatment of renal colic: a randomized, double-blind, double-dummy comparative trial
Authors Yakoot M , Salem A, Yousef S, Helmy S
Received 17 February 2014
Accepted for publication 13 March 2014
Published 2 May 2014 Volume 2014:8 Pages 405—410
DOI https://doi.org/10.2147/DDDT.S62571
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Video abstract presented by Mostafa Yakoot
Views: 2527
Mostafa Yakoot,1 Amel Salem,2 Sameh Yousef,2 Sherine Helmy3
1Green Clinic and Research Center, 2Alexandria Helmy Medical Center, 3Pharco Corporation, Alexandria, Egypt
Background: Renal colic is typically characterized by the sudden onset of severe pain radiating from the flank to the groin and its acute management in emergency departments essentially aims at rapid pain relief. Spasmofen® is a brand of Amriya Pharmaceutical Industries in the form of rectal suppositories containing ketoprofen 100 mg and hyoscine butylbromide 10 mg. This combination is intended for the rapid relief of severe colicky pain in the renal system, hepatobiliary system, or gastrointestinal tract. This trial aims to compare a single-dose of Spasmofen rectal suppository to a single intravenous (IV) ketorolac tromethamine 30 mg/2 mL dose in patients with acute renal colic.
Methods: A total of 80 eligible consecutive patients presenting to the emergency departments of two medical centers with acute renal colic were included in the study. Eligible patients who signed the informed consent were randomly assigned into two treatment groups: an experimental group (Spasmofen group) who received one Spasmofen rectal suppository plus an IV injection of 2 mL of normal saline solution; and a control group (ketorolac group) who received one ketorolac 30 mg/2 mL ampoule IV plus one placebo suppository. Treatment success, defined as a change in the verbal rating score from severe or moderate pain to none or mild at 60 minutes after the dose, was compared between groups using the chi-square/Fisher's exact test. Percentage reductions in visual pain analog scale (VPAS) scores at 15 and 60 minutes after the dose were compared between groups using the Z-test for proportions.
Results: Successful treatment at 60 minutes occurred in 35 of 40 (87.5%) of Spasmofen-treated patients and in 33 of 40 (82.5%) of ketorolac-treated patients. The difference was not statistically significant by Fisher's exact test (P=0.755). The mean percentage reduction of VPAS after 15 minutes was 61.82% in the Spasmofen-treated group and 64.76% in the ketorolac-treated group. The difference was also not statistically significant by the Z-test for proportions (P=0.795). Sixty minutes after being treated, Spasmofen was associated with a statistically significant greater reduction in VPAS (mean% reduction =92.36%) than ketorolac (75.06%; P=0.0466).
Conclusion: Single-dose Spasmofen rectal suppository might be a safe and effective first-aid treatment for the emergency department relief of acute renal colic.
Keywords: renal colic, ketoprofen, hyoscine butylbromide, ketorolac, RCT
Introduction
Renal colic is a rather common clinical presentation to emergency departments. It has an annual incidence of around 16 per 10,000 people and a lifetime incidence of 2%–5%.1,2
Renal colic is typically characterized by the sudden onset of severe pain radiating from the flank to the groin. It is most commonly caused by the passage of calculi through the urinary tract. Acute management of renal colic in the emergency department is primarily targeting rapid pain relief, confirmation of the diagnosis, and recognition of complications requiring immediate intervention.3 Non-steroidal anti-inflammatory drugs (NSAIDs), particularly administered intravenously, are commonly used to provide rapid pain relief4,5 and have been shown to be effective in the treatment of renal colic.6–16 Ketorolac tromethamine intravenous injection is commonly used in the emergency treatment of renal colic, as besides being proved efficacious, it does not require close patient monitoring and it is not associated with untoward sedation.15,16 Hyoscine butylbromide (scopolamine butylbromide) is an antispasmodic anticholinergic drug commonly used for the treatment of abdominal pain associated with smooth muscle spasms. Its antispasmodic effects are mainly due to blockade of the muscarinic receptors. It also binds to nicotinic receptors, where it can induce a ganglion-blocking effect.17 Hyoscine butylbromide combined with an analgesic such as acetaminophen, dipyrone, or ibuprofen in a suppository dosage form has been marketed in some countries for decades and is frequently used in the treatment of severe pain associated with smooth muscle spasm, such as in the case of severe biliary or renal colic.18
Spasmofen® is a brand of Amriya Pharmaceutical Industries (Alexandria, Egypt) in the form of a rectal suppository containing ketoprofen 100 mg and hyoscine butylbromide 10 mg. This combination is intended for the rapid relief of severe colicky pain in the renal system, hepatobiliary system, or gastrointestinal tract.
This trial compares a single-dose Spasmofen suppository to a single intravenous (IV) ketorolac dose (from the same company) in patients presenting with acute renal colic.
Objectives
To study the efficacy of the Spasmofen suppository in comparison to IV ketorolac as an active control with regards to the speed of onset of action, degree of analgesia, and the incidence of adverse effects.
Methods
Study design
A randomized, double-blind, double-dummy, active-controlled comparative study design.
Setting
Emergency department settings in two medical centers in Alexandria, Egypt.
Patients
Group sample sizes of 40 in group one and 40 in group two achieve 88% power to detect a difference of 0.2 between the group proportions. The test statistic used was the two-sided Z-test with pooled variance. The significance level of the test was targeted at 0.05.
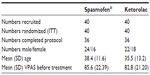
A total of 80 eligible consecutive patients presenting to the emergency departments of both centers from March to October 2007 were included in the study after approval of the study protocol by the local research ethical committee (Green Clinic and Research Center IRB: IRB00008268); each patient signed an informed consent form.
Patients were eligible for inclusion if they were adult of any sex, aged between 18 and 60 years old complaining of typical moderate to severe unilateral abdominal or flank pain that the treating emergency physician clinically diagnosed as renal colic with or without a positive imaging picture suggestive of renal calculi such as hydronephrosis, hydroureter, or a visible calculus on the affected side by ultrasonography. Exclusion criteria included: any suspected other cause of acute abdominal pain that could not be ruled out clinically or by ultrasonography in the emergency department; pregnancy or breastfeeding; patient size or weight far from the average for adult unit dose; history of allergy to an anticholinergic or any NSAID; and history of peptic ulcer disease, gastrointestinal bleeding, perforation, or inflammatory bowel disease.
Interventions
All patients underwent full history taking and physical examination at baseline to confirm eligibility and to record baseline demographic and clinical data by the attending investigators.
Eligible patients who signed the informed consent form were randomly assigned into two treatment groups: an experimental group (Spasmofen group) who received one Spasmofen rectal suppository plus an IV injection of 2 mL of normal saline solution, and a control group (ketorolac group) who received one ketorolac tromethamine (Amriya Pharmaceutical Industries) 30 mg/2 mL ampoule IV plus one placebo suppository. The randomization sequence was carried out centrally by a statistician independent from the study team through a computer-generated block randomization scheme. The study drugs were administered by research assistants in the treatment rooms according to the randomization scheme dictated by the statistician through a telephone call. Research assistants kept the patients blinded from the treatment allocations, whereas the assessors were also blinded as they checked the patients in rooms separate from the treatment rooms. The randomization sequences were kept in opaque, sealed envelopes and locked in the statistician’s office.
Efficacy, safety, and tolerability assessment
At 15, 30, 45, and 60 minutes after administration of the study medications, the following data were collected by the same attending physician (assessor) blinded to the treatment allocation:
Categorical pain assessment: subjects rated their ongoing pain as none, mild, moderate, or severe on a verbal rating scale (VRS).
Visual analog scale (VPAS) of pain severity: subjects grade their pain intensity by marking on a 100 mm horizontal line scale starting from the left side denoting “no pain” to end at the right side denoting “most severe pain imaginable”.
Vital signs, including temperature, blood pressure, pulse rate, and respiratory rate were recorded at baseline and every 15 minutes in the case report forms.
Any adverse effects reported by the patients at any time during the follow-up period were also recorded in the case report forms.
Outcome measures and statistical analyses
Treatment success, defined as a change in the VRS from severe or moderate to none or mild at 60 minutes after the dose, was compared between groups using chi-square/Fisher’s exact test. Patients who dropped out or needed rescue medication before the end of the study period were considered treatment failures. Percentage reductions of VPAS scores at 15 and 60 minutes after the dose were compared between groups using the Z-test for proportions. The data were analyzed using SPSS software (version 12; SPSS Inc., Chicago, IL, USA).
Results
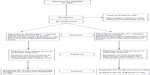
Eighty eligible patients were enrolled and received study drugs. At 15 minutes, two Spasmofen-treated patients and one ketorolac-treated patient withdrew from follow-up. At 30 minutes, two Spasmofen-treated patients and three ketorolac-treated patients dropped out, asking for rescue medication because of inadequate pain control, leaving 72 patients who completed the full study follow-up (Figure 1).
Baseline characteristics
Baseline demographic and clinical data are summarized in Table 1. The characteristics of the patients in the two groups were similar.
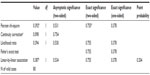
At 60 minutes post-administration, 35 out of the 40 Spasmofen-treated patients (87.5%) reported that their pain rating (VRS) decreased from severe or moderate to mild or none, denoting treatment success. This was reported in 33 out of the 40 ketorolac-treated patients (82.5%). The difference between both treatment groups was not statistically significant by Fisher’s exact test (P=0.755; Tables 2 and 3).
  | Table 2 Proportions of treatment success in both groups |
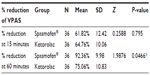
After 15 minutes, the mean percentage reduction of VPAS was 61.82% in the Spasmofen-treated group and 64.76% in the ketorolac-treated group. The difference was not statistically significant by the Z-test for proportions (P=0.795; Table 4). After 60 minutes of the dose, Spasmofen was associated with a statistically significant greater reduction in VPAS (mean% reduction =92.36%) than ketorolac (75.06%; P=0.0466).
Safety and tolerability
Four patients in the Spasmofen group complained of dry mouth, three complained of feeling giddiness and decreased alertness, and two complained of nausea and agitation. There were no statistically significant differences between treatment groups at any time interval for agitation, itchiness, nausea, nervousness, sweating, or vomiting. Dry mouth was reported in four cases treated with Spasmofen but it was mild and short-lived.
Discussion
In our study, there was a trend of greater reduction of VPAS in the ketorolac group than the Spasmofen group after 15 minutes of the dose. Although this difference was not found to be statistically significant, it can be explained by the fact that intravenous drug administration reaches the bloodstream immediately, as compared to rectal suppositories, which need some time for dissolution and absorption.
The effect at the end of 60 minutes showed a significantly greater percentage reduction in VPAS in the Spasmofen-treated group. The combination of an antispasmodic with a potent analgesic with a good bioavailability profile through the rectal route,18 as is the case in Spasmofen, although not as rapid in action as the intravenously administered route as it reaches its maximum effect a bit slower, could offer high efficacy in the control of renal colic, probably through the addition of two different mechanisms of action. Our results confirm the rationale of the combination of hyoscine butylbromide with an analgesic-like ibuprofen, acetaminophen, and dipyrone (Boehringer Ingelheim Limited) which have already been marketed in some countries for decades.18 To our knowledge, this study is the first to test the efficacy and safety of the combined ketoprofen with hyoscine butylbromide in a suppository dosage form. The efficacy of a combination of hyoscine butylbromide with diclofenac versus diclofenac alone had been studied by Sarfraz et al, their results also revealed the significantly higher efficacy of the combination versus diclofenac alone in the improvement of renal colic pain.19 Our study had many limitations including a small sample size and depending only upon subjective outcome measures, but we have made the best of our available resources.
Conclusion
Single-dose Spasmofen rectal suppository might be a safe, fast, and effective first-aid treatment for the emergency department relief of acute renal colic.
Disclosure
Amriya Pharmaceutical Industries, Alexandria, Egypt supported all study medications. The authors report no other conflicts of interest in this work.
References
Stewart C. Nephrolithiasis. Emerg Med Clin North Am. 1988;6(3):617–630. | |
Drach GW. Urinary lithiasis: etiology, diagnosis, and medical management. In: Walsh PC, Refik AB, Stamey TA, Vaughan ED, editors. Campbell’s Urology. 6th ed. Philadelphia, PA: WB Saunders; 1992:2085–2156. | |
Holdgate A, Hardcastle J. Renal colic: a diagnostic and therapeutic review. Emerg Med. 1999;11(1):9–16. | |
Curry C, Kelly AM. Intravenous tenoxicam for the treatment of renal colic. NZ Med J. 1995;108(1001):229–230. | |
Smally AJ. Analgesia in renal colic. Ann Emerg Med. 1997; 29(2):296. | |
Rosen P, Barkin R, Danzl D, editors. Emergency Medicine, Concepts and Clinical Practice. 4th ed. St Louis, MO: Mosby Year-Book; 1998:282. | |
Rosen P, Barkin R, Danzl D, editors. Emergency Medicine, Concepts and Clinical Practice. 4th ed. St Louis, MO: Mosby Year-Book; 1998:279. | |
Lundstam SO, Leissner KH, Wåhlander LA, Kral JG. Prostaglandin-synthetase inhibition with diclofenac sodium in treatment of renal colic: comparison with use of a narcotic analgesic. Lancet. 1982;1(8281):1096–1097. | |
Lundstam S, Wahjander L, Kral JG, Leissner K. Prostaglandin synthetase inhibition with diclofenac sodium in treatment of renal colic: comparison with use of a narcotic analgesic. Lancet. 1982;1(8281):1096–1097. | |
Vignoni A, Fierro A, MoreschiniG, et al. Diclofenac sodium in ureteral colic: a double blind comparison trial with placebo. J Int Med Res. 1983;11(5):303–307. | |
Grenabo L, Aurell M, Delin K, Holmlund D, Sjodin JG. Antidiuretic hormone levels and the effect of indomethacin on ureteric colic. J Urol. 1983;129(5):941–943. | |
Sommer P, Andersen K, Lendorf A, Lyngdorf P, Moller P. Analgesic effect and tolerance of Voltaren and Ketogan in acute renal or ureteric colic. Br J Urol. 1989;63(1):4–6. | |
Wolfson AB, Yealy DM. Oral indomethacin for acute renal colic. Am J Emerg Med. 1991;9(1):16–19. | |
Thompson JF, Pike JM, Chumas PD, Rundle JSH. Rectal diclofenac compared with pethidine injection in acute renal colic. Br Med J. 1989; 299:1140–1141. | |
Larsen LS, Miller A, Allegra JR. The use of intravenous ketorolac for the treatment of renal colic in the emergency department. Am J Emerg Med. 1993;11(3):197–199. | |
Cordell WH, Wright SW, Wolfson AB, et al. Comparison of intravenous ketorolac, Spasmofen, and both (balanced analgesia) for renal colic. Ann Emerg Med. 1996;28(2):151–158. | |
Tytgat GN. Hyoscine butylbromide: a review of its use in the treatment of abdominal cramping and pain. Drugs. 2007;67(9):1343–1357. | |
Sweetman SC, editor. Martindale: The Complete Drug Reference. 2007 edition. London, UK: Pharmaceutical Press. | |
Sarfraz K, Shehzad K, Sheikh I. Comparison between pain relief achieved by Diclofenac alone and the combination of Diclofenac and Hyoscine in the short-term treatment of ureteric colic. Pak Armed Forces Med J. 2008;58(2):136–140. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.