Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 16
Clinical Efficacy of Dapagliflozin in the Treatment of Patients with Diabetic Nephropathy and Its Effect on Proteinuria Level
Received 16 May 2023
Accepted for publication 18 July 2023
Published 22 July 2023 Volume 2023:16 Pages 2167—2175
DOI https://doi.org/10.2147/DMSO.S421579
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Prof. Dr. Antonio Brunetti
Ze-Jun Jin, Gen-Zhen Wang
Department of Nephrology, Anhui Wannan Rehabilitation Hospital, Wuhu, Anhui, People’s Republic of China
Correspondence: Gen-Zhen Wang, Email [email protected]
Objective: This study aimed to analyze the clinical efficacy of dapagliflozin in the treatment of diabetic kidney disease and its impact on proteinuria levels in patients.
Methods: Retrospective analysis of medical records of 176 patients with diabetic kidney disease treated at our hospital from January 2020 to January 2022. According to the different treatment methods, the patients were divided into a control group (n=88) receiving enalapril maleate treatment and an observation group (n=88) receiving dapagliflozin treatment. The clinical treatment effects, blood glucose levels, renal function indicators, inflammation factor indicators, and adverse reactions were compared between the two groups.
Results: The total effective rate of treatment (97.73%) in the observation group was significantly higher than that (79.55%) in the control group (P< 0.05). After treatment, the FPG, 2hPG, and HbAlc levels in the observation group were significantly lower than those in the control group (P< 0.05). After treatment, the Scr, BUN, UmAlb, UAER, UACR, and 24-hour urine protein quantitative levels in the observation group were significantly lower than those in the control group (P< 0.05). After treatment, the hs-CRP, IL-1β, and TNF-α levels in the observation group were significantly lower than those in the control group (P< 0.05). The incidence of adverse reactions in the observation group significantly lower than the control group (P< 0.05).
Conclusion: Compared with enalapril maleate alone, the combined application of dapagliflozin in the treatment of diabetic kidney disease has more significant clinical efficacy. It can further control patients’ blood sugar, reduce their body’s inflammatory response, alleviate or eliminate their proteinuria symptoms, promote the recovery of their renal function, and enhance the safety of their treatment to a certain extent, which helps to further improve the clinical treatment effect of patients.
Keywords: stroke, nursing, hemiplegic limb rehabilitation training nursing, effect analysis
Introduction
Diabetic nephropathy refers to kidney damage caused by diabetes, which is one of the most common complications of diabetes. The main manifestations of diabetic nephropathy are proteinuria, hypertension, and kidney function damage, which seriously affect the quality of life and long-term survival rate of patients.1 Currently, common treatments for diabetic nephropathy in clinical practice include blood glucose control, blood pressure control, and kidney protection therapy.2 Currently, agents are promising and have good availability to be used as inhibitors of DKD progression to new advanced stages of CKD and ACEI, ARB. As for monoclonal antibodies that play a role in inhibiting TGF-betha expression, they are still not available and expensive. Therefore, DM drugs given orally may have better availability. The progression of DKD to advanced stage of CKD is through increased expression of TGF-betha.3 TGF-betha expression was found to be higher in patients with persistent proteinuria.4
In recent years, an increasing number of studies5,6 have shown that dapagliflozin can not only control blood glucose levels in diabetic patients, but can also reduce their risk of cardiovascular events and death, and has significant renal protective effects. However, clinical studies on the treatment of diabetic nephropathy with dapagliflozin are still limited. Therefore, this study aims to analyze the clinical efficacy of dapagliflozin in treating diabetic nephropathy and its impact on patients’ proteinuria levels, providing reference for the clinical treatment of diabetic nephropathy. This study will compare the clinical efficacy and safety of treating diabetic nephropathy patients with either monotherapy of enalapril maleate or dapagliflozin, thereby providing new ideas for the treatment of diabetic nephropathy.
Objects and Methods
Study Objects
Retrospective analysis of medical records of 176 patients with diabetic kidney disease treated at our hospital from January 2020 to January 2022. Basic information such as gender, age, duration of diabetes, duration of diabetic nephropathy, and disease staging were collected. All patients met the inclusion criteria and were divided into two groups according to different treatment methods: the control group (n=88) received treatment with enalapril maleate, while the observation group (n=88) received treatment with dapagliflozin. This study has been approved by the ethics committee of Anhui Wannan Rehabilitation Hospital. Patients and their families were informed of the research content and voluntarily signed the informed consent. All the methods were carried out in accordance with the Declaration of Helsinki.
Inclusion and Exclusion Criteria
- Inclusion criteria: ① Patients aged 18 years or older; ② Patients diagnosed with diabetic nephropathy according to relevant diagnostic criteria; ③ Patients with a calculated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2 or a 24-hour urine protein excretion rate (24hUP) > 0.3g based on the CKD-EPI formula;7 ④ Patients who have not used other hypoglycemic drugs or dapagliflozin, or have stopped using these drugs for at least 4 weeks; ⑤ Patients have complete clinical data and relevant test data; ⑥ Patients or their family members agree to participate in this study and sign an informed consent form.
- Exclusion criteria: ① Patients with severe diseases or tumors of liver, kidney, heart, brain, or other organs; ② Patients with other significant diseases, such as infectious or autoimmune diseases, that may affect the judgment of study results; ③ Pregnant or lactating women; ④ Patients with alcohol abuse or drug addiction; ⑤ Patients who do not meet the complete inclusion criteria. ⑥Excluded non-compliant patients.
Methods
All patients were required to complete relevant tests upon enrollment, and their vital signs were closely monitored. The severity of the disease was assessed, and appropriate interventions such as hypoglycemic drugs and nutritional support were given based on the assessment results. Both groups of patients received a 12-week treatment.
- Control group: Patients in the control group were treated with oral administration of enalapril maleate (Changzhou Pharmaceutical Factory Co., Ltd., National Drug Approval H10930061), with a dose of 5 mg per administration, twice a day.
- Observation group: Patients in the observation group received treatment with oral administration of dapagliflozin (AstraZeneca Pharmaceuticals Co., Ltd., National Drug Approval J20170040), with a dose of 10 mg per administration, once a day.
Observational Indicators
- Clinical treatment efficacy: The efficacy evaluation criteria were as follows: markedly effective: disappearance of clinical symptoms such as proteinuria in patients, normalization of indicators such as 24-hour proteinuria, 24-hour urinary microalbumin, and blood glucose, and recovery of renal function; effective: basic disappearance of clinical symptoms such as proteinuria in patients, improvement of indicators such as 24-hour urinary microalbumin and 24-hour proteinuria by ≥50%, and basic recovery of renal function; ineffective: The clinical symptoms of the patient, such as proteinuria, have not disappeared and may have worsened. The indicators, including microalbuminuria in 24-hour urine and 24-hour proteinuria, have not improved. Renal function has not returned to normal. The total effective rate of treatment = (number of markedly effective cases + number of effective cases) / total cases × 100%.
- Blood glucose indicators: Before and after treatment, 3 mL of fasting and 2-hour postprandial venous blood samples were collected from the patients. The fasting plasma glucose (FPG) and 2-hour postprandial glucose (2hPG) levels were measured using the CX8 fully automated biochemical analyzer from Beckman, USA. The patients’ glycated hemoglobin (HbA1c) levels were measured using the glycated hemoglobin analyzer from Bio-Rad, USA.
- Renal function indicators: Before and after treatment, 3 mL of clear empty stomach venous blood was collected from each patient, routinely centrifuged for separation, and the levels of blood creatinine (Scr), blood urea nitrogen (BUN), and urinary microalbumin (UmAlb) were detected using an immunoturbidimetric method. The 24-hour urine was collected to determine urine protein quantification using a double reduction urea method, and the 24-hour urine protein excretion rate (UAER) was calculated. The concentration of creatinine (Cr) in the urine was detected using an enzymatic method, and the urine albumin to creatinine ratio (UACR) was calculated.
- Inflammatory factor indicators: Before and after treatment, 3 mL of fasting venous blood samples were collected from the patients. The samples were allowed to stand at room temperature for 1 hour and then centrifuged at 3000 rpm for 10–15 minutes to separate the serum, which was stored at −70°C until further use. The levels of high-sensitivity C-reactive protein (hs-CRP), interleukin-1β (IL-1β), and tumor necrosis factor-α (TNF-α) in the patients’ serum were measured using the Boruike ELISA kits (ELISA method) from Changsha Dalfo Bio-Tech Co., Ltd.
- Adverse reactions: The adverse reactions observed in this study included: diabetic ketoacidosis, hypoglycemia, nausea, vomiting, dizziness, and headache, and the occurrence of the above conditions were recorded by our hospital’s related medical staff.
Statistical Analysis
GraphPad Prism 8 was used for graphing and SPSS 22.0 was used for data analysis. For continuous data, the mean and standard deviation were used to describe the distribution, and t-tests or analysis of variance (ANOVA) were used to compare differences between two groups. For categorical data, frequency and percentage were used to describe the distribution, and chi-square tests or Fisher’s exact tests were used to compare differences between two groups. A P-value less than 0.05 indicated statistical significance. Multiple logistic regression is used to adjust for the relationship between exposure and outcome, thereby reducing the influence of confounding factors.
Results
Comparison of Baseline Data Between the Two Groups of Patients
Among the 88 patients in the control group, there were 44 males and 44 females, with an age range of 47–79 years and an average age of (65.13±3.79) years. The duration of diabetes ranged from 2 to 25 years, with an average duration of (17.34±2.69) years. The duration of diabetic nephropathy ranged from 0.5 to 5 years, with an average duration of (2.19±0.61) years. The disease stages were stage II in 49 cases and stage III in 39 cases. Among the 88 patients in the observation group, there were 42 males and 46 females, with an age range of 45–81 years and an average age of (65.32±3.86) years. The duration of diabetes ranged from 3 to 24 years, with an average duration of (17.21±2.58) years. The duration of diabetic nephropathy ranged from 0.5 to 6 years, with an average duration of (2.25±0.64) years. The disease stages were stage II in 47 cases and stage III in 41 cases. The baseline data of the two groups of patients were similar, and the difference was not statistically significant (P>0.05). See Table 1 for details on the comparison of baseline data between the two groups of patients.
 |
Table 1 Comparison of Baseline Data Between the Two Groups of Patients |
Comparison of Clinical Treatment Efficacy Between the Two Groups of Patients
The total effective rate of treatment in the control group was 79.55%, while that in the observation group was 97.73%. The total effective rate of treatment in the observation group was significantly higher than that in the control group (P<0.05). See Table 2 for details.
 |
Table 2 Comparison of Clinical Treatment Efficacy Between the Two Groups of Patients |
Comparison of Blood Glucose Indicators Between the Two Groups of Patients
As shown in Figure 1A–C, the FPG levels before and after treatment in the control group were (8.65±0.77, 6.78±0.54) and (12.27±2.41, 8.36±0.78) respectively, and the HbAlc levels were (7.86±0.88, 6.59±0.67). The FPG levels before and after treatment in the observation group were (8.54±0.79, 6.02±0.45) and (12.31±2.43, 7.41±0.72) respectively, and the HbAlc levels were (7.91±0.85, 6.11±0.54). Before treatment, there was no significant difference in FPG, 2hPG, and HbAlc levels between the two groups (P>0.05). After treatment, the FPG, 2hPG, and HbAlc levels in the observation group were significantly lower than those in the control group (P<0.05).
 |
Figure 1 Comparison of blood glucose indicators between the two groups of patients. (A) FPG levels. (B) 2hPG levels. (C) HbAlc levels. Note: *Means comparison P<0.05. |
Comparison of Renal Function Indicators Between the Two Patient Groups
As shown in Figure 2A–F, before treatment, the Scr levels of the control group were (89.59±5.71, 65.39±5.52), BUN levels were (17.68±3.26, 14.88±2.25), UmAlb levels were (248.32±5.36, 194.35±5.42), UAER levels were (158.36±12.18, 94.63±8.47), UACR levels were (178.32±12.56, 102.88±8.53), and 24h urine protein quantification levels were (289.94±16.85, 196.27±15.21); The Scr levels of the observation group before and after treatment were (89.77±5.54, 60.21±5.13), BUN levels were (17.55±3.31, 12.26±2.11), UmAlb levels were (248.35±5.21, 1176.32±5.37), UAER levels were (158.41±12.24, 85.72±7.53), UACR levels were (178.42±12.45, 83.89±7.09), and 24h urine protein quantification levels were (291.31±16.77, 152.75±12.79). Before treatment, there was no significant difference in Scr, BUN, UmAlb, UAER, UACR, and 24h urine protein quantification levels between the two groups (P>0.05); After treatment, the levels of Scr, BUN, UmAlb, UAER, UACR, and 24h urine protein quantification in the observation group were significantly lower than those in the control group (P<0.05).
Comparison of Inflammatory Marker Indices Between Two Groups of Patients
As shown in Figure 3A–C, before and after treatment, the levels of hs-CRP in the control group were (14.78±2.43, 9.53±1.62), IL-1β were (23.74±2.62, 16.42±2.31), and TNF-α were (7.68±0.85, 4.54±0.62), respectively. The levels of hs-CRP in the observation group before and after treatment were (14.69±2.51, 6.45±1.23), IL-1β were (23.69±2.65, 13.35±1.74), and TNF-α were (7.59±0.74, 3.25±0.53), respectively. Before treatment, there was no significant difference in the levels of hs-CRP, IL-1β, and TNF-α between the two groups of patients (P>0.05). After treatment, the levels of hs-CRP, IL-1β, and TNF-α in the observation group were significantly lower than those in the control group (P<0.05).
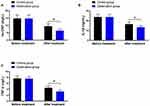 |
Figure 3 Comparison of inflammatory marker indices between two groups of patients. (A) Levels of hs-CRP. (B) Levels of IL-1β. (C) Levels of TNF-α. Note: *Indicates comparison P<0.05. |
Comparison of Adverse Reactions Between the Two Groups
The incidence of adverse reactions in the control group was 21.59%, while that in the observation group was 5.68%. The incidence of adverse reactions in the observation group was significantly lower than that in the control group (P<0.05). Refer to Table 3 for details.
 |
Table 3 Comparison of Adverse Reactions Between the Two Groups |
Discussion
In recent years, with the changes in lifestyle and dietary structure of Chinese residents, the incidence of diabetes has been increasing year by year. Furthermore, diabetic nephropathy caused by diabetes is also increasing along with the incidence of diabetes.8 Research9 shows that about 40% of diabetes patients will develop diabetic nephropathy. As a common complication of diabetes, diabetic nephropathy is the second leading cause of end-stage renal disease, and its harm to human health cannot be ignored. The pathogenesis of diabetic nephropathy is complex and mainly related to multiple factors such as hyperglycemia, hypertension, lipid abnormalities, activation of the renin-angiotensin-aldosterone system, insulin secretion defects, insulin resistance, and oxidative stress.10 In the early stage of diabetic nephropathy, the main features are glomerulosclerosis, microvascular damage, and microalbuminuria. At this stage, kidney damage has a certain degree of reversibility.11 Therefore, providing effective treatment and intervention for early diabetic nephropathy patients is of great significance in preventing the occurrence of kidney failure. The essential contributor of DKD-related CKD are proteinuria and the expression of TGF-betha. The known agent that have a potential effect in inhibiting CKD progression are monoclonal antibody. This antidiabetic agent potentially to be developed as a kidney protector.
ACE inhibitors are a class of commonly used antihypertensive drugs in clinical practice. Their main function is to inhibit the activity of angiotensin-converting enzyme, which reduces the generation of angiotensin II, decreases vascular constriction and tissue cell proliferation, and ultimately lowers blood pressure and reduces the burden on the heart.12 Enalapril maleate is a commonly used ACE inhibitor, which is widely used in the treatment of hypertension, heart failure, diabetic nephropathy, and other diseases.13 However, despite the various beneficial therapeutic effects of ACE inhibitors, their efficacy in diabetic nephropathy is not perfect and has certain limitations.14 Therefore, in recent years, researchers have started exploring the combined use of other drugs to enhance the efficacy of ACE inhibitors, in order to further improve patient treatment outcomes and quality of life.
SGLT-2 inhibitors are a new type of oral medication for diabetes, which can reduce blood glucose levels by inhibiting the function of the SGLT-2 protein in the renal tubules and reducing the reabsorption of glucose by the kidneys.15 Unlike traditional oral hypoglycemic drugs, SGLT-2 inhibitors not only lower blood glucose levels, but also have other effects such as weight loss, improving hypertension, and improving insulin resistance.16 Dapagliflozin is an oral SGLT-2 inhibitor that has been shown to have some efficacy in the treatment of type 2 diabetes.17 By blocking the reabsorption of glucose by the renal tubules, dapagliflozin can lower blood glucose levels and reduce insulin resistance. In addition, dapagliflozin can also improve kidney function by reducing proteinuria levels, reducing blood pressure, and improving vascular function by reducing the permeability of the glomerular filtration membrane.18 So far, more and more studies19,20 have shown that dapagliflozin has potential therapeutic effects in the treatment of diabetic nephropathy. Therefore, this study attempted to add dapagliflozin to the treatment with enalapril maleate and explore the clinical therapeutic effects of adding dapagliflozin on diabetic nephropathy.
The results of this study showed that after treatment, the levels of Scr, BUN, UmAlb, UAER, UACR, and 24-hour urine protein quantification in the observation group were significantly lower than those in the control group (P<0.05). This result suggests that the addition of dapagliflozin can promote better recovery of renal function in patients compared to treatment with only enalapril. The reason for this may be that dapagliflozin can have a greater impact on tubuloglomerular feedback and can also improve conditions such as tubular hypertrophy, thereby playing a protective role in kidney function. Regarding clinical efficacy and blood glucose control, the results of this study showed that the total effective rate of the control group was 79.55%, while that of the observation group was 97.73%, which was significantly higher than that of the control group (P<0.05). After treatment, the levels of FPG, 2hPG, and HbAlc in the observation group were significantly lower than those in the control group (P<0.05). These results are consistent with previous related studies,21,22 which confirmed that the use of dapagliflozin can not only stabilize the blood glucose levels of diabetic nephropathy patients but also improve their treatment outcomes. The reason for this may be related to the inhibitory effect of dapagliflozin on SGLT-2 in the proximal tubules of the kidney during application, and it can also promote the excretion of urinary glucose in patients to some extent. Regarding inflammatory factors and adverse reactions, the results of this study showed that after treatment, the levels of hs-CRP, IL-1β, and TNF-α in the observation group were significantly lower than those in the control group (P<0.05), and the incidence of adverse reactions in the control group was 21.59%, while that in the observation group was 5.68%, which was significantly lower than that in the control group (P<0.05). These results indicate that the addition of dapagliflozin can improve the inflammatory response of diabetic nephropathy patients, avoid adverse reactions such as hypoglycemia and ketoacidosis, and has a relatively high safety profile. The reason for this may be that dapagliflozin can inhibit the infiltration of inflammatory cells in the body, which can effectively reduce the release of inflammatory factors in the kidneys, and it can also improve the expression of oxidative stress markers, which plays a crucial role in improving the inflammatory response in diabetic nephropathy. Additionally, dapagliflozin has a unique mechanism for reducing blood glucose levels, which can achieve good results in controlling abnormal blood glucose levels. Furthermore, when used in combination with other hypoglycemic drugs, dapagliflozin can reduce the dosage of hypoglycemic drugs, avoid the accumulation of drugs in the body due to high doses, and thereby reduce the likelihood of adverse reactions.
There are still several limitations in this study, including: ① This study was a single-center study with a relatively small sample size, which may limit the generalizability and representativeness of the results. ② This study lacked analysis of patient factors such as different types of diabetes and complications, which may affect the efficacy of Dapagliflozin treatment. ③ This study lacked long-term follow-up data, which makes it impossible to evaluate the safety and effectiveness of Dapagliflozin in long-term use. ④ This study only evaluated the role of Dapagliflozin in treating diabetic kidney disease and did not study its application in treating other diseases. Therefore, in future research, we will make further improvements to address these limitations and expand the representativeness of the study to make the results more generalizable.
Conclusion
Compared with monotherapy using enalapril maleate, the combination therapy of dapagliflozin for treating diabetic nephropathy showed more significant clinical effects. It can further control patients’ blood glucose levels, reduce their systemic inflammatory response, alleviate or eliminate patients’ proteinuria symptoms, promote the recovery of their renal function. Additionally, the combination therapy of dapagliflozin can also improve the safety of patients’ treatment to some extent, which helps to further improve the clinical treatment effect of patients.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Samsu N, Bellini MI. Diabetic nephropathy: challenges in pathogenesis, diagnosis, and treatment. Biomed Res Int. 2021;2021:1497449. doi:10.1155/2021/1497449
2. Sagoo MK, Gnudi L. Diabetic nephropathy: an overview. Methods Mol Biol. 2020;2067:3–7.
3. Zhao L, Zou Y, Liu F. Transforming growth factor-beta 1 in diabetic kidney disease. Front Cell Dev Biol. 2020;8:187. doi:10.3389/fcell.2020.00187
4. Widiasta A, Wahyudi K, Sribudiani Y, et al. Thelevel of transforming growth factor-β as a possible predictor of cyclophosphamide response in children with steroid-resistant nephrotic syndrome. Biomedicine. 2021;11(3):68–75. doi:10.37796/2211-8039.1205
5. Mosenzon O, Wiviott SD, Cahn A, et al. Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: an analysis from the DECLARE-TIMI 58 randomised trial. Lancet Diabetes Endocrinol. 2019;7(8):606–617. doi:10.1016/S2213-8587(19)30180-9
6. Heerspink HJL, Jongs N, Chertow GM, et al. Effect of dapagliflozin on the rate of decline in kidney function in patients with chronic kidney disease with and without type 2 diabetes: a prespecified analysis from the DAPA-CKD trial. Lancet Diabetes Endocrinol. 2021;9(11):743–754. doi:10.1016/S2213-8587(21)00242-4
7. Das SK, Roy DK, Chowdhury AA, et al. Correlation of eGFR By MDRD and CKD-EPI formula with creatinine clearance estimation in CKD patients and healthy subjects. Mymensingh Med J. 2021;30(1):35–42.
8. Nagib AM, Elsayed Matter Y, Ashry Gheith O, et al. Diabetic nephropathy following posttransplant diabetes mellitus. Exp Clin Transplant. 2019;17(2):138–146. doi:10.6002/ect.2018.0157
9. Koch EAT, Nakhoul R, Nakhoul F, et al. Autophagy in diabetic nephropathy: a review. Int Urol Nephrol. 2020;52(9):1705–1712. doi:10.1007/s11255-020-02545-4
10. Tung CW, Hsu Y-C, Shih Y-H, et al. Glomerular mesangial cell and podocyte injuries in diabetic nephropathy. Nephrology. 2018;23(Suppl 4):32–37. doi:10.1111/nep.13451
11. Bonner R, Albajrami O, Hudspeth J, et al. Diabetic Kidney Disease. Prim Care. 2020;47(4):645–659. doi:10.1016/j.pop.2020.08.004
12. Zhang L, Miao R, Yu T, et al. Comparative effectiveness of traditional Chinese medicine and angiotensin converting enzyme inhibitors, angiotensin receptor blockers, and sodium glucose cotransporter inhibitors in patients with diabetic kidney disease: a systematic review and network meta-analysis. Pharmacol Res. 2022;177:106111. doi:10.1016/j.phrs.2022.106111
13. National Institute of Diabetes and Digestive and Kidney Diseases. Enalapril, in LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012.
14. Fu EL, Clase CM, Evans M, et al. Comparative effectiveness of renin-angiotensin system inhibitors and calcium channel blockers in individuals with advanced CKD: a nationwide observational cohort study. Am J Kidney Dis. 2021;77(5):719–729.e1. doi:10.1053/j.ajkd.2020.10.006
15. Yamada T, Wakabayashi M, Bhalla A, et al. Cardiovascular and renal outcomes with SGLT-2 inhibitors versus GLP-1 receptor agonists in patients with type 2 diabetes mellitus and chronic kidney disease: a systematic review and network meta-analysis. Cardiovasc Diabetol. 2021;20(1):14. doi:10.1186/s12933-020-01197-z
16. Moon JS, Hong JH, Jung YJ, et al. SGLT-2 inhibitors and GLP-1 receptor agonists in metabolic dysfunction-associated fatty liver disease. Trends Endocrinol Metab. 2022;33(6):424–442. doi:10.1016/j.tem.2022.03.005
17. Jongs N, Greene T, Chertow GM, et al. Effect of dapagliflozin on urinary albumin excretion in patients with chronic kidney disease with and without type 2 diabetes: a prespecified analysis from the DAPA-CKD trial. Lancet Diabetes Endocrinol. 2021;9(11):755–1766. doi:10.1016/S2213-8587(21)00243-6
18. McMurray JJV, Wheeler DC, Stefánsson BV, et al. Effects of dapagliflozin in patients with kidney disease, with and without heart failure. JACC Heart Fail. 2021;9(11):807–820. doi:10.1016/j.jchf.2021.06.017
19. Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;883(15):1436–1446. doi:10.1056/NEJMoa2024816
20. Jongs N, Chertow GM, Greene T, et al. Correlates and consequences of an acute change in eGFR in response to the SGLT2 inhibitor dapagliflozin in patients with CKD. J Am Soc Nephrol. 2022;33(11):2094–2107. doi:10.1681/ASN.2022030306
21. Heerspink HJL, Stefansson BV, Chertow GM, et al. Rationale and protocol of the Dapagliflozin and Prevention of Adverse outcomes in Chronic Kidney Disease (DAPA-CKD) randomized controlled trial. Nephrol Dial Transplant. 2020;35(2):274–282. doi:10.1093/ndt/gfz290
22. Tuttle KR, Brosius FC, Cavender MA, et al. SGLT2 Inhibition for CKD and cardiovascular disease in type 2 diabetes: report of a scientific workshop sponsored by the National Kidney Foundation. Am J Kidney Dis. 2021;77(1):94–109. doi:10.1053/j.ajkd.2020.08.003
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

