Back to Journals » International Journal of General Medicine » Volume 16
Clinical Effect of Platelet-Rich Fibrin Combined with BIO-GENE Artificial Bone Meal in Bone Defects After Jaw Cyst Surgery
Authors Li A, Piao H, Zhang J, Cheng Q, Piao F, Cao C, Yan Y, Li J , Jin B
Received 2 September 2023
Accepted for publication 1 November 2023
Published 10 November 2023 Volume 2023:16 Pages 5225—5234
DOI https://doi.org/10.2147/IJGM.S431638
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Luca Testarelli
An Li,1 Huxiong Piao,1 Jiamin Zhang,2 Qingtao Cheng,1 Fangyu Piao,1 Chang Cao,1 Yuqi Yan,1 Jingxu Li,1 Bin Jin1
1Stomatology, Affiliated Hospital of Yanbian University, Yanji, People’s Republic of China; 2Department of Periodontal Mucosal Disease, The Affiliated Stomatological Hospital of Southwest Medical University, Luzhou, People’s Republic of China
Correspondence: Jingxu Li; Bin Jin, Email [email protected]; [email protected]
Purpose: To compare the clinical repair effects of leaving the defect empty and using Platelet-Rich Fibrin (PRF) combined with BIO-GENE artificial bone powder in patients with bone defects 6 months after jaw cystectomy.
Patients and Methods: From June 2021 to June 2022, 70 patients who were admitted to the Department of Stomatology, Affiliated Hospital of Yanbian University, and were diagnosed with jaw cysts postoperatively were selected. All of the patients were divided into two groups according to random method, among which 35 patients who underwent cystectomy alone were recorded as group A, which served as blank control; 35 patients who underwent cystectomy and PRF combined with BIO-GENE artificial bone meal repaired bone defects during the same period were recorded as group B. 3D Slicer 5.0.3 software was used to reconstruct Cone Beam Computed Tomography (CBCT) after operation. In this study, the preoperative and postoperative CBCT data of the patients were analyzed using 3D Slicer 5.0.3 software in DICOM format to calculate the cleft volume before surgery and the newly formed bone volume after surgery. The osteogenesis rate was measured based on these calculations.The bone formation percentage in the bone defect area was recorded at 6 months, and the clinical curative effects of the two groups were compared.
Results: After 6 months of surgery, the patients showed varying degrees of restoration in the jaw cyst area.The osteogenesis rate at 6 months in group A was 76.06± 13.38%, while group B had a rate of 92.87± 5.72%.The CBCT values in group B were higher than those in group A at 6 months post-surgery (P< 0.05), t=− 6.84.Group A and Group B showed a statistically significant difference.
Conclusion: Compared with simple cystectomy, PRF combined with BIO-GENE artificial bone powder has a better effect on the speed of bone repair after cystectomy within 6 months and provides more favorable effects for the repair of postoperative dentition defects, and provides support to repair teeth after defects such as dental implants.
Keywords: PRF, bone transplantation, jaw cysts, bone regeneration
Introduction
Various methods have been explored to treat intrabony periodontal defects, such as bone grafts (BG), guided tissue regeneration, and growth factors. These regenerative modalities aim to restore and repair the periodontal tissues affected by the defects.1
BIO-GENE is an allogeneic bone composed mainly of inorganic bone mineral components. It retains the three-dimensional grid structure of natural bone tissue and exhibits good cytocompatibility and bone conduction. It facilitates the adhesion, growth, and gene expression of bone cells.Over time, this product is finally completely replaced by crawling, and after bone healing, it permanently becomes the bone tissue of the human body. Among platelet concentrates, Platelet-rich fibrin (PRF) is a type of second-generation blood autologous preparation, which initially described by Choukroun et al.2 During the PRF preparation process, a gel-like matrix is formed. This matrix contains a high concentration of non-activated and functional platelets. These platelets are embedded within a fibrin matrix,2,3 which prevents the PRF rapid decomposition after application and allows for the gradual release of growth factors. This promotes the growth of new angiogenesis and supports the growth and differentiation of bone cells4,5 Accelerating the healing process of transplanted bone or bone substitutes would be beneficial for patients. Platelet-rich plasma (PRP) has shown promise in this regard, although its use remains controversial.6–21 Recent histological studies showed that the combination of PRF and BG yields greater benefits during bone healing compared to BG alone in maxillary sinus augmentation.22 In terms of treatment, there are a variety of options available for addressing the muscular aspects of postures-related temporomandibular disorders. (TMD), including physical therapy, relaxation techniques and muscle exercises.23,24 Intra-articular injection of PRF after joint replacement has a better effect on temporomandibular joint osteoarthritis (TMJ-OA).25 There is limited information on the use of PRF combined with artificial BG for treating infrabony defects. This study aimed to examine how PRF combined with artificial bone graft affects the recovery of jaw cysts after surgery. The study involved 70 patients who were carefully selected.
Materials and Methods
Research Object
This study ran from April 2021 to January 2023.A retrospective analysis was performed on patients with postoperative pathological diagnosis of jaw cyst at the Department of Stomatology of Yanbian University Hospital from June 2021 to January 2023.The patients were notified about the study protocol, including the benefits and risks, we get the patient’s consent, and they provided written informed consent to participate (a parent or legal guardian of patients under 18 years of age provided informed consent).The study obtained approval from the ethics committee of affiliated hospital of Yanbian University where the research took place (Ethics: 2023204).The study complies with the Declaration of Helsinki.A total of 70 cases met the inclusion exclusion criteria. According to the random number method, it was divided into two groups, among which 35 cases of simple cystectomy were recorded as group A; At the same time,35 cases of PRF combined with BIO-GENE artificial bone meal implantation were recorded as group B.
Inclusion Criteria:
(1) The postoperative pathological findings were jaw cyst;
(2) Jaw cyst surgery for the first time;
(3) The patient’s medical record data is completed and there are completed postoperative review records.
Exclusion Criteria:
(1) Patients with poor habits such as smoking and alcohol abuse that could affect postoperative bone healing;
(2) Patients with systemic diseases such as diabetes, hypertension, and heart disease;
(3) Patients with solid tumors such as ameloblastoma.
The study is a randomized clinical trial (RCT) with a 1:1 allocation ratio between two parallel arms.Additionally, the statistician who carried out the study analysis and the outcome analyst were both blinded.
Group A: patients range in age from 15 to 80 years, with an average of (33.34±11.25) years; There were 24 males and 11 females.
Group B: patients ranged in age from 17 to 73 years, with an average of (40.83±12.31) years; There were 20 cases in males and 15 cases in females.Age differences between two groups of patients have no statistical significance, P>0.05.
Materials and Equipment
C-TECH Medical Centrifuge (Changsha High & New Technology Industrial Development Zone Xiangyi Centrifuge Instrument Co., Ltd, Changsha, China), 10mL sterile additive-free glass test tube, Bio-GENE bone meal (Beijing Kejian Biotechnology Co., Ltd, Beijing, China).(shown in Figure 1). Haio Dental Prosthetic Membrane (Yantai Zhenghai Biotechnology Co., Ltd, Yantai, China).
 |
Figure 1 Bone repair materials: BIO-GENE. |
Method
The observation group was treated with a combination of PRF and artificial bone meal.The PRF was prepared according to the protocol described by Choukroun et al.2 Ten milliliters of blood were taken from each patient’s foot vein on the day of operation.In a sterile glass test tube without any anticoagulant, blood was drawn.The obtained blood was then immediately centrifuged in a chilled centrifugal machine for 15 minutes at 1400 rpm.As a result of the differential densities, the centrifugation process led to the separation of three distinct fractions. At the bottom, there was a layer of red blood cells, while on the surface, there was acellular plasma. In between these two layers, a clot of PRF was formed. The middle layer (PRF) was removed and put in a sterile dish after the topmost layer was pumped out using a sterile dropper.The transplant material was combined with this clot, as depicted in Figure 2.
The patient received cefuroxime, and the oral and periodontal regions were disinfected and draped before surgery, selects the location of the intraoral incision according to the site and size of the cyst, incises the mucoperiosteum, flips the mucoperiosteal flap, removes the bone wall to expose the cyst cavity, carefully peels off the cyst wall, removes the cyst wall as completely as possible, and completely removes the cyst. In Group A, the patients were treated with normal saline rinsing, hemostasis, biofilm covering, and suturing of the wound. In Group B, 2 units of BIO-GENE artificial bone meal were mixed with PRF and used to suture the mucosa of the operative area.
Observation Indicators
Percentage of Osteogenesis at Bone Defects
In this investigation, we used a 3-dimensional (3D) reconstructed Cone Beam Computed Tomography (CBCT) to quantify the volume of the bone defects, following the methodology of Feng et al.26 Six months following surgery, the patient’s CBCT was rebuilt using 3D Slicer 5.0.3 software.Using a CS 9300 3D unit (Carestream Dental LLC, Atlanta, GA, USA) with a field of view of 50mm×50mm and voxel size of 0.09mm for a period of 19.96s for a 180-degree rotation, the CBCT pictures were acquired.The software is used to draw and measure the cyst cavity and osteogenic volume, and calculate the percentage of osteogenesis at the bone defect. To avoid systematic error, all measurements were averaged three times by the same physician on the same computer.
Bone generation rate of artificial bone meal = (bone volume implanted at some time after surgery) ÷ (bone volume implanted immediately after surgery) × 100%
Bone generation rate without artificial bone meal = (immediate postoperative cyst volume - postoperative capsule volume) ÷ (immediate postoperative cystic volume) × 100%
Statistical Analysis
The analysis of the data was carried out using the Statistical Package for Social Sciences (SPSS) v.23.0.0 software (IBM Corp, Armonk, NY, USA).To evaluate statistical significance, normalize the two sets of data and assess separately if they follow a normal distribution, and the independent-samples T-test was performed.Statistics were judged significant at P<0.05.
Results
In this study, 70 patients in total were enrolled using the comparative analysis of 3D reconstruction methodology. The CBCT scans and 3D reconstruction images taken six months following surgery for patients in groups A and B both revealed new bone development in the alveolar bone defect area and variable degrees of restoration of the patients’ original jaw cyst area’s fullness.The defect of the jawbone has been restored to varying degrees, and there is no clear boundary between the newly formed bone tissue and the surrounding original bone tissue.In terms of imaging density, as depicted in Figure 3, there appears to be no distinction from the typical bone tissue.Based on the reconstructed 3D image, the osteogenesis range was determined, as shown in Figure 4, and the osteogenic rate was calculated.The osteogenic rate data of the two components are shown in Table 1.The statistical analysis revealed that groups A and B’s bone formation rates followed a normal distribution. Figure 5 displays the statistical results of bone rates in each group, The osteogenesis rate at 6 months in group A was 76.06±13.38%, while group B had a rate of 92.87±5.72%.The osteogenesis rate of the BIO-GENE+PRF group was found to be statistically significant at P<0.05 compared to the group that underwent cystectomy alone after jaw cyst surgery, supporting that the combination of BIO-GENE+PRF is effective for repairing bone defects after jaw cyst surgery, as shown in Table 2. The CBCT values in group B were higher than those in group A at 6 months post-surgery.The study had 70 patients in total. There were 24 males and 11 females in the group A, ranging in age from 19 to 52 (average 33.34±11.25 years).20 males and 15 females, aged 18 to 58 (mean 40.83±12.31 years), made up the B group.The variations in osteogenesis rates between group A and group B were not statistically significant.
 |
Table 1 The osteogenic rate data |
 |
Table 2 (A) Independent-Samples T-test and (B) Group Statistics |
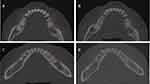 |
Figure 3 Image performance. (A) Group A before surgery. (B) A group of six months after surgery. (C) Group B before surgery. (D) B group of six months after surgery. |
 |
Figure 5 Group A and B component bone rate data conform to a normal distribution. |
Discussion
In recent years, autologous PRF has been affirmed and clinically applied by doctors because it brings together high concentrations of growth factors that promote bone and soft tissue repair and regeneration, and has affinity and no immune rejection.27 PRF can aid in the repair of the alveolar bone structure by promoting the growth and differentiation of homing stem cells.27,28 Platelets, white blood cells, growth factors, and fibrin are all abundant in PRF.When the platelets are activated to release a large number of growth factors, fibrinogen slowly polymerizes under the action of physiological thrombin, chemically combines the released growth factors and platelets, and slowly releases internal growth factors again with fiber degradation during fiber matrix reconstruction, prolongs the action time of growth factors, increases the effect of promoting osteogenesis, and induces microvascular formation, plays an effective role in oral soft and hard tissue trauma and defect repair, and has a good immunomodulatory effect. PRF has been used in periodontal therapy, implant surgery, TMD, jaw cyst and other oral related treatment. Cross-infection and immune rejection are well avoided.29 By using safe and effective bone grafting materials, the clinical efficacy of patients can be significantly improved.30 Various graft materials and biomaterials, such as allografts31 and autografts,32 are utilized in treating bone defects.33 The main focus of medical attention is the complicated process of bone repair.The synchronization of several cells and biological processes is essential for bone repair.34 The data from the study mentioned supports the clinical efficacy of combining PRF with BIO-GENE artificial bone meal has better prognosis in the repair of bone defects following jaw cyst surgery. These findings align with those presented by Miron et al in 2021.35 In simpler terms, some studies have shown that using PRF along with hydroxyapatite can provide significant benefits to patients. PRF has been found to have a positive effect on the cells responsible for bone formation in the alveolar bone and dental follicles. It promotes the growth of bone progenitor cells and enhances the differentiation of osteoblasts.36
Another study conducted on rats investigated the combination of PRF with a bone graft material called Bio-Oss. This study found a synergistic effect between PRF and Bio-Oss. Employing an autogenous graft and PRF combination was more successful than using an alternative different type of graft material, the difference was highly significant.37
The strengths of this study include having the same surgeon perform all surgeries, strict criteria for selecting patients, and the use of 3D reconstruction software for accurate evaluation of the rate of bone formation after grafting.Compared to earlier 2D evaluations, this is thought to be a more accurate predictor of bone healing.The limited sample size, the varied nature of the flaws, and the short-term follow-up are some of the study’s drawbacks.Histological evaluations of the postoperative healing process and the genuine nature of bone regeneration were not performed. Patient-related variables such to their age, sex, individual platelet counts, and biological particle sedimentation rates may also have had an impact on the quality of PRF generated.
Conclusion
Within the study’s limitations, we discovered that utilizing PRF in combination with BIO-GENE artificial bone meal after 6 months of jaw cyst surgery leads to a greater extent of osseous formation when compared to the control group. These findings suggest that the use of PRF combined with BIO-GENE artificial bone meal is a favorable choice for addressing bone defects following jaw cyst surgery, especially when rapid bone formation is prioritized in the short term.
Abbreviations
Abbreviations used in the text are in Table 3.
 |
Table 3 Abbreviations Used in the Text |
Funding
This research was supported by the International Exchange Program, Postdoctoral Management Office [2018] No.115.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Darby I. Darby I.Periodontal materials. Aust Dent J. 2011;56(Suppl 1):107–118. doi:10.1111/j.1834-7819.2010.01301.x
2. Dohan DM, Choukroun J, Diss A, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part I: technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(3):e37–e44. doi:10.1016/j.tripleo.2005.07.008
3. Davis VL, Abukabda AB, Radio NM, et al. Platelet-rich preparations to improve healing. Part II: platelet activation and enrichment, leukocyte inclusion, and other selection criteria. J Oral Implantol. 2014;40(4):511–521. doi:10.1563/AAID-JOI-D-12-00106
4. Tabrizi R, Arabion H, Karagah T. Does platelet-rich fibrin increase the stability of implants in the posterior of the maxilla? A split-mouth randomized clinical trial. Int J Oral Maxillofac Surg. 2018;47(5):672–675. doi:10.1016/j.ijom.2017.07.025
5. Dohan Ehrenfest DM, Rasmusson L, Albrektsson T. Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol. 2009;27(3):158–167.
6. Sánchez AR, Sheridan PJ, Kupp LI. Is platelet-rich plasma the perfect enhancement factor? A current review. Int J Oral Maxillofac Implants. 2003;18(1):93–103.
7. Froum SJ, Wallace SS, Tarnow DP, Cho SC. Effect of platelet-rich plasma on bone growth and osseointegration in human maxillary sinus grafts: three bilateral case reports. Int J Periodontics Restorative Dent. 2002;22(1):45–53.
8. Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR. Platelet-rich plasma: growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85(6):638–646. doi:10.1016/S1079-2104(98)90029-4
9. Zechner W, Tangl S, Tepper G, et al. Influence of platelet-rich plasma on osseous healing of dental implants: a histologic and histomorphometric study in minipigs. Int J Oral Maxillofac Implants. 2003;18(1):15–22.
10. Wiltfang J, Schlegel KA, Schultze-Mosgau S, Nkenke E, Zimmermann R, Kessler P. Sinus floor augmentation with beta-tricalciumphosphate (beta-TCP): does platelet-rich plasma promote its osseous integration and degradation? Clin Oral Implants Res. 2003;14(2):213–218. doi:10.1034/j.1600-0501.2003.140212.x
11. Soffer E, Ouhayoun JP, Anagnostou F. Fibrin sealants and platelet preparations in bone and periodontal healing. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95(5):521–528. doi:10.1067/moe.2003.152
12. Aghaloo TL, Moy PK, Freymiller EG. Investigation of platelet-rich plasma in rabbit cranial defects: a pilot study. J Oral Maxillofac Surg. 2002;60(10):1176–1181. doi:10.1053/joms.2002.34994
13. Aghaloo TL, Moy PK, Freymiller EG. Evaluation of platelet-rich plasma in combination with anorganic bovine bone in the rabbit cranium: a pilot study. Int J Oral Maxillofac Implants. 2004;19(1):59–65.
14. Choi BH, Im CJ, Huh JY, Suh JJ, Lee SH. Effect of platelet-rich plasma on bone regeneration in autogenous bone graft. Int J Oral Maxillofac Surg. 2004;33(1):56–59. doi:10.1054/ijom.2003.0466
15. Grageda E. Platelet-rich plasma and bone graft materials: a review and a standardized research protocol. Implant Dent. 2004;13(4):301–309. doi:10.1097/01.id.0000148555.91063.06
16. Jakse N, Tangl S, Gilli R, et al. Influence of PRP on autogenous sinus grafts An experimental study on sheep. Clin Oral Implants Res. 2003;14(5):578–583. doi:10.1034/j.1600-0501.2003.00928.x
17. Jensen TB, Rahbek O, Overgaard S, Søballe K. Platelet rich plasma and fresh frozen bone allograft as enhancement of implant fixation An experimental study in dogs. J Orthop Res. 2004;22(3):653–658. doi:10.1016/j.orthres.2003.10.006
18. Jensen TB, Rahbek O, Overgaard S, Søballe K. No effect of platelet-rich plasma with frozen or processed bone allograft around noncemented implants. Int Orthop. 2005;29(2):67–72. doi:10.1007/s00264-004-0622-6
19. Mazor Z, Peleg M, Garg AK, Luboshitz J. Platelet-rich plasma for bone graft enhancement in sinus floor augmentation with simultaneous implant placement: patient series study. Implant Dent. 2004;13(1):65–72. doi:10.1097/01.ID.0000116454.97671.40
20. Oyama T, Nishimoto S, Tsugawa T, Shimizu F. Efficacy of platelet-rich plasma in alveolar bone grafting. J Oral Maxillofac Surg. 2004;62(5):555–558. doi:10.1016/j.joms.2003.08.023
21. Wiltfang J, Kloss FR, Kessler P, et al. Effects of platelet-rich plasma on bone healing in combination with autogenous bone and bone substitutes in critical-size defects An animal experiment. Clin Oral Implants Res. 2004;15(2):187–193. doi:10.1111/j.1600-0501.2004.00980.x
22. Bölükbaşı N, Yeniyol S, Tekkesin MS, Altunatmaz K. The use of platelet-rich fibrin in combination with biphasic calcium phosphate in the treatment of bone defects: a histologic and histomorphometric study. Curr Ther Res Clin Exp. 2013;75:15–21. doi:10.1016/j.curtheres.2013.05.002
23. Minervini G, Franco R, Marrapodi MM, Ronsivalle V, Shapira I, Cicciù M. Prevalence of temporomandibular disorders in subjects affected by Parkinson disease: a systematic review and metanalysis. J Oral Rehabil. 2023;50(9):877–885. doi:10.1111/joor.13496
24. Minervini G, Franco R, Marrapodi MM, et al. Correlation between Temporomandibular Disorders (TMD) and Posture Evaluated trough the Diagnostic Criteria for Temporomandibular Disorders (DC/TMD): a systematic review with meta-analysis. J Clin Med. 2023;12(7):2652. doi:10.3390/jcm12072652
25. Işık G, Kenç S, Özveri Koyuncu B, Günbay S, Günbay T. Injectable platelet-rich fibrin as treatment for temporomandibular joint osteoarthritis: a randomized controlled clinical trial. J Craniomaxillofac Surg. 2022;50(7):576–582. doi:10.1016/j.jcms.2022.06.006
26. Feng B, Jiang M, Xu X, Li J. A new method of volumetric assessment of alveolar bone grafting for cleft patients using cone beam computed tomography. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;124(2):e171–e182. doi:10.1016/j.oooo.2017.04.003
27. Ray HL, Marcelino J, Braga R, Horwat R, Lisien M, Khaliq S. Long-term follow up of revascularization using platelet-rich fibrin. Dent Traumatol. 2016;32(1):80–84. doi:10.1111/edt.12189
28. Chang YC, Yu HC, Huang FM. Anterior maxillary ridge splitting with simultaneous implant placement using platelet-rich fibrin as the sole grafting material. J. Dent Sci. 2016;11(1):110–112. doi:10.1016/j.jds.2015.11.001
29. Kökdere NN, Baykul T, Findik Y. The use of platelet-rich fibrin (PRF) and PRF-mixed particulated autogenous bone graft in the treatment of bone defects An experimental and histomorphometrical study. Dent Res J (Isfahan). 2015;12(5):418–424. doi:10.4103/1735-3327.166188
30. Thomas B, Stedman M, Davies L. Grade as a prognostic factor in oral squamous cell carcinoma: a population-based analysis of the data. Laryngoscope. 2014;124(3):688–694. doi:10.1002/lary.24357
31. Bullens PH, Minderhoud NM, de Waal Malefijt MC, Veth RP, Buma P, Schreuder HW. Survival of massive allografts in segmental oncological bone defect reconstructions. Int Orthop. 2009;33(3):757–760. doi:10.1007/s00264-008-0700-2
32. Bigham-Sadegh A, Oryan A. Basic concepts regarding fracture healing and the current options and future directions in managing bone fractures. Int Wound J. 2015;12(3):238–247. doi:10.1111/iwj.12231
33. Dreyer CH, Rasmussen M, Pedersen RH, Overgaard S, Ding M. Comparisons of efficacy between autograft and allograft on defect repair in vivo in normal and osteoporotic rats. Biomed Res Int. 2020;2020:9358989. doi:10.1155/2020/9358989
34. Einhorn TA, Gerstenfeld LC. Fracture healing: mechanisms and interventions. Nat Rev Rheumatol. 2015;11(1):45–54. doi:10.1038/nrrheum.2014.164
35. Miron RJ, Moraschini V, Fujioka-Kobayashi M, et al. Use of platelet-rich fibrin for the treatment of periodontal intrabony defects: a systematic review and meta-analysis. Clin Oral Investig. 2021;25(5):2461–2478. doi:10.1007/s00784-021-03825-8
36. Zumstein MA, Berger S, Schober M, et al. Leukocyte- and platelet-rich fibrin (L-PRF) for long-term delivery of growth factor in rotator cuff repair: review, preliminary results and future directions. Curr Pharm Biotechnol. 2012;13(7):1196–1206. doi:10.2174/138920112800624337
37. Oliveira MR, deC Silva A, Ferreira S, Avelino CC, Garcia IR, Mariano RC. Influence of the association between platelet-rich fibrin and bovine bone on bone regeneration. A histomorphometric study in the calvaria of rats. Int J Oral Maxillofac Surg. 2015;44(5):649–655. doi:10.1016/j.ijom.2014.12.005
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


