Back to Journals » Journal of Inflammation Research » Volume 16
Clinical Effect of Electroacupuncture on Acute Pancreatitis: Efficacies and Mechanisms
Received 1 March 2023
Accepted for publication 20 July 2023
Published 28 July 2023 Volume 2023:16 Pages 3197—3203
DOI https://doi.org/10.2147/JIR.S410618
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Xianqiang Yu,1,2 Qian Zhu3
1Women and Children’s Hospital Affiliated to Qingdao University, Qingdao, People’s Republic of China; 2University of California, Los Angeles, Department of Cardiology, Los Angeles, CA, USA; 3Department of Hepatobiliary Surgery, Zhongnan Hospital of Wuhan University, Wuhan, People’s Republic of China
Correspondence: Qian Zhu, Department of Hepatobiliary and Pancreatic Surgery, Pancreatic Surgery Center, Zhongnan Hospital of Wuhan University, Wuhan, Hubei Province, 430071, People’s Republic of China, Email [email protected]
Abstract: Acute pancreatitis (AP) is a common acute abdomen and the number one cause of acute digestive hospitalizations in the United States. Abdominal pain is the main clinical manifestation of abdominal symptoms, so reducing the abdominal symptoms caused by inflammation is very important to alleviate the pain of patients. Electroacupuncture (EA) as a traditional Chinese medicine (TCM) therapy has significant conditioning effects on various inflammatory diseases including AP. Continuous studies in recent years have shown that EA conditioning has significant effects on reducing inflammation and regulating gastrointestinal symptoms in AP. At the same time, there is sufficient evidence to further elucidate the mechanism of EA. In this review, we will summarize the effect of EA on AP and its mechanism, so as to better serve clinical practice in the treatment of AP in the future.
Keywords: electroacupuncture, acute pancreatitis, traditional Chinese medicine, TCM
Introduction
Acute pancreatitis (AP) is the most common cause of gastroenterology-related hospitalization in the United States with more than 220,000 annual admissions.1,2 At present, it is generally believed that AP is an inflammatory disease activated by trypsin initiated by biliary diseases, hyperlipidemia, alcohol and other reasons, which further leads to the self-digestion, edema, bleeding and even necrosis of pancreatic tissues.3–7 The severity of pancreatic inflammation varies. Mild acute pancreatitis (MAP) can be basically recovered within 3–5 days after conservative treatment, but about 20% AP still develops into severe acute pancreatitis (SAP) with systemic inflammatory response and multiple organ failure.5,8 When the disease progresses to severe stages, it can further lead to multiple organ dysfunction syndrome (MODS) and in some cases organ failure or even death.9 AP and some of its complications are often treated with symptomatic support (eg fluid resuscitation, enteral feeding, antibiotics).10–13 At present, there is no specific treatment or drug for AP.
In clinical practice, the main treatment means of Western medicine is supportive therapy, with symptomatic treatment according to the body symptoms, such as fasting, water abstinence, gastric empty-out, inhibition of gastric acid secretion, anti-infection, antispasmolysis and analgesia.14 Modern pharmacokinetic evidence shows that Traditional Chinese Medicine (TCM) therapy can simultaneously regulate anti-inflammatory factors and pro-inflammatory factors, so as to treat inflammatory diseases more effectively.15–18 In the Chinese Guidelines for the Diagnosis and Treatment of Acute Pancreatitis, single rhubarb and compound preparations, such as Qingyi Decoction and ChaishaoChengqi decoction, have been included in the treatment regimen.19 Acupuncture as a characteristic intervention means of traditional Chinese medicine has positive clinical significance for analgesia, promoting gastrointestinal creep and regulating gastrointestinal immune function.20 Studies have shown that when acute pancreatitis progresses to SAP stage, it will cause intestinal paralysis, and then abdominal distension, abdominal pain, and anal stop exhaust defecation.19 Acupuncture has been studied for its potential analgesic effects in various pain conditions. A study investigated the use of acupuncture for pain relief in patients with chronic pancreatitis.21 The study reported that acupuncture provided significant pain relief and improved quality of life in the participants. However, more research is needed to evaluate the efficacy of acupuncture specifically for AP.
Electroacupuncture (EA), another traditional Chinese treatment, has also been used to manage AP.19,22–26 Studies demonstrated that EA may promote analgesia and anti-inflammatory effects in patients with back pain and arthritis, while another study showed that it may regulate inflammatory responses in rats with AP.27,28 EA treatment has a good effect in the rat model of AP, especially in recent years, clinical studies have shown that EA treatment of AP may benefit patients to a large extent.29,30 From this perspective, EA has a landmark significance in changing the clinical course of AP and improving the prognosis of patients and makes it possible for this traditional Chinese treatment method to be widely recommended in clinical practice.
EA plays a role in regulating gastrointestinal function by stimulating acupoints (Figure 1). Some scholars used EA to stimulate the lung Shu point (BL13) to regulate inflammatory mediators, thus reducing the severity of viral pneumonia.31 Studies have also shown that electrical stimulation of Zusanli can significantly reduce abdominal pain and abdominal distension and other gastrointestinal symptoms caused by severe acute pancreatitis.32–34 Although the mechanism by which EA alleviates gastrointestinal symptoms has not been fully elucidated, its clinical effect of significantly regulating inflammation and alleviating disease has potential value. The clinical effects of EA in Western medicine are primarily studied and reported in the context of pain management. EA is a technique that combines traditional acupuncture with electrical stimulation, and it has been explored as an adjunct therapy to conventional Western medicine interventions. Clinical trials have shown that EA can provide pain relief and improve symptoms in conditions such as chronic musculoskeletal pain, osteoarthritis, postoperative pain, and chemotherapy-induced neuropathy.33 EA has also been investigated for its potential role in rehabilitation and functional improvement. Studies have suggested that it may help improve motor function and physical performance in conditions of stroke.35 It’s important to note that the clinical effects of electroacupuncture can vary depending on the specific condition being treated, individual patient factors, and the expertise of the practitioner.
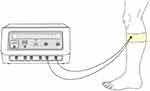 |
Figure 1 Electroacupuncture treatment site. |
Therefore, exploring the specific mechanism of EA regulating gastrointestinal function will help to understand the principle of its function. In this review, we will discuss the potential effects of EA on AP and its mechanisms.
Mechanisms
The esophagus is the passage that carries food to the stomach. At the gastroesophageal junction (GEJ), there is a thickened layer of muscle called the lower esophageal sphincter (LES). Esophageal motility abnormalities are classified into different types according to the function and contraction mode of LES.36 Recent studies have reported the effect of EA on esophageal dyskinesia. EA stimulation increases LES pressure (LESP) and peak peristalsis amplitude.37
Gastric motility is one of the most important physiological functions of the human intestine. Without coordinated movement, digestion and absorption of dietary nutrients cannot occur. To perform its function efficiently, the intestine needs to produce not only simple contractions, but also coordinated contractions to produce transport (peristalsis) of luminal contents. Studies have shown that EA at ST36 restored the impaired gastric regulation induced by vagal nerve transection in dogs, but had no effect on gastric regulation in normal dogs.38 In addition, electrical stimulation significantly increased the number and amplitude of peak gastric EMG activity, suggesting increased gastric contractions after stimulation.39
The small intestine moves in two different patterns: fasting and eating. The typical manifestation of fasting is the migration motor complex (MMC). Intestinal dyskinesia include loss of MMC, MMC damage. In experiments with rats, the investigators observed that EA at hind limb points (ST36 and SP6) significantly enhanced small intestinal transport.40
There are staged contractions and large transitional contractions in the colon. Disrupted colonic motility is associated with a variety of functional disorders, such as irritable bowel syndrome (IBS), constipation, and diarrhea. EA stimulation increased colonic transport processes through the sacral parasympathetic efferent pathway.41 Similarly, EA stimulation of ST36 significantly increased contractility in the distal colon.
In 2021, Ma et al conducted an in-depth study on the neuroanatomy of EA stimulation therapy, and they confirmed that the physiological mechanism of electroacupuncture stimulation at ST36 site in mice is realized by driving the vagal adrenal axis.42 Therefore, EA has become an important method for neuroimmune regulation of AP to play an anti-inflammatory role, and provides a theoretical basis for us to understand its working mechanism (Figure 2).
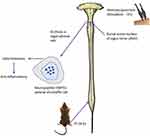 |
Figure 2 Schematic diagram of the mechanism of electroacupuncture. |
Significance
EA has been found to provide effective pain relief in various acute and chronic pain conditions. It can be used as an adjunct therapy alongside conventional pain management approaches, such as medication or physical therapy. The electrical stimulation delivered during electroacupuncture is believed to enhance the analgesic effects of traditional acupuncture, potentially leading to improved pain control and patient outcomes. With growing concerns about the overuse and side effects of pain medications, non-pharmacological approaches have gained significance in Western medicine. EA offers a non-invasive, drug-free option for pain management, providing an alternative or complement to traditional pharmacotherapy. This makes it a valuable therapeutic option, particularly for individuals who prefer non-pharmacological interventions or who may have limitations in using certain medications. EA aligns with the principles of holistic and patient-centered care. It acknowledges the interconnectedness of physical, mental, and emotional well-being and seeks to address pain and related symptoms from a comprehensive perspective. By considering the individual patient’s needs and preferences, EA contributes to personalized pain management approaches that focus on the overall well-being of the patient. While research on EA continues to evolve, there is a growing body of evidence supporting its effectiveness in pain management. Randomized controlled trials, systematic reviews, and meta-analyses have provided insights into the analgesic effects of EA. This accumulating evidence helps validate its significance in Western medicine and guides its integration into clinical practice.
Discussion
AP is a common inflammatory disease of the pancreas with an unpredictable clinical course and increasing clinical and economic burden. Studies have shown that AP costs account for 0.1% of total annual medical costs or more than US $2 billion in hospitalization costs, placing a severe financial burden on the health care system.43 It is clear that AP has brought considerable financial burden and suffering to patients including those in China. SAP is often caused by a variety of pathological mechanisms of intestinal paralysis, such as abdominal distension, abdominal pain and anus stop exhaust defecation and other symptoms.19 Duration of intestinal paralysis is an important factor affecting the natural course of SAP, and the incidence of systemic inflammatory response syndrome (SIRS) and MODS increases significantly with the course of SAP.24 The mechanism of its occurrence is the injury of acini cells caused by different etiologies, excessive activation of neutrophils and release of a large number of inflammatory transmitters, and then damage multiple organs through complex chain reaction and amplification effect.44 Therefore, effective and affordable new therapies are urgently needed for AP, and the high mortality associated with organ failure will only be reduced when specific treatment options are developed to target the fundamental factors that drive the pathophysiology of SIRS and MODS. A large number of studies have also shown that acupuncture and moxibustion intervention has positive clinical significance in analgesia, promoting gastrointestinal motility and regulating gastrointestinal immune function.45–47 In addition, acupuncture and moxibustion treatment of chronic gastritis, digestive ulcer and other digestive system diseases have also achieved good effect.48–50
Based on the therapeutic effect of acupuncture and moxibustion, some scholars have tried to use EA stimulation to treat AP and obtained positive effects. Zhu et al found that EA treatment may reduce the severity of AP by increasing the production of IL-10 and shorten the time to oral re-feeding in patients with both mild and severe AP.25 This is consistent with Li et al conclusion that the possible explanation is that increased IL-10 levels may reduce the risk of SIRS.26 In addition, both Li Ming’s and Wang Ming’s studies showed significant differences in the three indicators (recovery time of bowel sound, remission time of abdominal pain and recovery time of defecation) before and after EA treatment, which further verified the regulatory effect of EA on gastrointestinal function of AP.22,24 This fully shows that EA has the same effect of regulating gastrointestinal function as acupuncture. At the same time, the study of Zhu et al, Zhao et al and Luo et al found that EA treatment of AP significantly shortened the hospitalization time, which was consistent with its therapeutic effect.22,24–26 The positive results of these indicators after integrated analysis fully indicate that EA therapy for AP plays a key role in regulating gastrointestinal peristalsis and gastrointestinal immunity. EA as a convenient and effective treatment strategy seems to bring a new therapeutic concept to AP.
According to the classical Chinese medicine “the abdomen viscera unobstructed is the key, if not unobstructed will pain” theory, the treatment should be purging the bowels, clearing away heat and detoxification, removing blood stasis and guiding stagnation, invigorating and dispelling pathogenic factors.22 At present, there is still no good method for intestinal paralysis caused by SAP progressing to multi-organ dysfunction. In particular, these gastrointestinal dynamic changes further exacerbate the breakdown of intestinal barrier integrity, leading to severe bacterial displacement, systemic inflammatory response, which further aggravates the enterogenic endotoxemia.23
Studies have showed that EA can regulate the release of inflammatory factors and immunologic function.51 The pituitary-adrenal cortical system can play a bidirectional benign regulation role when stimulating Zusanli acupoint and improve the immune level.52 More high-quality RCTs with suitable study cohorts are needed to ascertain the efficacy of EA for AP.
It is worth affirming that none of the patients who received EA experienced serious discomfort or complications, suggesting that EA is a safe intervention for AP. In addition, some patients are reluctant to accept this novel intervention in clinical practice, which means that the correct promotion of the therapeutic effect of EA and the selection of experienced operators are the key to clinical application. What’s more, as a classic treatment method in traditional Chinese medicine, EA can meet the needs of patients under different conditions due to its easy operation and repeatable use of equipment.
Despite the high quality of the current RCTS, these articles are not double-blinded design, which may affect the objectivity of the results and cause subjective bias. We could not infer the long-term efficacy of EA and traditional treatment for treatment of AP. Secondly, at present most of the studies on EA conditioning AP did not stratify the severity of AP. In spite of this, we hope that the novel EA method for AP introduced in this article can be applied in clinical treatment more quickly and widely. However, there are few RCTs on EA in the treatment of AP at present, so more high-quality studies are needed.
Conclusion
In this review, we found the therapeutic effect of EA is obvious than that of traditional therapy on major clinical outcomes in patients with AP. In particular, EA can improve gastrointestinal function by regulating intestinal paralysis. The available research suggests that electroacupuncture may have potential benefits in modulating inflammatory responses in acute pancreatitis. However, due to the limited number of studies and the lack of well-designed clinical trials, further research is needed to establish the efficacy, safety, and optimal application of electroacupuncture specifically for acute pancreatitis. Consulting with healthcare professionals experienced in acupuncture and integrative medicine can provide further guidance on the potential use of electroacupuncture in the management of acute pancreatitis. We look forward to more high-quality RCT to further validate the therapeutic value of EA for AP.
Data Sharing Statement
The datasets used or analysed during the current study are available from the corresponding author on reasonable request.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Program of Excellent Doctoral (Postdoctoral) of Zhongnan Hospital of Wuhan University (Grant No. ZNYB2019007), the National Natural Science Foundation of China (Grant No. 82002589), and the Cancer Research and Transformation Platform Project of Zhongnan Hospital of Wuhan University (Grant No. ZLYNXM202004).
Disclosure
The authors declare that they have no competing interests.
References
1. Peery AF, Dellon ES, Lund J, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143(5):1179–1187.e3. doi:10.1053/j.gastro.2012.08.002
2. Fagenholz PJ, Fernández-del Castillo C, Harris NS, et al. Direct medical costs of acute pancreatitis hospitalizations in the United States. Pancreas. 2007;35(4):302–307. doi:10.1097/MPA.0b013e3180cac24b
3. Yang AL, Vadhavkar S, Singh G, Omary MB. Epidemiology of alcohol-related liver and pancreatic disease in the United States. Arch Intern Med. 2008;168(6):649–656. doi:10.1001/archinte.168.6.649
4. Windsor JA, Escott A, Brown L, et al. Novel strategies for the treatment of acute pancreatitis based on the determinants of severity. J Gastroenterol Hepatol. 2017;32(11):1796–1803. doi:10.1111/jgh.13784
5. Forsmark CE, Vege SS, Wilcox CM, Campion EW. Acute Pancreatitis. N Engl J Med. 2016;375(20):1972–1981. doi:10.1056/NEJMra1505202
6. Parniczky A, Lantos T, Tóth EM, et al. Antibiotic therapy in acute pancreatitis: from global overuse to evidence based recommendations. Pancreatology. 2019;19(4):488–499. doi:10.1016/j.pan.2019.04.003
7. Lerch MM. Classifying an unpredictable disease: the revised Atlanta classification of acute pancreatitis. Gut. 2013;62(1):2–3. doi:10.1136/gutjnl-2012-303724
8. Trikudanathan G, Wolbrink DRJ, van Santvoort HC, Mallery S, Freeman M, Besselink MG. Current concepts in severe acute and necrotizing pancreatitis: an evidence- based approach. Gastroenterology. 2019;156(7):1994–2007.e3. doi:10.1053/j.gastro.2019.01.269
9. Cosen-Binker LI, Binker MG, Negri G, et al. Acute pancreatitis possible initial triggering mechanism and prophylaxis. Pancreatology. 2003;3(6):445–456. doi:10.1159/000074972
10. Wu C, Wang X, Jiang T, et al. Improved effect of continuous renal replacement therapy in metabolic status and body composition of early phase of acute pancreatitis. Int J Artif Organs. 2015;38(10):523–529. doi:10.5301/ijao.5000444
11. Li G, Pan Y, Zhou J, et al. Enteral nutrition tube placement assisted by ultrasonography in patients with severe acute pancreatitis: a novel method for quality improvement. Medicine. 2017;96(45):e8482. doi:10.1097/MD.0000000000008482
12. Tong Z, Shen X, Ke L, et al. The effect of a novel minimally invasive strategy for infected necrotizing pancreatitis. Surg Endosc. 2017;31(11):4603–4616. doi:10.1007/s00464-017-5522-0
13. Jiang W, Tong Z, Yang D, et al. Gastrointestinal fistulas in acute pancreatitis with infected pancreatic or peripancreatic necrosis: a 4-year single-center experience. Medicine. 2016;95(14):e3318. doi:10.1097/MD.0000000000003318
14. Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology. 2013;13(Suppl 2):e1–e15. doi:10.1016/j.pan.2013.07.063
15. Li J, Xu L, Sang R, et al. Immunomodulatory and anti-inflammatory effects of total flavonoids of Astragalus by regulating NF-KappaB and MAPK signalling pathways in RAW 264.7 macrophages. Pharmazie. 2018;73(10):589–593. doi:10.1691/ph.2018.8633
16. Pan MH, Chiou YS, Tsai M-L, et al. Anti-inflammatory activity of traditional Chinese medicinal herbs. J Tradit Complement Med. 2011;1(1):8–24. doi:10.1016/S2225-4110(16)30052-9
17. Zou YH, Zhao L, Xu Y-K, et al. Anti-inflammatory sesquiterpenoids from the Traditional Chinese Medicine Salvia plebeia: regulates pro-inflammatory mediators through inhibition of NF-kappaB and Erk1/2 signaling pathways in LPS-induced Raw264.7 cells. J Ethnopharmacol. 2018;210:95–106. doi:10.1016/j.jep.2017.08.034
18. Kodithuwakku ND, Pan M, Zhu Y-L, et al. Anti-inflammatory and antinociceptive effects of Chinese medicine SQ gout capsules and its modulation of pro-inflammatory cytokines focusing on gout arthritis. J Ethnopharmacol. 2013;150(3):1071–1079. doi:10.1016/j.jep.2013.10.016
19. Li J, Zhao Y, Wen Q, et al. 电针干预重症急性胰腺炎伴麻痹性肠梗阻:随机对照研究 [Electroacupuncture for severe acute pancreatitis accompanied with paralytic ileus: a randomized controlled trial]. Zhongguo Zhen Jiu. 2016;36(11):1126–1130. Chinese. doi:10.13703/j.0255-2930.2016.11.002
20. Zhang SY, Du YQ. 温针灸对肠癌术后患者胃肠功能及免疫功能的影响 [Effects of warming needle moxibustion on improvement of gastrointestinal and immune function in patients with postoperation of colorectal cancer]. Zhongguo Zhen Jiu. 2011;31(6):513–517. Chinese.
21. Juel J, Liguori S, Liguori A, et al. Acupuncture for pain in chronic pancreatitis: a single-blinded randomized crossover trial. Pancreas. 2017;46(2):170–176. doi:10.1097/MPA.0000000000000749
22. Zhao L, Li X, Shi Z, et al. 电针大肠俞、上巨虚穴辅助乌司他丁治疗重症急性胰腺炎临床观察 [Clinical observation on severe acute pancreatitis treated with electroacupuncture at Dachangshu (BL 25) and Shangjuxu (ST 37) combined with ulinastatin]. Zhongguo Zhen Jiu. 2018;38(2):132–136. Chinese. doi:10.13703/j.0255-2930.2018.02.005
23. Wang XY. 电针治疗急性胰腺炎及对患者肠道通透性的影响 [Electroacupuncture for treatment of acute pancreatitis and its effect on the intestinal permeability of the patient]. Zhongguo Zhen Jiu. 2007;27(6):421–423. Chinese.
24. Luo YH, Zhong GW, Zhao S-P, et al. 应用电针干预重症早期急性胰腺炎并发肠麻痹 [Efficacy observation of electroacupuncture intervention on severe acute pancreatitis at early stage complicated with intestinal paralysis]. Zhongguo Zhen Jiu. 2011;31(2):105–109. Chinese.
25. Zhu SF, Guo H, Zhang -R-R, et al. Effect of electroacupuncture on the inflammatory response in patients with acute pancreatitis: an exploratory study. Acupunct Med. 2015;33(2):115–120. doi:10.1136/acupmed-2014-010646
26. Li L, Yu J, Mu R, et al. Clinical effect of electroacupuncture on lung injury patients caused by severe acute pancreatitis. Evid Based Complement Alternat Med. 2017;2017:3162851. doi:10.1155/2017/3162851
27. Ahsin S, Saleem S, Bhatti AM, et al. Clinical and endocrinological changes after electro-acupuncture treatment in patients with osteoarthritis of the knee. Pain. 2009;147(1–3):60–66. doi:10.1016/j.pain.2009.08.004
28. Zhu J, Chen XY, Li L-B, et al. Electroacupuncture attenuates collagen-induced arthritis in rats through vasoactive intestinal peptide signalling-dependent re-establishment of the regulatory T cell/T-helper 17 cell balance. Acupunct Med. 2015;33(4):305–311. doi:10.1136/acupmed-2014-010732
29. Xue QM, Ning L, Xue P, et al. 电针“足三里”对急性胰腺炎大鼠血清促炎因子及胰腺核因子-κB活性的影响 [Effect of electroacupuncture on serum proinflammatory cytokine levels and pancreatic nuclear factor kappa-b expression in acute pancreatitis rats]. Zhen Ci Yan Jiu. 2011;36(4):272–277. Chinese.
30. Li J, Shi XF, Zhou L-Y, et al. 电针对急性胰腺炎大鼠胃肠运动功能的影响 [Experimental study on electroacupuncture for strengthening gastrointestinal motility in the rat with acute pancreatitis]. Zhongguo Zhen Jiu. 2008;28(5):365–368. Chinese.
31. Luo W, Wang JY, Liu C-L, et al. 针灸“肺俞”穴对病毒性肺炎小鼠肺指数及相关炎性因子的影响 [Effect of EA stimulation of “Feishu” (BL 13) on lung index, serum and lung IL-10 and TNF-alpha levels in mice with viral pneumonia]. Zhen Ci Yan Jiu. 2014;39(4):293–297. Chinese.
32. Li J, Zhao Y, Wen Q, Xue Q, Lv J, Li N. EA for severe acute pancreatitis accompanied with paralytic ileus: a randomized controlled trial. Zhongguo Zhen Jiu. 2016;36(11):1126–1130.
33. Wang X. EA for treatment of acute pancreatitis and its effect on the intestinal permeability of the patient. Zhongguo Zhen Jiu. 2007;27(6):421–423.
34. Zhao L, Li X, Shi Z. Clinical observation on severe acute pancreatitis treated with EA at Dachangshu (BL 25) and Shangjuxu (ST 37) combined with ulinastatin. Zhongguo Zhen Jiu. 2018;38(2):132–136.
35. Choi TY Y, Ang L, Jun JH, et al. Acupuncture and electroacupuncture for stroke: a protocol for overview of systematic review and meta-analysis. Medicine. 2022;101(1):e28496. doi:10.1097/MD.0000000000028496
36. Nebel OT, Fornes MF, Castell DO. Symptomatic gastroesophageal reflux: incidence and precipitating factors. Am J Dig Dis. 1976;21(11):953–956. doi:10.1007/BF01071906
37. Shuai X, Xie P, Liu J, Xiang Y, Li J, Lan Y. Different effects of EA on esophageal motility and serum hormones in cats with esophagitis. Dis Esophagus. 2008;21(2):170–175. doi:10.1111/j.1442-2050.2007.00757.x
38. Ouyang H, Xing J, Chen J. EA restores impaired gastric accommodation in vagotomized dogs. Dig Dis Sci. 2004b;49(9):1418–1424. doi:10.1023/B:DDAS.0000042240.05247.01
39. Tabosa A, Yamamura Y, Forno ER, Mello LE. A comparative study of the effects of EA and moxibustion in the gastrointestinal motility of the rat. Dig Dis Sci. 2004;49(4):602–610. doi:10.1023/B:DDAS.0000026305.20852.41
40. Iwa M, Matsushima M, Nakade Y, Pappas TN, Fujimiya M, Takahashi T. EA at ST-36 accelerates colonic motility and transit in freely moving conscious rats. Am J PhysiolGastrointest Liver Physiol. 2006a;290(2):G285–G292. doi:10.1152/ajpgi.00068.2005
41. Luo D, Liu S, Xie X, Hou X. EA at acupoint ST-36 promotes contractility of distal colon via a cholinergic pathway in conscious rats. Dig Dis Sci. 2008;53(3):689–693. doi:10.1007/s10620-007-9929-7
42. Liu S, Wang Z, Su Y, et al. A neuroanatomical basis for electroacupuncture to drive the vagal-adrenal axis. Nature. 2021;598(7882):641–645. doi:10.1038/s41586-021-04001-4
43. Smith C, Cowan C, Sensenig A, et al. Health spending growth slows in 2003. Health Aff. 2005;24:185–194.
44. Escobar J, Pereda J, Arduini A, et al. Cross-talk between oxidative stress and pro-inflammatory cytokines in acute pancreatitis: a key role for protein phosphatases. Curr Pharm Des. 2009;15(26):3027–3042. doi:10.2174/138161209789058075
45. He BM, Li WS, Li W-Y, et al. 头针超前镇痛对肠癌患者术后硬膜外吗啡镇痛的影响 [Effect of previous analgesia of scalp acupuncture on post-operative epidural morphine analgesia in the patient of intestinal cancer]. Zhongguo Zhen Jiu. 2007;27(5):369–371. Chinese.
46. Takahashi T. Effect and mechanism of acupuncture on gastrointestinal diseases. Int Rev Neurobiol. 2013;111:273–294.
47. Li HF, Hu GQ, Liu W-W, et al. 针刺夹脊穴对脓毒症胃肠功能障碍炎性反应指标的影响 [Clinical observation on the inflammatory indexes in septic gastrointestinal dysfunction treated with acupuncture at Jiaji (EX-B 2)]. Zhongguo Zhen Jiu. 2019;39(10):1055–1058. Chinese. doi:10.13703/j.0255-2930.2019.10.006
48. Ma D, He H. One hundred and six cases of chronic gastritis treated by acupuncture. J Tradit Chin Med. 2004;24(3):170–171.
49. Zhang C, Zhou ZL. 针灸结合中药治疗慢性胃炎 110例报告 [Chronic gastritis treated by acupuncture combined with Chinese herbs: a report of 110 cases]. Zhong Xi Yi Jie He Xue Bao. 2003;1(2):141, 159. Chinese. doi:10.3736/jcim20030217
50. Zhou EH, Liu HR, Wu H-G, et al. Down-regulation of protein and mRNA expression of IL-8 and ICAM-1 in colon tissue of ulcerative colitis patients by partition-herb moxibustion. Dig Dis Sci. 2009;54(10):2198–2206. doi:10.1007/s10620-008-0620-4
51. Li G, Li S, An L, Wang B. Electroacupuncture alleviates intraoperative immunosuppression in patients undergoing supratentorial craniotomy. Acupunct Med. 2013;31(1):51–56. doi:10.1136/acupmed-2012-010254
52. Gao W, Huang Y, Chen H, et al. Regulatory effects of electro-acupuncture at Zusanli on brain-gut peptide contents inrats’pituitaryglandandblood. J Fourth Milt Med Univ. 2001;22:793–796.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
