Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 18
Clinical Diagnostic Value of Serum GABA, NE, ET-1, and VEGF in Chronic Obstructive Pulmonary Disease with Pulmonary Hypertension
Authors Yan J, Duan Y, Cheng M
Received 24 April 2023
Accepted for publication 8 August 2023
Published 19 August 2023 Volume 2023:18 Pages 1803—1813
DOI https://doi.org/10.2147/COPD.S418478
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Min Zhang
Jing Yan,1,* Yajing Duan,2,* Mengyu Cheng3,4
1Department of Respiratory and Critical Care Medicine, Lvliang People’s Hospital Affiliated to Shanxi Medical University, Lvliang City, Shanxi Province, 033000, People’s Republic of China; 2Department of Intensive Care Unit, Key Laboratory for Critical Care Medicine of the Ministry of Health, Emergency Medicine Research Institute, Tianjin First Center Hospital, Nankai University, Tianjin, 300192, People’s Republic of China; 3Department of Respiratory and Critical Care Medicine, Shanxi Bethune Hospital, Shanxi Academy of Medical Sciences, Tongji Shanxi Hospital, Third Hospital of Shanxi Medical University, Taiyuan, Shanxi, 030032, People’s Republic of China; 4Department of Respiratory and Critical Care Medicine, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, 430030, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Mengyu Cheng, Department of Respiratory and Critical Care Medicine, Shanxi Bethune Hospital, Shanxi Academy of Medical Sciences, Tongji Shanxi Hospital, Third Hospital of Shanxi Medical University, No. 99, Longcheng Street, Taiyuan, Shanxi, People’s Republic of China, Tel +8613994237390, Email [email protected]
Background: Pulmonary hypertension (PH) is the one of the most common complications of chronic obstructive pulmonary disease (COPD). Whereas, the associated diagnostic factors are uncertain. The present study aims to investigate useful diagnostic factors in patients with COPD and PH (COPD-PH).
Patients and Methods: A total of 111 patients with COPD in Shanxi Bethune Hospital from December 2019 to December 2020 were divided into COPD (PASP≤ 50 mmHg) and COPD-PH groups (PASP> 50 mmHg). Pulmonary function and chest CT results were collected. Routine blood, biochemical, and blood coagulation function indices were examined for all patients. Arterial blood gas analysis and serum cytokines were also measured. Differences in the distribution of the above indicators between the two groups were analyzed using binary logistic regression analysis to identify the risk factors of COPD-PH, and multiple linear regression analysis to determine the factors affecting PASP. The influencing factors and joint predictive factors of the above linear regression analysis were analyzed using the ROC curve. The area under the curve and the best cut-off value were calculated, and their predictive value and clinical significance in disease diagnosis were discussed.
Results: A total of 27 indexes with statistically significant differences between the two groups were identified (P < 0.05). Binary Logistic regression analysis showed that the factors influencing the diagnosis of pulmonary hypertension were serum GABA, NE, VEGF, BUN, and LYM% levels (P < 0.05). Multiple linear regression showed that the factors influencing PASP were serum NE, ET-1, GABA, and VEGF levels, and the goodness of fit of the model was 0.748 (P < 0.001). ROC curve showed that the AUC of the combined diagnosis of serum NE, ET-1, GABA, and VEGF levels was 0.966 (sensitivity, 87.5%; specificity, 93.65%).
Conclusion: Serum NE and ET-1 are risk factors for COPD-PH, whereas serum GABA and VEGF are protective factors against COPD-PH. The combined diagnostic value of serum NE, ET-1, GABA, and VEGF levels was the highest.
Keywords: diagnosis, chronic obstructive pulmonary disease, pulmonary hypertension, cytokine
Introduction
Chronic obstructive pulmonary disease (COPD) manifests as persistent respiratory symptoms and progressive airflow obstruction and can further develop into pulmonary heart disease. COPD is a common chronic disease caused by respiratory failure.1 Cigarette smoke and other harmful pollutants are common risk factors for COPD and usually cause abnormal pulmonary inflammatory response.2 More than three million people die annually from COPD.3
Pulmonary hypertension (PH) is one of the most common complications of COPD. Compared to COPD patients without PH, presence of PH showed worse clinical symptoms, higher medical costs, increased exacerbations, and prognosis.4,5 Repeated infections and airflow limitations can cause endothelial cell dysfunction, and finally induced PH. Inflammatory injury and repair can lead to pulmonary vascular remodeling and eventually PH. Studies suggest that PH is an independent risk factor for increased mortality in COPD.5–8 Therefore, early diagnosis of PH is essential. Right heart catheterization is the gold standard for diagnosing PH;4 however, it is invasive and unsuitable to most patients. Moreover, the overlapping symptoms of PH and COPD obstructive pulmonary disease present diagnostic challenges. Therefore, there is an urgent need to identify new biomarkers of COPD and PH (COPD-PH).
The occurrence and progression of COPD-PH are related to inflammatory responses, and previous studies have found that many cytokines are related to PH. Norepinephrine (NE) is one of the most excitatory sympathetic neurotransmitters highly expressed in animals with PH.9,10 While γ-aminobutyric acid (GABA) is one of the inhibitory sympathetic neurotransmitters; and the heart feature is related to decreased inhibitory role of GABA.11 The application of GABA can inhibit the release of NE and decrease peripheral sympathetic activity, thereby decreasing pulmonary arterial pressure.10 Endothelin-1 (ET-1), secreted by endothelial cells, is the strongest vasoconstricting substance in development of PH. ET-1’s main role is binding to ET-A in the pulmonary vascular smooth muscle.12,13 As for the role of vascular endothelial growth factor (VEGF), still remains controversial; although few studies recommend it is a protecting factor,14 other suggests that it promotes pulmonary vascular remodeling.15 Currently, the focus on these cytokines in COPD-PH remains limited.
In the present study, we generated some clinical indices of patients with COPD and COPD-PH and determined cytokine levels to identify the key protective and risk factors in patients with PH. Moreover, we evaluated the diagnostic effectiveness of the key factors, providing a basis for the early diagnosis of COPD-PH.
Materials and Methods
Participant
A total of 111 patients with COPD from the Respiratory and Critical Care Department of Shanxi Bethune Hospital between December 2019 and December 2020 were included in this study. All patients underwent transthoracic echocardiography in accordance with the diagnostic standards of the 2019 Global Initiative for Chronic Obstructive Lung Disease (GOLD)16 and performed transthoracic echocardiography (TTE). Each participant’s COPD diagnosis was based on the Global Initiative for Chronic Obstructive Lung Disease guideline standards (forced expiratory volume during the first second of forced breath [FEV1] to forced vital capacity [FVC] ratio after bronchodilation <0.70). Based on pulmonary artery systolic pressure (PASP), all patients were divided into COPD and COPD-PH groups according to the Chinese Guidelines for the Diagnosis and Treatment of Pulmonary Hypertension 2018.17 COPD groups was defined as with PASP≤50 mmHg or peak tricuspid regurgitation ≤ 3.4 m/s; COPD-PH groups was defined as with PASP>50 mmHg or peak tricuspid regurgitation > 3.4 m/s. All enrolled patients signed informed consent forms prior to their participation this study. This study was approved by the risk factor for increased mortality in Ethics Committee of the Third Hospital of Shanxi Medical University (approval no. YXLL-2020-073) and complied with the Declaration of Helsinki.
Exclusion Criterion
The following were the exclusion criteria: (1) patients with various diseases of the blood system; (2) patients with severe nervous system disorders; (3) patients with severe liver and kidney dysfunction; (4) patients with a history of hemodialysis in the last three months; (5) patients with a history of blood transfusion in the last 2 weeks; (6) patients with a history of lung surgery; (7) severe valvular disease and right ventricular outflow tract disease; (8) PH caused by a malignant tumor, rheumatic disease, left heart failure, pulmonary thromboembolism, and other unknown causes.
Information Collection
General information, such as sex, age, body mass index (BMI), and smoking index, was collected after admission. Routine blood indices, including white blood cell count (WBC), neutrophil count (NEUT), neutrophil percentage (NETU%), lymphocyte count (LYM), lymphocyte percentage (LYM%), eosinophil count (EOS), eosinophil percentage (EOS%), red blood cell count (RBC), hemoglobin (Hb), red blood cell distribution width (RDW), and platelet count were collected. Serum biochemical parameters, including serum creatinine (Scr), blood urea nitrogen (BUN), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) were collected. Coagulation function indices such as prothrombin time (PT), activated partial thromboplastin time (APTT), and international normalized ratio (INR) were also collected. Finally, the procalcitonin (PCT), N-terminal pro-brain natriuretic peptide (NT-proBNP), arterial blood gas, TTE, and chest CT results were recorded.
Lung function test results of patients were obtained after admission for three months. Serum specimen was collected and GABA, NE, ET-1, VEGF, hypoxia inducible factor-1α (HIF-1α), interleukin-6 (IL-6), homocysteine (Hcy), and tumor necrosis factor-α (TNF-α) were determined.
Arterial Blood Gas Analysis
We collected 2 mL arterial blood samples within half an hour of admission. Arterial blood gas analysis was performed using a GEM-3500 blood gas analyzer within half an hour. Hydrogen potential (pH), partial pressure of oxygen (PO2), partial pressure of carbon dioxide (PCO2), and fraction of inspiration O2 (FiO2) were recorded. Finally, the oxygenation index (OI) was calculated as follows: OI= PO2/FiO2×100%.
Cardiac Color Ultrasound
Color Doppler imaging was used to measure the maximum regurgitation velocity and peak velocity of the valve orifice using Vivid E9 echocardiography (GE Healthcare, USA). Systolic pulmonary artery pressure was measured by a senior professional physician. PASP was estimated from the tricuspid regurgitation velocity after excluding valvular disease and obstructive disease of the right heart. The PASP was determined by averaging multiple measurements.
Determination of Lung Function
A master screen pulmonary function instrument (CareFusion, Germany) was used to determine lung function. The optimal value was calculated three consecutive times by experienced pulmonary function technicians. Forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1), forced expiratory volume in 1 second as percentage of predicted value (FEV1%pred, and forced expiratory volume in 1second/forced vital capacity (FEV1/FVC) were recorded.
Chest CT
After admission, patients underwent chest CT using the German SOMATOM Definition Flash CT system, and CT scans were performed at the end of maximum inspiration by radiologists. All images were reviewed by two experienced radiologists, and other diseases that may cause respiratory symptoms and airflow limitations were excluded.
Determination of Cytokine Level
We collected 5 mL fasting venous blood within 24 h of admission. The blood was centrifuged at 3000r/min for 10 min and the supernatant was quick-frozen in liquid nitrogen and stored at −80°C. Serum GABA, NE, ET-1, VEGF, HIF-1α, IL-6, Hcy, and TNF-α were detected using a kit from Shanxi Boao Xinyuan Biotechnology Co., LTD. The determination method used was enzyme-linked immunosorbent assay (ELISA). The procedure was performed strictly in accordance with the manufacturer’s instructions.
Statistical Analysis
All the analyses were conducted in SPSS 26.0. Variables with normal distribution are presented as mean ± standard deviation (SD), and variables with non-normal distribution are presented as medians (interquartile ranges). For the comparison between the two groups, the Mann–Whitney test was adopted for non-normally distributed data, and two independent sample t-tests were adopted for normally distributed measurement data. With PH as the dependent variable and indicators with statistically significant differences between the two groups as independent variables, a multivariate binary logistic regression analysis was performed using the Wald method. Variables with P < 0.05 were considered as risk factors for COPD-PH. Multiple linear regression was performed with logistic regression risk factors and ET-1 as independent variables, and PASP as the dependent variable to determine the factors influencing PASP. Receiver operating characteristic (ROC) curve analysis was performed on the influencing factors and combined predictors.
Results
Information About the Patients
A total of 111 patients were included in this study, 63 with COPD and 48 with COPD-PH. There were no significant differences in age, BMI, or smoking index between the COPD and COPD-PH groups (Table 1). These results suggested that there was no heterogeneity in the selected samples.
 |
Table 1 Normal Information of the Patients |
Differences in Arterial Blood Gas Analysis, Blood Routine Index, Coagulation Function Index, PCT, NT-proBNP, Serum Biochemical Index, Lung Function, and Cytokines
Arterial blood was collected during calm oxygen inhalation (FiO2=29%), and the pH, PCO2, and OI were calculated. The pH and PCO2 levels were not significantly different between the COPD and COPD-PH groups. The OI was lower in the COPD-PH group than in the COPD group (Table 2, t = 2.484, P =0.014).
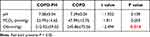 |
Table 2 Differences in Arterial Blood Gas Analysis |
Fasting blood samples were collected within 24h of admission to complete the first routine blood examination. The results showed that The levels of NETU%, LYM%, LYM, EOS%, EOS, RBC, Hb, RDW, and PLT were significantly different between the COPD and COPD-PH groups (Table 3; P < 0.05).
 |
Table 3 Difference in Blood Routine Index |
Fasting blood samples were collected within 24h after admission, and the coagulation function index, PCT, and NT-proBNP were examined. The results showed that PT, APTT, INR, PCT, and NT-proBNP levels were significantly different between the COPD and COPD-PH groups (Table 4, P < 0.05).
 |
Table 4 Differences in Coagulation Function Index, PCT, and NT-proBNP |
Fasting blood samples were collected within 24h after admission. Scr, BUN, ALT, AST, and Na+ levels differed significantly between the COPD and COPD-PH groups (Table 5, P < 0.05).
 |
Table 5 Differences in Serum Biochemical Index |
Lung function was determined within three months after admission. FEV1, FVC, FEV1%, and FEV1/FVC were significantly different between the COPD and COPD-PH groups (Table 6; P < 0.05).
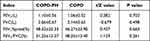 |
Table 6 Differences in Lung Function |
Fasting blood samples were collected for enzyme-linked immunosorbent assay. GABA, VEGF, Hcy, HIF-1α, NE, ET-1, and TNF-α were significantly different between COPD and COPD-PH groups (Table 7, P < 0.05).
 |
Table 7 Differences in Cytokines |
Logistic and Linear Regression Analysis for Identification of Diagnostic Factors for COPD-PH
After the above analysis, factors that showed significant differences between the COPD and COPD-PH groups were included in multivariate binary logistic regression. The potential factors influencing COPD-PH included serum GABA, NE, VEGF, BUN, and LYM% levels (Table 8; P < 0.05). Among these, GABA, VEGF, and LYM% were protective factors, while NE and BUN were risk factors. The goodness-of-fit of the model was 0.748. The diagnostic accuracy was 93.7%, with 3 misdiagnoses and 4 missed diagnoses (Table 9).
 |
Table 8 Logistic Regression Analysis |
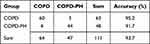 |
Table 9 Diagnostic Accuracy of the Logistic Model |
Considering that most publications have shown that ET-1 is a risk factor for PH,12,13 we combined ET-1 and the above significant factors (GABA, NE, VEGF, BUN, and LYM%) in the multiple linear regression analysis. NE, ET-1, GABA, and VEGF were confirmed as potential key factors in PH and were significantly correlated with PASP (Table 10, P < 0.001). The goodness-of-fit of the linear model was 0.784.
 |
Table 10 Multiple Linear Regression Analysis |
Diagnostic Value of the Combined Factors
Receiver operating characteristic (ROC) curve analyses were performed to evaluate the diagnostic ability of these factors. The results showed that area under ROC curve (AUC) for NE, ET-1, GABA, and VEGF were 0.934, 0.822, 0.727, and 0.637, respectively (Table 11 and Figure 1). More importantly, the combined diagnostic ability of these factors was the most effective with an AUC of 0.966.
 |
Table 11 Diagnostic Ability of the Factors |
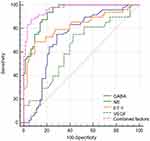 |
Figure 1 The receiver operator characteristic (ROC) curve analysis for the key factors. |
Discussion
The prevalence of COPD is extremely high, and COPD-PH is an independent predictive factor for worsening disease and shortened life expectancy.5–8 Therefore, early diagnosis of PH is important. Nevertheless, the gold diagnostic standard of right heart catheterization for PH is not acceptable for most patients, and the overlapping symptoms of PH and COPD suggest a diagnostic challenge. In the present study, we generated the clinical factors and some cytokines factors including GABA, NE, ET-1, VEGF, HIF-1α, IL-6, Hcy, and TNF-α for further analysis. The regression and ROC curve analysis found that among these factors, NE, ET-1, GABA, and VEGF are the effective diagnostic factors in COPD-PH. Moreover, the combined diagnostic effect of these cytokines was valuable with an AUC of 0.966.
Previous studies have emphasized the important roles of NE, ET-1, GABA, and VEGF in COPD and PH. NE is a catecholamine derived from catecholamines that are circulating and released by sympathetic nerve fibers in the middle of blood vessels.18 In patients with PH and PH rat models, the sympathetic nervous system is activated, and NE levels are elevated.9,10,19 In chronic cor pulmonale, serum NE levels increase, and the reason is that chronic hypoxia induces a hyperadrenergic state.20 In a study of 21 patients with idiopathic PH, NE is positively correlated with pulmonary arterial pressure (r=0.66, P < 0.01) and pulmonary vascular resistance (r=0.69, P < 0.001),21 which is consistent with our results. In patients with COPD, the sympathetic nervous system is also activated and NE level increased, which exerts vasoactive effects by binding to α- and β-adrenergic receptors.22 Stimulation of α1-adrenergic receptors located on pulmonary vascular smooth muscle causes pulmonary vasoconstriction, and stimulation of endothelial α2-adrenergic receptors causes pulmonary vasodilatation. Ciarka et al demonstrated that hypersympathetic activity in patients with PH is associated with disease progression.19 Nevertheless, a previous study suggested that patients with PH have normal sympathetic nervous system activity, basal plasma catecholamine levels, and stimulated plasma catecholamine levels,23 which demonstrates that the current results need further exploration.
ET-1 is the strongest vasoconstrictor produced by endothelial cells and acts by binding to ET-A or ET-B receptors in lung vessels. ET-1 levels in idiopathic PH are elevated and is positively related to right atrial pressure (r=0.74, P < 0.0001).21 HouWeling et al demonstrated a correlation between pulmonary vascular resistance and circulating ET-1 levels, supporting the role of ER-1 in PH.24 In our study, ET-1 expression increased in patients with COPD-PH, which is in agreement with the above studies. Elevated ET levels in COPD-PH may be the result of decreased ET clearance, and increased ET production. When ET-1 binds to the ET-A receptor on pulmonary artery smooth muscle, intracellular Ca2+ levels increase rapidly and then activate PKC, causing contraction and proliferation of pulmonary artery smooth muscle.25 Endothelium-dependent ET-B1 receptor-mediated vasodilation is impaired in PH rats, while the ET-A synergic ET-B2 receptor-mediated vasodilation response is enhanced.26 Therefore, we believe that ET-1 plays a major role in the imbalance of vasodilating-systolic function in PH and is involved in the abnormal proliferation of pulmonary vascular smooth muscle and pulmonary vascular remodeling. Selective blocking of the ET-A and ET-B2 receptors may be an effective treatment for COPD-PH.
GABA is the main inhibitory neurotransmitter in the human body and is found not only in the central nervous system but also in the peripheral system. Most studies on GABA are related to neurological and psychiatric diseases. It has been proved that in schizophrenia, the inhibitory effect of GABA on dopaminergic neural pathways is weakened, leading to dopamine hyperactivity.27 The extent and density of sympathetic innervation in the pulmonary arteries vary among species, and dense sympathetic innervation is observed in human pulmonary vessels.28 Sympathetic ganglia have receptors for GABA and its related amino acids; when activated, GABA receptors can depolarize neurons.29 A previous study detects an inhibitory effect of GABA on sympathetic nerve terminals.30 It has also been found that injection of the GABA receptor antagonist into the paraventricular nucleus of the hypothalamus can excite sympathetic nerves and cause a strong pressure response.31 The pathogenesis of COPD-PH is not fully found, but sympathetic hyperactivity has been shown to be involved.32 Our results showed that serum GABA levels in patients with COPD-PH were significantly lower than those in the COPD group, which was a protective factor against PH. Low concentrations of GABA attenuate sympathetic inhibition, while sympathetic excitation is characterized by elevated NE levels and gamma-aminobutyric acid transaminase (GABA-T) activity.33 NE stimulates the α1-adrenergic receptor located in the pulmonary vascular smooth muscle, leading to the proliferation disorder of pulmonary artery smooth muscle and abnormal contraction of pulmonary vessels. At the same time, it can also affect the formation of TXA2 and PGI2, eventually leading to PH. The latter can degrade GABA in the body, and a decrease in GABA content weakens the inhibition of sympathetic nerves, increases the release of NE, and increases the activity of sympathetic nerves. At the same time, the inhibition of the renin-angiotensin-aldosterone system is weakened, the secretion of angiotensin is increased, and the contraction of vascular smooth muscle is enhanced, which eventually leads to PH. At present, researchers have blocked or inhibited the sympathetic nerve pathway using surgery or drugs to reduce sympathetic nerve tension, thereby reducing pulmonary hypertension and reversing right ventricular remodeling.9,34–36 This may be because, after thoracic sympathetic nerve block, the sympathetic pathway is inhibited, similar to the inhibitory effect of GABA on sympathetic nerves, resulting in vasodilation. A study has suggested that GABA treatment can reverse pulmonary hypertension by inhibiting the sympathetic nervous system of PH rats and significantly reducing the plasma NE level.10 In addition, the GABA-T inhibitor vigabatrin (GVG) can reverse the increase in NE levels and decrease in GABA levels, thereby reversing PH and right ventricular remodeling.33 A previous study shows that serum GABA can reduce idiopathic PH by inhibiting the expression of VEGF, VEGF mRNA, and MMP-9, thereby reducing pulmonary vascular remodeling.37 Recent studies have confirmed that the inert gas argon reduces pulmonary vascular tone in rats and humans by activating GABA receptors;38 therefore, argon may be suitable for patients with PH and right heart failure. These methods provide new ideas for the treatment of COPD-PH; however, no large clinical trials have been conducted for such therapies, and their specific roles in COPD-PH remain unclear and require further research. In the future, we may attempt to increase the level of GABA in the blood of patients by consuming GABA-rich foods, which may delay or improve the occurrence and development of PH to a certain extent and increase patient compliance.
VEGF is a cytokine secreted by endothelial cells that induces fibroblast proliferation and promotes neovascularization. VEGF has been demonstrated to be involved in the occurrence and development of COPD and eventually leads to PH.39 COPD patients usually have a history of smoking and hypoxemia, and inducible HIF-1α can stimulate VEGF production, which increases reactive oxygen species (ROS) production through the transforming growth factor-β (TGF-β)/NADPH oxidase pathway and promotes pulmonary vascular remodeling.15 However, the results of the present study showed that serum VEGF in patients with COPD-PH was lower than that in patients with COPD, which is a protective factor against PH, which is inconsistent with the above results. This may be due to the small sample size, or it may be confirmed by many researchers that serum VEGF levels are elevated in patients with COPD and mild-to-moderate PH. As the disease progresses, pulmonary vascular destruction and apoptosis of pulmonary vascular endothelial cells decrease VEGF as a protective factor. Interestingly, recent studies have shown that the loss of VEGF-specific receptors may exacerbate PH.14 The roles of VEGF and its receptor in the pathogenesis of COPD-PH remain controversial and require further investigation.
Nevertheless, the present study has some limitations. First, the included patients were mostly those with acute exacerbation of COPD, and participants in the stable period were absent. Second, the pulmonary arterial pressure of the patients was estimated by echocardiography and not measured by the right heart floating catheter, which may have caused some errors. Third, the sample size in the present study was small and needs to be further verified in prospective, large-scale, and multicenter clinical trials.
Conclusion
Serum NE and ET-1 are risk factors for COPD-PH, whereas serum GABA and VEGF are protective factors. The combination of serum NE, ET-1, GABA, and VEGF levels had the highest diagnostic value.
Ethics Approval and Informed Consent
The research was approved from the Ethics Committee of the Third Hospital of Shanxi Medical University, Written informed consents were obtained from all participants. This study was conducted in accordance with the Declaration of Helsinki.
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Jing Yan, Yajing Duan and Mengyu Cheng. The first draft of the manuscript was written by Jing Yan and Mengyu Cheng and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript and have agreed on the journal to which the article has been submitted. All authors agree to be accountable for all aspects of the work.
Funding
Funding was provided by the Natural Science Foundation of Shanxi Province (No. 201901D111414). Funding was provided by the Research Project Supported by Shanxi Scholarship Council of China (No. 2020-178). Funding was provided by the Fund Program for the Scientific Activities of Selected Returned Overseas Professionals in Shanxi Province (No. 20200029).Funding was provided by the Tianjin Medical Construction of Key Discipline (Specialty) Funded Project (TJYXZDXK-013A).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Labaki WW, Rosenberg SR. Chronic obstructive pulmonary disease. Ann Intern Med. 2020;173(3):Itc17–itc32. doi:10.7326/AITC202008040
2. Christenson SA, Smith BM, Bafadhel M, Putcha N. Chronic obstructive pulmonary disease. Lancet. 2022;399(10342):2227–2242. doi:10.1016/S0140-6736(22)00470-6
3. Rabe KF, Watz H. Chronic obstructive pulmonary disease. Lancet. 2017;389(10082):1931–1940. doi:10.1016/S0140-6736(17)31222-9
4. Cassady SJ, Reed RM. Pulmonary hypertension in COPD: a case study and review of the literature. Medicina (Kaunas, Lithuania). 2019;55(8). doi:10.3390/medicina55080432
5. Nathan SD, Barbera JA, Gaine SP, et al. Pulmonary hypertension in chronic lung disease and hypoxia. Eur Respir J. 2019;53(1):1801914. doi:10.1183/13993003.01914-2018
6. Andersen K, Iversen M, Kjaergaard J, et al. Prevalence, predictors, and survival in pulmonary hypertension related to end-stage chronic obstructive pulmonary disease. J Heart Lung Transpl. 2012;31(4):373–380. doi:10.1016/j.healun.2011.11.020
7. Weitzenblum E, Hirth C, Ducolone A, Mirhom R, Rasaholinjanahary J, Ehrhart M. Prognostic value of pulmonary artery pressure in chronic obstructive pulmonary disease. Thorax. 1981;36(10):752–758. doi:10.1136/thx.36.10.752
8. Oswald-Mammosser M, Weitzenblum E, Quoix E, et al. Prognostic factors in COPD patients receiving long-term oxygen therapy. Importance of pulmonary artery pressure. Chest. 1995;107(5):1193–1198. doi:10.1378/chest.107.5.1193
9. Bogaard HJ, Natarajan R, Mizuno S, et al. Adrenergic receptor blockade reverses right heart remodeling and dysfunction in pulmonary hypertensive rats. Am J Respir Crit Care Med. 2010;182(5):652–660. doi:10.1164/rccm.201003-0335OC
10. Suzuki R, Maehara R, Kobuchi S, Tanaka R, Ohkita M, Matsumura Y. Beneficial effects of γ-aminobutyric acid on right ventricular pressure and pulmonary vascular remodeling in experimental pulmonary hypertension. Life Sci. 2012;91:693–698. doi:10.1016/j.lfs.2012.04.006
11. Wang R, Zeng Q, Wang W, Wang W. GABA(A) and GABA(B) receptor-mediated inhibition of sympathetic outflow in the paraventricular nucleus is blunted in chronic heart failure. Clin Exp Pharmacol Physiol. 2009;36:516–522. doi:10.1111/j.1440-1681.2008.05101.x
12. Benza R, Gomberg-Maitland M, Demarco T, et al. Endothelin-1 pathway polymorphisms and outcomes in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2015;192(11):1345–1354. doi:10.1164/rccm.201501-0196OC
13. Dai Y, Chen X, Song X, et al. Immunotherapy of endothelin-1 receptor type A for pulmonary arterial hypertension. J Am Coll Cardiol. 2019;73(20):2567–2580. doi:10.1016/j.jacc.2019.02.067
14. Hwangbo C, Lee H-W, Kang H, et al. Modulation of endothelial bone morphogenetic protein receptor type 2 activity by vascular endothelial growth factor receptor 3 in pulmonary arterial hypertension. Circulation. 2017;135(23):2288–2298. doi:10.1161/CIRCULATIONAHA.116.025390
15. Wang YL. 慢性阻塞性肺疾病患者血清VEGF、bFGF水平与肺动脉高压的关系研究 [Relationship between serum VEGF and bFGF levels and pulmonary arterial hypertension in patients with chronic obstructive pulmonary disease]. J Clin Pulm Med. 2019;2019:849–852. Chinese.
16. Singh D, Agusti A, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the GOLD science committee report 2019. Eur Respir J. 2019;53(5):1900164. doi:10.1183/13993003.00164-2019
17. 中华医学会心血管病学分会肺血管病学组;中华心血管病杂志编辑委员会. 中国肺高血压诊断和治疗指南2018 [Chinese guidelines for the diagnosis and treatment of pulmonary hypertension 2018]. Zhonghua xin xue guan bing za zhi. 2018;46(12):933–964. Chinese. doi:10.3760/cma.j.issn.0253-3758.2018.12.006
18. Knight D, Ellison J, Hibbs R, Hyman A, Kadowitz P. A light and electron microscopic study of the innervation of pulmonary arteries in the cat. Anat Rec. 1981;201(3):513–521. doi:10.1002/ar.1092010308
19. Ciarka A, Doan V, Velez-Roa S, Naeije R, van de Borne P. Prognostic significance of sympathetic nervous system activation in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2010;181(11):1269–1275. doi:10.1164/rccm.200912-1856OC
20. Watanabe E, Ogawa K, Ban M, Satake T. Sympathetic nervous systems in chronic cor pulmonale. Jpn Circ J. 1981;45(6):646–653. doi:10.1253/jcj.45.646
21. Nootens M, Kaufmann E, Rector T, et al. Neurohormonal activation in patients with right ventricular failure from pulmonary hypertension: relation to hemodynamic variables and endothelin levels. J Am Coll Cardiol. 1995;26(7):1581–1585. doi:10.1016/0735-1097(95)00399-1
22. Pepke-Zaba J, Higenbottam T, Dinh-Xuan A, Ridden C, Kealey T. Alpha-adrenoceptor stimulation of porcine pulmonary arteries. Eur J Pharmacol. 1993;235:169–175. doi:10.1016/0014-2999(93)90133-3
23. Richards A, Ikram H, Crozier I, Nicholls M, Jans S. Ambulatory pulmonary arterial pressure in primary pulmonary hypertension: variability, relation to systemic arterial pressure, and plasma catecholamines. Br Heart J. 1990;63(2):103–108. doi:10.1136/hrt.63.2.103
24. Houweling B, Merkus D, Sorop O, Boomsma F, Duncker D. Role of endothelin receptor activation in secondary pulmonary hypertension in awake swine after myocardial infarction. J Physiol. 2006;574:615–626. doi:10.1113/jphysiol.2006.107060
25. Guo Q, Xu H, Yang X, et al. Notch activation of Ca-sensing receptor mediates hypoxia-induced pulmonary hypertension. Hypertens Res. 2017;40(2):117–129. doi:10.1038/hr.2016.118
26. Wang Z, Li A, Guo Q, et al. Effects of cyclic intermittent hypoxia on ET-1 responsiveness and endothelial dysfunction of pulmonary arteries in rats. PLoS One. 2013;8(3):e58078. doi:10.1371/journal.pone.0058078
27. Cotter D, Landau S, Beasley C, et al. The density and spatial distribution of GABAergic neurons, labelled using calcium binding proteins, in the anterior cingulate cortex in major depressive disorder, bipolar disorder, and schizophrenia. Biol Psychiatry. 2002;51(5):377–386. doi:10.1016/S0006-3223(01)01243-4
28. Barnes P, Liu S. Regulation of pulmonary vascular tone. Pharmacol Rev. 1995;47(1):87–131.
29. Adams P, Brown D. Actions of gamma-aminobutyric acid on sympathetic ganglion cells. J Physiol. 1975;250(1):85–120. doi:10.1113/jphysiol.1975.sp011044
30. Bowery N, Hudson A. Gamma-aminobutyric acid reduces the evoked release of [3H]-noradrenaline from sympathetic nerve terminals [proceedings]. Br J Pharmacol. 1979;66(1):108P.
31. Kenney M, Weiss M, Patel K, Wang Y, Fels R. Paraventricular nucleus bicuculline alters frequency components of sympathetic nerve discharge bursts. Am J Physiol Heart Circ Physiol. 2001;281(3):H1233–41. doi:10.1152/ajpheart.2001.281.3.H1233
32. Velez-Roa S, Ciarka A, Najem B, Vachiery J-L, Naeije R, van de Borne P. Increased sympathetic nerve activity in pulmonary artery hypertension. Circulation. 2004;110(10):1308–1312. doi:10.1161/01.CIR.0000140724.90898.D3
33. Lingeshwar P, Kaur G, Singh N, et al. A study on the involvement of GABA-transaminase in MCT induced pulmonary hypertension. Pulm Pharmacol Ther. 2016;36:10–21. doi:10.1016/j.pupt.2015.11.002
34. Chen S-L, Zhang -F-F, Xu J, et al. Pulmonary artery denervation to treat pulmonary arterial hypertension: the single-center, prospective, first-in-man PADN-1 study (First-in-Man Pulmonary Artery Denervation for Treatment of Pulmonary Artery Hypertension). J Am Coll Cardiol. 2013;62(12):1092–1100. doi:10.1016/j.jacc.2013.05.075
35. Huang Y, Liu YW, Xiang L, et al. 经胸肺动脉去神经支配术在肺动脉高压大鼠模型中的治疗作用[J].中国循环杂志 [Therapeutic Effects of Transthoracic Pulmonary Artery Denervation in a Rat Model of Pulmonary Arterial Hypertension]. Chin Circ J. 2018;12:616–620. Chinese.
36. Ishikawa M, Sato N, Asai K, Takano T, Mizuno K. Effects of a pure alpha/beta-adrenergic receptor blocker on monocrotaline-induced pulmonary arterial hypertension with right ventricular hypertrophy in rats. Circ J. 2009;73(12):2337–2341. doi:10.1253/circj.CJ-09-0213
37. Chen G, Song J, Xiong H, Zhang L, Lv J. γ-氨基丁酸对肺动脉高压大鼠肺组织VEGF、MMP-9表达的影响[J].重庆医学 [Effect of GABA on expression of VEGA and MMP-9 in monocrotaline-induced pulmonary hypertension rats]. Chongqing Med J. 2014;36:4043–4045. Chinese.
38. Suleiman S, Klassen S, Katz I, et al. Argon reduces the pulmonary vascular tone in rats and humans by GABA-receptor activation. Sci Rep. 2019;9(1):1902. doi:10.1038/s41598-018-38267-y
39. Ma Z, Yu Y-R, Badea CT, et al. Vascular endothelial growth factor receptor 3 regulates endothelial function through β-Arrestin 1. Circulation. 2019;139(13):1629–1642. doi:10.1161/CIRCULATIONAHA.118.034961
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
