Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 15
Clinical Courses and Outcomes of Patients with Chronic Obstructive Pulmonary Disease During the COVID-19 Epidemic in Hubei, China
Authors Hu W, Dong M, Xiong M, Zhao D, Zhao Y, Wang M, Wang T, Liu Z, Lu L, Hu K
Received 28 May 2020
Accepted for publication 20 August 2020
Published 23 September 2020 Volume 2020:15 Pages 2237—2248
DOI https://doi.org/10.2147/COPD.S265004
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 6
Editor who approved publication: Dr Richard Russell
Weihua Hu,1,* Minglin Dong,1,* Mengqing Xiong,1,* Dong Zhao,1 Yang Zhao,1 Mengmei Wang,1 Tao Wang,1 Zhenlian Liu,2 Li Lu,3 Ke Hu1
1Department of Respiratory and Critical Care Medicine, Renmin Hospital of Wuhan University, Wuhan, People’s Republic of China; 2Fever Clinic, The East Campus, Renmin Hospital of Wuhan University, Wuhan, People’s Republic of China; 3Department of Respiratory Medicine, The East Campus, Renmin Hospital of Wuhan University, Wuhan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Ke Hu
Department of Respiratory and Critical Care Medicine, Renmin Hospital of Wuhan University, Zhangzhidong Road No. 99, Wuhan 430060, People’s Republic of China
Tel +86-18971035988
Fax +86-027-88042292
Email [email protected]
Purpose: In this study, we investigated the acute exacerbation and outcomes of COPD patients during the outbreak of COVID-19 and evaluated the prevalence and mortality of COPD patients with confirmed COVID-19.
Methods: A prospectively recruited cohort of 489 COPD patients was retrospectively followed-up for their conditions during the COVID-19 pandemic from December 2019 to March 2020 in Hubei, China. In addition, the features of 821 discharged patients with confirmed COVID-19 were retrospectively analyzed.
Results: Of the 489 followed-up enrolled COPD patients, 2 cases were diagnosed as confirmed COVID-19, and 97 cases had exacerbations, 32 cases of which were hospitalized, and 14 cases died. Compared with the 6-month follow-up results collected 1 year ago, in 307 cases of this cohort, the rates of exacerbations and hospitalization of the 489 COPD patients during the last 4 months decreased, while the mortality rate increased significantly (2.86% vs 0.65%, p=0.023). Of the 821 patients with COVID-19, 37 cases (4.5%) had pre-existing COPD. Of 180 confirmed deaths, 19 cases (10.6%) were combined with COPD. Compared to COVID-19 deaths without COPD, COVID-19 deaths with COPD had higher rates of coronary artery disease and/or cerebrovascular diseases. Old age, low BMI and low parameters of lung function were risk factors of all-cause mortality for COVID-19 patients with pre-existing COPD.
Conclusion: Our findings imply that acute exacerbations and hospitalizations of COPD patients were infrequent during the COVID-19 pandemic. However, COVID-19 patients with pre-existing COPD had a higher risk of all-cause mortality.
Keywords: chronic obstructive pulmonary disease, COPD, exacerbation, mortality, novel coronavirus pneumonia, COVID-19, novel coronavirus, SARS-CoV-2
Key Messages
What is the key question?
◆ It is unknown whether patients with COPD have an increased risk of SARS-CoV-2 infection, more acute exacerbations and/or a poorer prognosis.
What is the bottom line?
◆This study shows that acute exacerbations and hospitalizations of COPD patients were infrequent during the COVID-19 pandemic, however, the fatality rate of COPD patients during the epidemic period was significantly higher than that during the non-epidemic period.
Why read on?
◆ COPD seemed to be not common in the comorbidities for patients with COVID-19, but COVID-19 patients with pre-existing COPD had a higher risk of all-cause mortality.
Introduction
COVID-19 has disseminated across the world and has caused numerous infections and very high numbers of deaths. The world’s population is generally susceptible to SARS-CoV-2, especially elderly subjects with chronic diseases such as hypertension, diabetes and cardio-cerebrovascular diseases.1,2 In addition, elderly patients infected with SARS-CoV-2 were more likely to be in critical condition and had a higher mortality rate.2,3 A study of 1099 confirmed COVID-19 cases indicated that the median age of the patients was 47.0 years, 15.1% of whom were aged ≥65 years, and 27.0% of the patients in critical condition were aged ≥65 years.4 Another investigation of 4021 confirmed COVID-19 cases showed that the mortality rate of patients aged ≥60 years (5.3%) was significantly higher than that of patients ˂ 60 years (1.4%), most of which had chronic diseases.3
Chronic obstructive pulmonary disease (COPD) is one of the most common chronic diseases among the elderly in China,5 and its prevalence increases with age and the quantity of cigarettes consumed.6 Acute exacerbation (AE) of COPD is defined as an acute worsening of respiratory symptoms that results in additional therapy.6 AEs of COPD are important events for they negatively impact the health status and are associated with increased hospitalizations and readmissions, disease progression and increased mortality of COPD patients.7,8 Several factors can precipitate AEs of patients with COPD and respiratory viral infections are very common causes.9 However, the detection rate of a viral etiology is below 40% in the elderly, and this also holds true for AEs of chronic respiratory illnesses.10 Data regarding new viruses and elderly subjects are scarce, including patients with severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) associated coronaviruses, NL63, HKU1 and potential respiratory pathogens.10 The roles of coronaviruses in AEs of patients with COPD have already been explored to some extent, but the results are not consistent.11–13 One investigation demonstrated that SARS-associated coronavirus assessed using RT-PCR was not present in lower respiratory tract specimens of 141 COPD patients with AEs one year after the first outbreak.11 However, patients with MERS and comorbidities, including those who smoke and have COPD, may have worse outcomes.12,13 Furthermore, a retrospective study suggested that it was unlikely for human bocavirus infections to have a major role in adult COPD patients with AEs.14 Up to now, no study has been published that analyzed the clinical characteristics and mortality in COPD patients with COVID-19. Therefore, it is unknown whether patients with COPD have an increased risk of SARS-CoV-2 infection, more exacerbations and/or a poorer prognosis. The purpose of this study was to identify the prevalence of COVID-19, AEs and outcomes in patients with COPD during the COVID-19 epidemic in Hubei province, China. Those goals were achieved by following-up the recruited COPD patients in an existing database, and by analyzing the comorbidities of admitted confirmed cases in a designated hospital for COVID-19.
Methods
Data Sources and COVID-19 Subjects
Since January 2016, we have been participating in a project entitled “Study on the diagnosis and treatment of complications and comorbidities in patients with chronic obstructive pulmonary disease”, which is supported by the National Key Research and Development Program of China (project number: 2016YFC1304403) and is registered in ClinicalTrials.gov (Clinical Trials ID: NCT 03182309). Up to December 2019, 558 COPD patients diagnosed by lung function had been recruited for that study. We obtained written informed consent from all participants or from their family members and established an electronic database to collect their personal information, previous history, medication history, pulmonary function parameters, laboratory testing and sleep-breathing monitoring results. The patients enrolled in the database have been routinely followed-up once every six months. To understand the AEs and outcomes of this cohort during the epidemic of COVID-19, we carried out an additional follow-up by telephone and short message service at the end of March 2020.
Meanwhile, we collected data of 821 discharged laboratory confirmed cases, including demographics, laboratory results, comorbidities and outcomes, from the information system of a designated hospital for severe or critical patients with COVID-19. The details of confirmed COVID-19 deaths of patients with COPD and the COPD deaths in followed-up patients were analyzed. The prevalence of all-cause mortality and risk factors of deaths in COVID-19 patients with COPD were also investigated.
Ethics Approval and Consent to Participate
This study procedure was reviewed and approved by the Ethics Committee of Renmin Hospital of Wuhan University (Nos. 2017K-C014 and WDRY2020-K019). Under exceptional circumstances for reducing exposure to SARS-CoV-2, informed verbal consent was obtained from the COVID-19 patients or written informed consent were obtained from family members verbally authorized by the COVID-19 patients, and a waiver of written informed consent from the hospitalized confirmed cases was applied and obtained agreement of the Ethics Committee. This study was conducted in accordance with the Declaration of Helsinki.
Follow-Up Protocol
A questionnaire was designed specifically for follow-up by telephone and text messaging on a cell phone (short message service, SMS). A short message containing the questionnaire was sent to each patient’s cell phone one day before the follow-up by telephone. The patients’ conditions and outcomes in the last four months were collected during the follow-up, which began on December 01, 2019 and ended on March 31, 2020, covering the whole COVID-19 pandemic in Hubei province, China. Figure 1 shows a flowchart of the follow-up process. By the end of March 2020, 558 enrolled patients with COPD in the database were followed-up through SMS and telephone, which were carried out by three professional investigators. Twenty-eight subjects were excluded because of losing contact, and 41 cases were excluded who could not answer the questions accurately or completely. In total, 489 patients were included in this study. In order to explore whether their conditions and/or outcomes had changed during the COVID-19 outbreak, we compared their responses with the 6-month follow-up results from October 2018 to March 2019 in 307 cases of this cohort.
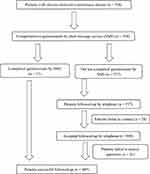 |
Figure 1 Flow chart of the follow-up design and process. |
Questionnaire
We designed a questionnaire with 12 questions (Supplementary Table 1) related to where the patients had lived during the last four months, whether they were diagnosed with COVID-19, their infection severity, AEs of COPD or hospitalization, morbidity and outcome.
Confirmed Criteria for COVID-19 and Disease Severity
Laboratory confirmed cases met the criteria in the Guidelines on the Diagnosis and Treatment of Novel Coronavirus Infected Pneumonia,15 ie, the cases had a history of epidemiological exposure, typical symptoms and X-ray imaging, positive for SARS-COV-2 nucleic acid by real-time RT-PCR, or had SARS-COV-2 specific IgM and IgG in their serum.
The disease severity was classified as mild, moderate, severe or critical.15 Severe cases had at least one of the following: respiratory distress (≥30 breaths/min); oxygen saturation ≤93% at rest; arterial partial pressure of oxygen (PaO2)/fraction of inspired oxygen (FiO2) ≤300 mm Hg; obvious lesion progression on chest imaging and >50% within 24–48 hours. Critical cases refer to any of the following: respiratory failure and needing mechanical ventilation; shock; other organ failure requiring ICU care.
Detection of SARS-CoV-2 Nucleic Acid
Nasonasopharyngeal swab specimens were collected from all patients, preserved in viral-transport medium and submitted for analysis by trained technicians. SARS-CoV-2 nucleic acid was detected by reverse-transcriptase RT-RCR, which was completed in the biosafety level 2 clinical laboratory of Renmin Hospital of Wuhan University. RT-PCR detection reagents for SARS-CoV-2 nucleic acid open reading frame 1ab and nucleocapsid protein were provided by GeneoDx Biotech Co., LTD, Shanghai, China.
Detection of Antibodies
Serum specific IgG and IgM antibodies against SARS-CoV-2 were characterized using a full-automatic chemiluminescence immunoassay analyzer (UniCel DxI800, Beckman Coulter, Inc., USA) according to the manufacturer’s instructions. The measured relative light unit (RLU) was used as the indicator of immune activity. That analyzer automatically converts the RLU from each immunoassay into an absolute unit (AU) by fitting it to a standard master calibration curve. The kits were provided by Shenzhen YHLO Biotech Co., Ltd., China. A cutoff value of >10.0 AU/mL is considered as positive for antibodies.
Study Endpoints
In this study, one endpoint was to investigate the AEs, hospitalizations and outcomes of enrolled COPD patients during the COVID-19 pandemic by follow-up; the other endpoint was to analyze the prevalence rate of COPD in hospitalized confirmed COVID-19 cases and their deaths.
Statistical Analysis
SPSS 21.0 software was used for statistical analysis. Data are expressed as medians (interquartile range [IQR]) or absolute numbers and percentages [n/N (%)], where N represents the total number of patients with available data. P values were calculated from the Mann–Whitney U-test, the χ2 test or Fisher’s exact test. A P value is considered significant if it is less than 0.05. Logistic regression analysis was used to assess the risk of all-cause mortality in follow-up COPD patients (Table 1) and in confirmed COVID-19 cases with COPD (Table 2).
 |
Table 1 Risk Factors Associated with Death of the Followed-Up Patients with COPD During the Epidemic (n = 489) |
 |
Table 2 Risk Factors Associated with Death of Confirmed COVID-19 Patients (n=821) |
Results
Prevalence of COVID-19 in Followed-Up COPD Patients
Of the 489 successfully followed-up enrolled COPD patients, two patients (0.41%) were diagnosed as having confirmed COVID-19. Neither of those 2 patients had a history of exposure to the Huanan seafood market in Wuhan, but there were infections in their families. One of those patients was an 86-year-old male with a BMI of 15.5 kg/m2, who lived in Wuhan and had a history of COPD for 20 years with grade 4 of GOLD airflow limitation classification. He died of respiratory failure and multiple organ failure 19 days after infection with COVID-19 in a designated hospital. The other confirmed case was a 68-year-old male resident of Wuhan with grade 2 of lung function classification; he recovered after 26 days of hospitalization.
Acute Exacerbations and Hospitalizations in Followed-Up COPD Patients
Of the 489 patients with COPD, 324 were residents of Wuhan and 165 were nonresidents of Wuhan in the Hubei province (Supplementary Figure 1). In the 4 months of follow-up during the outbreak, 392 cases (80.16%) were in stable condition and 97 cases (19.84%) had AEs of COPD, 32 cases (6.54%) of which were hospitalized and 14 cases (2.86%) died. Compared with 6-month follow-up results collected 1-year ago, in 307 cases of this cohort, both the rates of AE and hospitalization during the last 4 months in 489 patients decreased, however, the fatality rate significantly increased (2.86% vs 0.65%, p=0.023) (Table 3).
 |
Table 3 Comparison of COPD Patients Conditions During Periods of Epidemic and Non-Epidemic |
Deaths of COPD Patients During Follow-Up
Of the 489 patients with COPD, 14 cases died during the 4 months from Dec. 2019 to Mar. 2020, of which 7 cases died in Dec. 2019, 3 patients died in Jan. 2020 and 4 patients died in Feb. 2020. There were no deaths of those patients in March. Nine cases died of respiratory failure caused by AEs of COPD, however, their deaths cannot be definitely associated with COVID-19 due to the lack of data. One case died 3 days after failure of lung transplantation, 2 cases died from hematological malignancies and 1 died from cerebrovascular disease. Only 1 case died of confirmed COVID-19 (see above).
The followed-up COPD patients were divided into a survivor group and a deceased group (Table 4). Of baseline data at enrollment, the deceased group was significantly older than the survivor group (74.07±7.75 vs 68.15±8.71, p=0.012), had a significantly lower BMI (20.40±4.99 vs 22.91±3.85, p=0.017) and lower spirometry function parameters (FVC, FVC% and FEV1). There was no significant statistical difference between the two groups in other baseline data regarding gender composition, smoking status, prevalence of comorbidity or sleep-breathing parameters (neck circumference, AHI, T90, Min SpO2, Mean SpO2).
 |
Table 4 Baseline Data of the Followed-Up Patients with COPD (n = 489) |
Logistic regression analysis showed that older age, low BMI, poor lung function and COVID-19 were risk factors of all-cause mortality for COPD patients, in which, older age was an independent risk factor (Table 1).
Comorbidities and Deaths in Confirmed COVID-19 Cases
We analyzed the prevalence of comorbidity, mortality and risk factors of all-cause mortality in confirmed COVID-19 patients (Table 5). Of 821 discharged cases, 335 cases (40.8%) had comorbidities and 486 cases (59.2%) did not. The order of comorbidity rate was hypertension (27.3%), diabetes (10.6%), coronary artery disease (7.3%), cerebrovascular diseases (4.5%), COPD (4.5%), malignancies (3.3%), chronic kidney disease (2.3%) and chronic liver disease (2.3%). However, of 180 confirmed deaths with COVID-19, 92 cases (51.1%) were combined with hypertension, 33 cases (18.3%) with diabetes, 32 cases (17.8%) with coronary artery disease, 27 (15.0%) with cerebrovascular diseases, 19 (10.6%) with COPD, 14 (7.8%) with malignancies, 14 (7.8%) with chronic kidney disease and 4 (2.2%) with chronic liver disease (Table 5).
 |
Table 5 Comorbidities and Deaths in 821 Patients with Confirmed COVID-19 |
The clinical features of the 180 deaths with confirmed COVID-19 are shown in Table 6. Of the 37 confirmed COVID-19 patients with pre-existing COPD, 19 cases died, a mortality rate of 51.4% (Table 5). Compared to COVID-19 deaths of patients without COPD, the COVID-19 deaths of patients with COPD had a higher rate of coronary artery disease or cerebrovascular diseases, more expectoration. There was no significant difference between the two groups in terms of gender, age, severity of disease, other first-episode symptoms and time of death after hospitalization. The first-episode syndromes and comorbidities in confirmed COVID-19 deaths with or without COPD are shown in Table 6. Compared with the deaths of patients without COPD, the patients with COPD who died had higher levels of CRP, fibrinogen and IL-4, and lower levels of albumin, LDH and D-dimer (Table 7). Furthermore, the characteristics of COVID-19 deaths of patients with COPD and the followed-up COPD deaths during the pandemic were compared (Table 8), and older age, more comorbidities and lower lung function parameters were their common features. Risk factors associated with the death of confirmed COVID-19 patients were male, old age and comorbidities (Table 2).
 |
Table 6 The First-Episode Syndromes and Comorbidities in Confirmed COVID-19 Deaths with or without COPD |
 |
Table 7 Laboratory Results in Confirmed COVID-19 Deaths with or without COPD |
 |
Table 8 The Characteristics of COPD Deaths in Confirmed COVID-19 Cases and During the Followed-Up |
Discussion
In this study, we explored whether the infection by SARS-CoV-2 increases the risks of AEs and all-cause mortality in patients with COPD during the breakout of COVID-19. We found that AEs and hospitalizations of patients with COPD were infrequent, but the risk of all-cause mortality increased in hospitalized COVID-19 patients with pre-existing COPD.
In a total of 489 COPD patients followed-up during the four months of the epidemic, only 2 of them were diagnosed as confirmed cases of COVID-19, 1 of which died. Ninety-seven cases (19.84%) had AEs of COPD, 32 cases (6.54%) of which were hospitalized. Both the rates of AEs and hospitalizations (events per person per month) were lower than the prevalence rates in 307 cases of this cohort during the non-epidemic period 1-year ago. In addition, 4.5% of hospitalized patients with COVID-19 and 10.6% of confirmed deaths overlapped COPD, in which, the rates of COPD were much lower than other comorbidities, such as hypertension, diabetes, cardiovascular disease and cerebrovascular diseases. The results indicated that COVID-19 had a slight effect on AEs and hospitalization in COPD patients, which was consistent with a study of SARS patients16 and a literature review on published cases of COVID-19.17 That review concluded that the COVID-19 epidemic was a low rate associated with previous chronic pulmonary diseases, eg COPD, asthma, and bronchiectasis.17
We also found that patients with COVID-19 had a high prevalence rate (40.8%) of comorbidities except for COPD (4.5%), including hypertension (27.3%), diabetes (10.6%), cardio-cerebrovascular diseases (4.5% and 7.3%), malignancies (3.3%), chronic kidney disease (2.3%) and chronic liver disease (2.3%). The deaths with COVID-19 had a much higher prevalence of comorbidities (shown in Table 6), 10.6% of which were combined with COPD. These findings are in agreement with the results published by Zhou et al18 and Wu et al19 from China, and Grasselli et al from Italy.20 However, our patients did not overlap with those studied by Zhou et al and Wu et al, which were from two other hospitals in Wuhan.
Another important finding in the present study was that the risk of all-cause mortality increased in hospitalized COVID-19 patients with pre-existing COPD. Of the 37 confirmed COVID-19 patients with pre-existing COPD, 19 cases died, a mortality rate of 51.4%. COVID-19 patients with pre-existing COPD simultaneously combined with cardio-cerebrovascular co-morbidities are at a higher risk of all-cause mortality (Table 7). Moreover, we found that 14 patients with COPD died during the 4-month follow-up period of the COVID-19 epidemic in Hubei. For causes of all-cause mortality, only 1 case died of confirmed COVID-19 and 4 cases died of comorbidities, eg hematological malignancies, cerebrovascular disease and failure of lung transplantation. Nine cases died of respiratory failure caused by AEs of COPD, however, it was not clear if those deaths were associated with COVID-19 due to a lack of data. Old age, low BMI, poor lung function and COVID-19 may be risk factors of all-cause mortality for COPD patients during the epidemic of COVID-19, which is consistent with previous findings about risk factors of all-cause mortality in COPD patients during the non-epidemic period.21,22 A meta-analysis study revealed that COVID-19 patients with pre-existing COPD had a 5.9-fold higher risk of aggravation than patients without COPD.23 However, body mass index (BMI) has different effects on all-cause mortality of COVID-19 or COPD, obesity may be a risk factor for poor outcome in COVID-19-induced lung injury24 and low BMI is associated with a higher risk of all-cause mortality for patients with COPD.25 In the present study, we found that low BMI was related with worse prognosis in COVID-19 subjects with pre-existing COPD.
The roles of coronaviruses on AEs of patients with COPD have already been investigated to some extent, but the results have not been consistent.11–13 The SARS coronavirus has a 70% homology with SARS-CoV-2.26 Although chronic diseases are risk factors for SARS infection and progression toward death, COPD was not the most common comorbidity and its prevalence was lower than cardiovascular disease and diabetes in SARS patients.16,27 The SARS associated coronavirus was not detected by RT-PCR in lower respiratory tract specimens of COPD patients with AEs.11 However, smokers and patients with COPD were more susceptible to MERS coronavirus infections28 and may have worse outcomes,12,13 which could be partially explained by upregulated levels of MERS-CoV receptors in the lungs of smokers and COPD patients.29 Angiotensin-converting enzyme-2 (ACE-2) receptor could be an adhesion molecule for SARS-CoV-2 that causes COVID-19,30 the known receptor for both the SARS-coronavirus31 and the human respiratory coronavirus NL63.32 Cigarette smoking and COPD may up-regulate ACE-2 expression in lower airways,33,34 and one might anticipate that these populations would be at increased risk of SARS-CoV-2 infections and more severe presentations of COVID-19.34 In a systematic review, Alqahtani and colleagues35 concluded that COPD and ongoing smoking is most likely associated with the negative progression and adverse outcomes of COVID-19. However, it is striking that patients with chronic respiratory diseases, particularly COPD and asthma, appear to be under-represented in the comorbidities reported for patients with COVID-19.20,36 As for the correlation between coronavirus infection and pre-existing chronic pulmonary disease, our findings are somewhat similar to the SARS study other than the MERS results, and therefore, it needs to be further characterized.
The strength of this study lies in the fact that we not only conducted a retrospective analysis of patients with COVID-19, but also targeted a prospectively recruited cohort of COPD patients in the Hubei province to retrospectively follow-up their conditions during the whole COVID-19 pandemic, which may better reflect the conditions of COPD patients in the epidemic and non-epidemic periods.
We also acknowledge several limitations of this study. First, data of lung function and BMI could not be obtained for many patients due to the epidemic period, which may influence analysis of the results. Second, it is unclear about the exact causes and the relationship with COVID-19 in the deaths of 9 patients with COPD during the follow-up, after all, second-hand reports via phone interviews with family members cannot be as effective as first-hand medical data. Third, many patients were instructed to stay home due to strict isolation measures during the pandemic. Severe patients had to visit the hospital, having greater mortality risk and biasing outcomes.
Conclusions
Our study revealed that: (a) compared with the non-pandemic period, the incidence of AEs and hospitalization in COPD patients was significantly reduced during the epidemic period; (b) the all-cause mortality of COPD patients during the epidemic period was significantly higher than that during the non-epidemic period; (c) COPD patients with advanced age, lower BMI or poorer lung functions were more likely to die during the outbreak of COVID-19.
ClinicalTrials
NCT 03182309.
Role of the Sponsor
The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication.
Data Sharing Statement
No additional data are available.
Acknowledgment
This work was supported by the National Key Research and Development Program of China (project number: 2016YFC1304403) and the Science and technology key project on novel coronavirus pneumonia, Hubei province, China (project number: 2020FCA002).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Dong M and Xiong M contributed equally with Hu W. All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none were declared.
References
1. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi:10.1016/S0140-6736(20)30211-7
2. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel Coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061. doi:10.1001/jama.2020.1585.
3. Yang Y, Lu QB, Liu MJ, et al. Epidemiological and clinical features of the 2019 novel coronavirus outbreak in China. medRxiv. 2020. doi:10.1101/2020.02.10.20021675.
4. Guan WJ, Ni ZY, Hu Y, et al. China medical treatment expert group for COVID–19. Clinical characteristics of Coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi:10.1056/NEJMoa2002032.
5. Wang C, Xu J, Yang L, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet. 2018;391(10131):1706–1717. doi:10.1016/S0140-6736(18)30841-9
6. Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Am J Respir Crit Care Med. 2017;195(5):557–582. doi:10.1164/rccm.201701-0218PP
7. Li MH, Fan LC, Mao B, et al. Short-term exposure to ambient fine particulate matter increases hospitalizations and mortality in COPD: a systematic review and meta-analysis. Chest. 2016;149(2):447–458. doi:10.1378/chest.15-0513
8. Liu S, Zhou Y, Liu S, et al. Association between exposure to ambient particulate matter and chronic obstructive pulmonary disease: results from a cross-sectional study in China. Thorax. 2017;72(9):788–795. doi:10.1136/thoraxjnl-2016-208910
9. Woodhead M, Blasi F, Ewig S, et al. Guidelines for the management of adult lower respiratory tract infections. Eur Respir J. 2005;26(6):1138–1180. doi:10.1183/09031936.05.00055705
10. Jartti L, Langen H, Söderlund-Venermo M, et al. New respiratory viruses and the elderly. Open Respir Med J. 2011;5:61–69. doi:10.2174/1874306401105010061
11. Rohde G, Borg I, Arinir U, et al. Evaluation of a real-time polymerase chain reaction for severe acute respiratory syndrome (SARS) associated coronavirus in patients with hospitalized exacerbation of COPD. Eur J Med Res. 2004;9(11):505–509.
12. Arabi YM, Arifi AA, Balkhy HH, et al. Clinical course and outcomes of critically ill patients with Middle East respiratory syndrome coronavirus infection. Ann Intern Med. 2014;160:389e397. doi:10.7326/M13-2486
13. Kapoor M, Pringle K, Kumar A, et al. Clinical and laboratory findings of the first imported case of Middle East respiratory syndrome coronavirus to the United States. Clin Infect Dis. 2014;59:1511e1518. doi:10.1093/cid/ciu635
14. Ringshausen FC, Tan AYM, Allander T, et al. Frequency and clinical relevance of human bocavirus infection in acute exacerbations of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2009;4:111–117. doi:10.2147/COPD.S4801
15. National Health Commission of the People’s Republic of China. Diagnosis and treatment of novel coronavirus infected pneumonia (trial 7th edition) [EB/OL]; 2020. Available from: http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989/files/ce3e6945832a438eaae415350a8ce964.pdf.
16. Peiris JSM, Yuen KY, Osterhaus ADME, Stöhr K. The severe acute respiratory syndrome. N Engl J Med. 2003;349(25):2431–2441. doi:10.1056/NEJMra032498
17. Lupia T, Scabini S, Mornese Pinna S, Di Perri G, De Rosa FG, Corcione S. 2019 novel coronavirus (2019-nCoV) outbreak:A new challenge. J Glob Antimicrob Resist. 2020;21:22–27. doi:10.1016/j.jgar.2020.02.021.
18. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi:10.1016/S0140-6736(20)30566-3
19. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with Coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934. doi:10.1001/jamainternmed.2020.0994.
20. Grasselli G, Zangrillo A, Zanella A, et al. COVID-19 lombardy ICU network. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323(16):1574. doi:10.1001/jama.2020.5394.
21. Ji Z, de Miguel-diez J, Castro-Riera CR, et al. Differences in the outcome of patients with COPD according to body mass index. J Clin Med. 2020;9(3):710. doi:10.3390/jcm9030710
22. Mullerova H, Maselli DJ, Locantore N, et al. Hospitalized exacerbations of COPD: risk factors and outcomes in the ECLIPSE cohort. Chest. 2015;147(4):999–1007. doi:10.1378/chest.14-0655
23. Wang B, Li R, Lu Z, Huang Y. Does comorbidity increase the risk of patients with COVID-19: evidence from meta-analysis. Aging (Albany NY). 2020;12. doi:10.18632/aging.103000.
24. Memtsoudis SG, Ivascu NS, Pryor KO, Goldstein PA. Obesity as a risk factor for poor outcome in COVID-19-induced lung injury: the potential role of undiagnosed obstructive sleep apnoea. Br J Anaesth. 2020;125(2):e262–e263. doi:10.1016/j.bja.2020.04.078
25. Guo Y, Zhang T, Wang Z, et al. Body mass index and mortality in chronic obstructive pulmonary disease: a dose-response meta-analysis. Medicine. 2016;95(28):e4225. doi:10.1097/MD.0000000000004225
26. Gorbalenya AE, Baker SC, Baric RS, de Groot RJ, Drosten C, Gulyaeva AA. Severe acute respiratory syndrome-related coronavirus: the species and its viruses–a statement of the Coronavirus Study Group. bioRxiv. 2020. doi:10.1101/2020.02.07.937862.
27. Wang M, Du L, Zhou DH, et al. Study on the epidemiology and measures for control on severe acute respiratory syndrome in Guangzhou city [Article in Chinese]. Zhonghua Liu Xing Bing Xue Za Zhi. 2003;24(5):353–357.
28. Seys LJM, Widagdo W, Verhamme FM, et al. DPP4, the middle east respiratory syndrome coronavirus receptor, is upregulated in lungs of smokers and chronic obstructive pulmonary disease patients. Clin Infect Dis. 2018;66(1):45–53. doi:10.1093/cid/cix741
29. Meyerholz DK, Lambertz AM, McCrayJr PB. Dipeptidyl peptidase 4 distribution in the human respiratory tract: implications for the middle east respiratory syndrome. Am J Pathol. 2016;186(1):78–86. doi:10.1016/j.ajpath.2015.09.014
30. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi:10.1038/s41586-020-2012-7
31. Li W, Moore MJ, Vasilieva N, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi:10.1038/nature02145
32. Hofmann H, Pyrc K, van der Hoek L, Geier M, Berkhout B, Pöhlmann S. Human coronavirus NL63 employs the severe acute respiratory syndrome coronavirus receptor for cellular entry. Proc Natl Acad Sci USA. 2005;102:7988–7993. doi:10.1073/pnas.0409465102
33. Brake SJ, Barnsley K, Lu W, McAlinden KD, Eapen MS, Sohal SS. Smoking upregulates angiotensin- converting enzyme-2 receptor: a potential adhesion site for novel Coronavirus SARS-CoV-2 (Covid-19). J Clin Med. 2020;9(3):
34. Leung JM, Yang CX, Tam A, et al. ACE-2 expression in the small airway epithelia of smokers and COPD patients: implications for COVID-19. Eur Respir J. 2020;55(5):
35. Alqahtani JS, Oyelade T, Aldhahir AM, et al. Prevalence, severity and mortality associated with COPD and smoking in patients with COVID-19: a rapid systematic review and meta-analysis. PLoS One. 2020;15(5):e0233147. doi:10.1371/journal.pone.0233147
36. Guan WJ, Liang WH, Zhao Y, et al. China medical treatment expert group for Covid-19. Comorbidity and its impact on 1590 patients with Covid-19 in China: a nationwide analysis. Eur Respir J. 2020;55(5):
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
