Back to Journals » Infection and Drug Resistance » Volume 16
Clinical Characteristics of Severe COVID-19 Patients During Omicron Epidemic and a Nomogram Model Integrating Cell-Free DNA for Predicting Mortality: A Retrospective Analysis
Authors Lu Y , Xia W , Miao S, Wang M, Wu L, Xu T, Wang F, Xu J, Mu Y , Zhang B, Pan S
Received 1 August 2023
Accepted for publication 13 October 2023
Published 18 October 2023 Volume 2023:16 Pages 6735—6745
DOI https://doi.org/10.2147/IDR.S430101
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Yanfei Lu,1,2,* Wenying Xia,1,2,* Shuxian Miao,1,2 Min Wang,1,2 Lei Wu,1,2 Ting Xu,1,2 Fang Wang,1,2 Jian Xu,1,2 Yuan Mu,1,2 Bingfeng Zhang,1,2 Shiyang Pan1,2
1Department of Laboratory Medicine, Jiangsu Province Hospital and Nanjing Medical University First Affiliated Hospital, Nanjing, People’s Republic of China; 2National Key Clinical Department of Laboratory Medicine, Nanjing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Shiyang Pan, Department of Laboratory Medicine, Jiangsu Province Hospital and Nanjing Medical University First Affiliated Hospital, Guangzhou Street No. 300, Nanjing, 210029, People’s Republic of China, Tel +8625-6830-6287, Fax +8625-8372-4440, Email [email protected]
Objective: This study aimed to investigate the clinical characteristics and risk factors of death in severe coronavirus disease 2019 (COVID-19) during the epidemic of Omicron variants, assess the clinical value of plasma cell-free DNA (cfDNA), and construct a prediction nomogram for patient mortality.
Methods: The study included 282 patients with severe COVID-19 from December 2022 to January 2023. Patients were divided into survival and death groups based on 60-day prognosis. We compared the clinical characteristics, traditional laboratory indicators, and cfDNA concentrations at admission of the two groups. Univariate and multivariate logistic analyses were performed to identify independent risk factors for death in patients with severe COVID-19. A prediction nomogram for patient mortality was constructed using R software, and an internal validation was performed.
Results: The median age of the patients included was 80.0 (71.0, 86.0) years, and 67.7% (191/282) were male. The mortality rate was 55.7% (157/282). Age, tracheal intubation, shock, cfDNA, and urea nitrogen (BUN) were the independent risk factors for death in patients with severe COVID-19, and the area under the curve (AUC) for cfDNA in predicting patient mortality was 0.805 (95% confidence interval [CI]: 0.713– 0.898, sensitivity 81.4%, specificity 75.6%, and cut-off value 97.67 ng/mL). These factors were used to construct a prediction nomogram for patient mortality (AUC = 0.856, 95% CI: 0.814– 0.899, sensitivity 78.3%, and specificity 78.4%), C-index was 0.856 (95% CI: 0.832– 0.918), mean absolute error of the calibration curve was 0.007 between actual and predicted probabilities, and Hosmer-Lemeshow test showed no statistical difference (χ 2=6.085, P=0.638).
Conclusion: There was a high mortality rate among patients with severe COVID-19. cfDNA levels ≥ 97.67 ng/mg can significantly increase mortality. When predicting mortality in patients with severe COVID-19, a nomogram based on age, tracheal intubation, shock, cfDNA, and BUN showed high accuracy and consistency.
Keywords: severe COVID-19, Omicron, clinical characteristics, mortality, CfDNA, predicting nomogram
Introduction
Coronavirus disease 2019 (COVID-19) first broke out in Wuhan, China, in December 2019. Subsequently, COVID-19 spread rapidly and widely, causing a pandemic.1 It has emerged as a well-known human pandemic over the last three years due to its high pathogenicity and infectivity, which posed serious risks to public health.2 According to data from the World Health Organization (https://covid19.who.int), there were more than 750 million confirmed cases of COVID-19 and more than 6.94 million deaths worldwide as of May 31, 2023.
Severe acute respiratory syndrome-related coronavirus-2 (SARS-CoV-2), the pathogen that causes COVID-19, has been mutating throughout transmission.2 Due to the rapid spread of Omicron variants across China in late 2022, the high transmission of SARS-CoV-2 Omicron variants and the vast population base, the infection spread widely and resulted in a significant increase in hospitalizations, critically ill patients, and deaths.3 According to the data from the Chinese Center for Disease Control (CDC) and Prevention, the number of patients with COVID-19 hospitalized peaked at 1.625 million per day, with severe cases peaking at 1.280 million per day on January 5, 2023. Omicron variants caused all COVID-19 cases from September 26, 2022 to April 27, 2023 (https://www.chinacdc.cn/).
Although COVID-19 symptoms are generally mild, mainly when caused by the Omicron variants, the disease can worsen in some populations, with adverse outcomes. Previous research has demonstrated that patients with cardiovascular disease or increased cardiovascular risk, renal failure, cancer chemotherapy, diabetes, and hypertension are significantly more likely to progress to a severe form of COVID-19, even resulting in death.4–8 However, earlier reports of risk factors for mortality in patients with severe COVID-19 were primarily based on data collected in 2020, during which the Omicron variant had not yet been discovered.9–11 A better understanding of the clinical characteristics and prognostic risk factors of patients with severe COVID-19 caused by the Omicron variant still needs further study.
Cell-free DNA (cfDNA), an extracellular nucleic acid, was first reported in 1948. It can be secreted by cells, and it can be released into the bloodstream post-apoptosis and post-necrosis.12 cfDNA is only found in trace amounts and has a short half-life in healthy individuals.13,14 Pathological conditions can cause tissue damage and cell necrosis, leading to significantly higher circulating concentrations of cfDNA. CfDNA is an extremely sensitive biomarker for tissue damage. It has been demonstrated to be a valuable and promising non-invasive biomarker in liquid biopsy that can be used for diagnosing and monitoring several diseases.14–17
In this study, we analyzed the clinical data and laboratory indicators of patients with severe COVID-19, measured the level of cfDNA using a duplex real-time polymerase chain reaction (PCR) assay we previously developed,18 investigated the risk factors, and constructed a nomogram model for predicting the mortality of patients with severe COVID-19 to better understand the COVID-19 caused by Omicron variants and provide evidence for early clinical intervention and reduce mortality. Our study was approved by the Ethics Committee of Jiangsu Province Hospital, and prior to the trial, patients gave their informed consent. The study complied with the Declaration of Helsinki and maintained patient privacy and data confidentiality.
Materials and Methods
Study Population
This single-center retrospective cohort study was conducted at the Jiangsu Province Hospital from December 17, 2022 to January 27, 2023. Severe COVID-19 patients admitted to the hospital during this period were enrolled in this study. COVID-19 was confirmed based on a positive result of nucleic acid test for SARS-CoV-2 from patients’ throat swabs using PCR. The diagnosis of severe COVID-19 was based on the guidelines for the diagnosis and treatment of novel coronavirus infection (Trial 10th version), released by the National Health Commission of People’s Republic of China.19 Severe patients with COVID-19 included those who were severely ill or critically ill. A severely ill case was considered as meeting any of the following criteria: 1. Shortness of breath with a breathing rate of ≥30 beats/min; 2. oxygen saturation (SpO2, inhaling air, resting state) ≤93%; 3. the arterial partial pressure of oxygen (PaO2)/oxygen inhalation concentration (FiO2) ≤300 mmHg; 4. progressive aggravation of clinical symptoms and lung imaging demonstrated a significant lesion progression of >50% within 24–48 h. A critically ill case was considered as one that met one of the following criteria: 1. respiratory failure and the need for mechanical ventilation; 2. shock; 3. combined with other organ failures and requiring intensive care unit (ICU) monitoring and treatment. The patients were excluded from this study once they met the following criteria: 1. incomplete or missing clinical data; 2. patients who rejected or did not cooperate with active treatments; 3. patients with immunosuppression (including transplant recipients, hematologic neoplasms, chemotherapy, those on long-term use of immunosuppressants); 4. patients receiving continuous renal replacement therapy (CRRT). A total of 1048 patients were deemed positive for SARS-CoV-2 nucleic acid from throat swab samples; from these 766 cases were excluded based on the exclusion criteria and, finally, 282 cases were enrolled in this study (Figure 1).
 |
Figure 1 Study flow diagram of severe COVID-19 patient enrollment. |
Data Collection
Clinical data were collected by reviewing the patient’s electronic medical records. We collected the data on the age, sex, time from symptom onset to hospital admission, the length of stay (LOS), COVID-19 vaccine information, and ICU stay or not; comorbidities (including hypertension, diabetes mellitus, pulmonary diseases, cerebral infarction, coronary heart disease [CHD], renal diseases, and malignancy); clinical manifestations (including coughing, breathing difficulty, fever, chest tightness, asthma, unconsciousness, acute respiratory distress syndrome [ARDS], respiratory failure or not, and SpO2 value on admission); treatments (including invasive operations, and therapeutics); complications (including shock, cardiac insufficiency, hepatic insufficiency, renal insufficiency, hypoproteinemia, gastrointestinal bleeding [GIB], and combined infections), and patients’ prognoses (survival or death in 60 days). We also collected data on laboratory indicators at the time of admission, including white blood cell (WBC), lymphocyte (L), neutrophil (N), neutrophil to lymphocyte ratio (NLR), C-reactive protein (CRP), procalcitonin (PCT), prothrombin time (PT), international normalized ratio (INR), activated partial thromboplastin time (APTT), thrombin time (TT), fibrinogen (FIB), D dimer, Troponin T, creatine kinase-MB (CK-MB), myohemoglobin (Mb), brain natriuretic peptide (BNP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), urea nitrogen (BUN), creatinine (Cr), total protein (TP), albumin (ALB), and fasting blood-glucose (Glu) levels.
Quantitative Detection of cfDNA
The QLAamp DNA Blood Mini Kit (50) (QIAGEN, Germany) was used to extract DNA from 200 μL of the patients’ plasma samples on admission as per the internal standard and as recommended by the manufacturer. A plasma cell-free DNA quantitative detection kit (Code Biotech, Jiangsu, China) was used to quantify the concentration of cfDNA by duplex real-time PCR assay; the detailed steps of the procedure have been described previously.18
Statistical Analysis
Statistical analysis and nomogram establishment were performed with SPSS 24.0 software (IBM Corp, Armonk, NY, USA) and the R software (version 4.2.3). Categorical variables were expressed as frequency (percentage). Continuous variables were expressed as the mean ± SD when normally distributed and median (Quartile 25%, Quartile 75%) when non-normally distributed. Categorical variables were compared by the χ2 test or Fisher’s exact test and continuous variables were compared by Student’s t-test or Mann–Whitney U-test, as deemed appropriate. The clinical characteristics and laboratory findings of severe COVID-19 patients in the survival and death groups were primarily compared through univariate analysis, and variables with p < 0.05 were entered into forward conditional multivariate regression analyses to identify the independent risk factors for death in severe COVID-19 patients. A predicting nomogram model of severe COVID-19 patient mortality based on the above mentioned independent risk factors was established using the R software package with the Rms. Internal validation of the established nomogram was performed by 1000 bootstrap samples (repeated sampling of the original data 1000 times), C-index, calibration curve, and receiver operating characteristic (ROC) curve; Hosmer—Lemeshow test was used to assess the performance and accuracy of the model.
Results
Clinical Characteristics and Laboratory Findings
The clinical characteristics of 282 patients with severe COVID-19 caused by SARS-CoV-2 are shown in Table 1. The ages of the patients ranged from 32 to 100 years, with a median age of 80 years. One hundred ninety-one patients (67.7%) were male and 91 (32.3%) were female. Only 10 out of 282 patients had ever received the COVID-19 vaccine before infection. In addition, 90.1% (254/282) of these patients had comorbidities, such as hypertension (63.8%), diabetes mellitus (40.8%), CHD (29.1%), and cerebral infarction (27.3%) being the most common. Fever (61.0%), cough (52.5%), and respiratory failure (49.6%) were the three primary clinical manifestations. Moreover, 27.7% of the patients progressed to ARDS. Treatments for patients with severe COVID-19 were generally based on symptomatic supportive therapy, with 62.4% (176/282) of them taking antivirals for SARS-CoV-2 such as Azvudine, Paxlovid, or a combination of both. The most common complications were cardiac insufficiency (52.1%), renal insufficiency (35.5%), and hypoproteinemia (22.3%), affecting 78.4% (221/282) of patients. During the study period, 157 patients died and 125 patients survived; the mortality rate was 55.7% (157/282). The laboratory findings of patients with severe COVID-19 are shown in Table 2. The concentrations of N, CRP, PCT, APTT, FIB, D-dimer, Troponin T, Mb, BNP, BUN, and Glu were higher than normal. In contrast, L, TP, and ALB concentrations were lower than normal. The median value of cfDNA was 112.98 (65.23, 233.51) ng/mL.
 |
Table 1 Clinical Characteristics of Severe COVID-19 Patients Caused by SARS-CoV-2 |
 |
Table 2 Laboratory Findings of Severe COVID-19 Patients Caused by SARS-CoV-2 |
Comparison Between Patients in the Survival and the Death Groups
We compared the clinical characteristics, traditional laboratory findings, and cfDNA levels between the two groups. According to univariate analyses, age, LOS, ICU stay, hypertension, unconsciousness, ARDS, respiratory failure, SpO2 level, tracheal intubation, mechanical ventilation, CRRT, antiviral drug use, shock, cardiac insufficiency, renal insufficiency, GIB, pulmonary fungal infections, WBC, L, N, NLR, CRP, PCT, PT, INR, D-dimer, Troponin T, CK-MB, Mb, BNP, AST, BUN, Cr, Glu, and cfDNA were significantly different in the two groups (Table 1 and Table 2). The results of the multivariate logistic regression model using significantly different variables showed that age, tracheal intubation, shock, cfDNA, and BUN were the independent risk factors for death in patients with severe COVID-19 (Table 3). The ROC curves for age, cfDNA, and BUN for predicting mortality of patients with severe COVID-19 are shown in Figure 2. The area under the curve (AUC) for age, cfDNA, and BUN were 0.660 (95% CI: 0.603–0.730, sensitivity 73.9%, specificity 52.8%, and cut-off value 76.5 years), 0.805 (95% CI: 0.713–0.898, sensitivity 81.4%, specificity 75.6%, and cut-off value 97.67 ng/mL), and 0.638 (95% CI: 0.573–0.703, sensitivity 73.2%, specificity 50.4%, and cut-off value 7.67 μmol/L), respectively.
 |
Table 3 Logistic Multivariable Regression Analysis of Risk Factors for Death in Severe COVID-19 Patients Caused by SARS-CoV-2 |
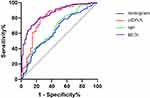 |
Figure 2 The ROC curves for variables predicting mortality in severe COVID-19 patients. |
Establishment and Validation of the Nomogram for Patient Mortality
A nomogram model for predicting mortality of patients with severe COVID-19 was constructed based on the independent risk factors. As shown in Figure 3, the value of each variable corresponded to a specific score, the sum of which was the total score, and the corresponding point on the predicted probability of death axis was the probability that patients with severe COVID-19 would die. The calibration curve revealed that the mean absolute error between the actual and predicted probabilities was 0.007, suggesting that the nomogram model had a high consistency between the actual and the predicted probabilities (Figure 4). The ROC curve (Figure 2) and C-index were used to assess the predictive ability of the established nomogram. AUC was 0.856 (95% CI: 0.814, 0.899), sensitivity 78.3%, specificity 78.4%, the C-index of the nomogram was 0.856 (95% CI: 0.832, 0.918), and Hosmer-Lemeshow test showed no statistically significant difference (χ2 = 6.085, P = 0.638). The results showed high accuracy and uniqueness of this prediction nomogram.
 |
Figure 3 Predicting nomogram model for severe COVID-19 patient mortality. 0: No; 1: Yes. |
 |
Figure 4 The calibration curves of the nomogram. |
Discussion
In our study, the mortality rate for patients with severe COVID-19 was 55.7%, similar to other patients with severe COVID-19, which ranged from 48.1% to 52.2%.20–22 The overall pooled case fatality rate of COVID-19 was only 10.0%. Still, it varied significantly for different populations, highlighting the significance of performing stratified analysis to better understand the impact of the disease on other groups of patients.1
According to previous studies, age has been a significant risk factor for developing severe diseases and death in patients with COVID-19.10,23–25 It was once again demonstrated in our research, but interestingly, we discovered that the median age of patients with severe COVID-19 in this study was greater than previously reported. The findings indirectly showed the reduced pathogenicity of the Omicron variants compared to the original strain or other variants.23,25 In our study, male patients were more likely to develop severe symptoms. Tu et al incorporated gender as a risk factor into the nomogram model for predicting patients with severe COVID-19.26 Vaccination against SARS-CoV-2 is the most effective measure to reduce hospitalization, severe COVID-19, and death.27 Moghadas et al reported that vaccination can reduce the overall morbidity rate by 4.4%, and the effect is more obvious in people over 65 years old, meanwhile, vaccination can reduce the non-ICU hospitalization, ICU hospitalization rate, and mortality rate by 63.5%, 65.6%, and 69.3%, respectively.28 Only 3.5% of the patients in this study had received the COVID-19 vaccine prior to the infection; this finding may help to explain why these patients developed severe infections despite the low pathogenicity of the Omicron strain. According to research, comorbidities affected over 50% of inpatients with COVID-19 and increased to 75% in patients with severe COVID-19.10,29 The percentage in our study increased to 90.1%, this may be associated with our elderly patients, who were more likely to have underlying diseases. The most common comorbidities were hypertension, diabetes mellitus, and CHD, as reported in previous literature.23 These three diseases have a history of being associated with severe infections.23,24,29 Tumor chemotherapy, renal failure, increased cardiovascular risk despite having no cardiovascular disease, or obesity have all been associated with serious COVID-19 outcomes.4–6,29 Therefore, the population at high risk of severe COVID-19 should focus on preventing and controlling primary diseases to reduce the incidence of severe diseases when COVID-19 occurs. This group should also be promptly monitored, and interventional measures should be implemented.
The rapid disease progression in patients with severe COVID-19 that resulted in ARDS, respiratory failure, other complications, or even multiple organ failure was identified. Angiotensin-converting enzyme 2, the primary cell entry receptor for SARS-CoV-2, is widely distributed in bodily tissues, which allows it to attack several other organs in addition to the respiratory system, such as the kidneys, liver, cardiovascular system, and gastrointestinal tract.30,31 On the other hand, multiple organ failure and disease aggravation were also a result of the cytokine storm caused by COVID-19.32 The use of broad-spectrum antibiotics also creates ideal conditions for the colonization and infection of fungi20 and other pathogen infections observed in patients with severe COVID-19 and inflamed alveolar space caused by SARS-CoV-2.
When patients with severe COVID-19 were admitted, laboratory results showed increased inflammatory factors, abnormal coagulation parameters and cardiac function parameters, decreased protein levels, and increased blood glucose levels, which are signs of multi-organ damage. Previous research suggested that CRP, TP, D-dimer, ALB, lymphocyte count and IL-6 can predict patients with severe COVID-19.2,26 Additionally, we observed high concentrations of cfDNA in patients with severe COVID-19.
The high concentration of cfDNA was initially observed in systemic lupus erythematosus. It was discovered that cfDNA can be also increased in various diseases, including tumors, trauma, infection, stroke, myocardial infarction, etc.33 According to studies, cfDNA levels are an independent factor of ICU admission and positively correlate with the severity of COVID-19.13 Temesgen et al found that cfDNA hospitalized patients with COVID-19 source from hematopoietic cells and hepatocytes, adipocytes, vascular endothelium, lung, heart, and renal cells, indicating multi-organ damage. In addition, cfDNA may cause tissue injury and aggravate inflammation as a danger-associated molecular pattern by binding to toll-like receptor 9.16 Although cfDNA appears to be a valuable biomarker, it is not frequently used in clinical settings. The main reasons for the limitations of cfDNA in clinical applications are the significant discrepancies in quantitative analysis of cfDNA from various laboratories and the lack of accurate and precise quantitative methods. The method presented in our study has been demonstrated to have adequate sensitivity, repeatability, precision, and accuracy. This duplex real-time PCR assay can also eliminate variations and have stable analytical performance in the quantification of cfDNA.18
Age, tracheal intubation, shock, cfDNA, and BUN were identified as the independent risk factors for death in patients with severe COVID-19 by multivariate logistic regression analysis findings. Age is already a known risk factor. Age >70 years old have been documented.10,11 The cut-off age in our study was 76.5 years. Treatment for patients with severe COVID-19 who developed acute hypoxemic and respiratory failure included tracheal intubation.34 However, this is a high-risk procedure, and peri-intubation complications such as cardiovascular instability, hemodynamic changes, ventilator-induced lung injury, and secondary infections may expose patients to a higher risk of mortality.35–37 Shock is a severe clinical condition that causes organs and peripheral tissues to receive insufficient perfusion because of acute circulatory failure.38 If not treated promptly and effectively, it can quickly result in multi-organ failure and, ultimately, death. In this study, shock in patients with severe COVID-19 can increase the risk of death by 26-fold. Early identification of the onset of shock and the type of shock, along with prompt and appropriate treatment measures, may significantly improve the prognosis of patients with severe diseases.39 The AUC of cfDNA was up to 0.805, indicating a good diagnostic efficiency for predicting the death of the patient. cfDNA and BUN were the only two laboratory indicators included in our logistic model. Patients at risk of severe disease and death can be specifically identified by their cfDNA profile at admission.13,16 According to a systematic review, acute renal injury was a predictor of mortality in patients with COVID-19, and BUN can significantly increase in death.40
Then, to more clearly comprehend the connection between these distinct risk variables and mortality in patients with severe COVID-19,10 we created a prediction nomogram model for mortality based on five risk factors. A nomogram is a graphic representation of the outcomes of a regression model. It is a graphical tool that combines disconnected line segments to depict the functional relationship between various variables in a plane coordinate. Each variable is given a score based on how much it contributes to the outcome variable, and by adding the scores for the many variables, the probability of the anticipated occurrence may be easily determined. Because of its visualization function, nomogram is frequently used in tumor risk prediction and prognosis assessment and has a promising future in this field.41 As a new biomarker, cfDNA was initially included in the nomogram for predicting the mortality of patients with severe COVID-19 when compared to previously reported nomogram models predicting death, and this prediction model demonstrated good predictive ability.
Our research has some limitations. First, because this is a single-center retrospective study, the population may contain certain biases. Second, we did not compare the clinical characteristics with those of common patients with COVID-19, the clinical data of common COVID-19 caused by Omicron variants was relatively hard to collect as the shortage of medical resources resulted in most non-severe patients were not hospitalized during this period. Finally, the developed nomogram model was not validated using any external data.
Conclusion
This study examined severe COVID-19 infections in a tertiary general hospital in East China. Most patients with severe COVID-19 were elderly males with underlying diseases; they were prone to complications and had high mortality rates. cfDNA levels ≥97.67 ng/mg can significantly increase the mortality rate of patients with severe COVID-19. A nomogram based on age, tracheal intubation, shock, cfDNA, and BUN had high accuracy and consistency when predicting mortality in patients with severe COVID-19.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Alimohamadi Y, Tola HH, Abbasi-Ghahramanloo A, Janani M, Sepandi M. Case fatality rate of COVID-19: a systematic review and meta-analysis. J Prev Med Hyg. 2021;62(2):E311–E320. doi:10.15167/2421-4248/jpmh2021.62.2.1627
2. Chang Y, Wan X, Fu X, et al. Severe versus common COVID-19: an early warning nomogram model. Aging. 2022;14(2):544–556. doi:10.18632/aging.203832
3. Jing W, Ding F, Zhang Y, et al. Trends of SARS-CoV-2 infection in rural area in sentinel community-based surveillance - China, December 2022 to January 2023. China CDC Wkly. 2023;5(11):241–247. doi:10.46234/ccdcw2023.044
4. Warren-Gash C, Davidson JA, Strongman H, et al. Severe COVID-19 outcomes by cardiovascular risk profile in England in 2020: a population-based cohort study. Lancet Region Health Europe. 2023;27:100604. doi:10.1016/j.lanepe.2023.100604
5. Zarębska-Michaluk D, Jaroszewicz J, Rogalska M, et al. Impact of kidney failure on the severity of COVID-19. J Clin Med. 2021;10(9):2042. doi:10.3390/jcm10092042
6. Rakhsha A, Azghandi S, Taghizadeh-Hesary F. Decision on chemotherapy amidst COVID-19 pandemic: a review and a practical approach from Iran. Infect Chemother. 2020;52(4):496–502. doi:10.3947/ic.2020.52.4.496
7. Sen S, Chakraborty R, Kalita P, Pathak MP. Diabetes mellitus and COVID-19: understanding the association in light of current evidence. World J Clin Cases. 2021;9(28):8327–8339. doi:10.12998/wjcc.v9.i28.8327
8. Peng M, He J, Xue Y, Yang X, Liu S, Gong Z. Role of hypertension on the severity of COVID-19: a review. J Cardiovasc Pharmacol. 2021;78(5):e648–e655. doi:10.1097/FJC.0000000000001116
9. Thakur V, Ratho RK. OMICRON (B.1.1.529): a new SARS-CoV-2 variant of concern mounting worldwide fear. J Med Virol. 2022;94(5):1821–1824. doi:10.1002/jmv.27541
10. Yang Y, Zhu XF, Huang J, et al. Nomogram for prediction of fatal outcome in patients with severe COVID-19: a multicenter study. Military Med Res. 2021;8(1):21. doi:10.1186/s40779-021-00315-6
11. Moon HJ, Kim K, Kang EK, Yang H-J, Lee E. Prediction of COVID-19-related mortality and 30-day and 60-day survival probabilities using a nomogram. J Korean Med Sci. 2021;36(35):e248. doi:10.3346/jkms.2021.36.e248
12. Liu JP, Zhang SC, Pan SY. Value of dynamic plasma cell-free DNA monitoring in septic shock syndrome: a case report. World J Clin Cases. 2020;8(1):200–207. doi:10.12998/wjcc.v8.i1.200
13. Bello S, Lasierra AB, López-Vergara L, et al. IL-6 and cfDNA monitoring throughout COVID-19 hospitalization are accurate markers of its outcomes. Respir Res. 2023;24(1):125. doi:10.1186/s12931-023-02426-1
14. Xia WY, Gao L, Dai EH, et al. Liquid biopsy for non-invasive assessment of liver injury in hepatitis B patients. World J Gastroenterol. 2019;25(29):3985–3995. doi:10.3748/wjg.v25.i29.3985
15. Rhodes A, Cecconi M. Cell-free DNA and outcome in sepsis. Crit Care. 2012;16(6):170. doi:10.1186/cc11508
16. Andargie TE, Tsuji N, Seifuddin F, et al. Cell-free DNA maps COVID-19 tissue injury and risk of death and can cause tissue injury. JCI Insight. 2021;6(7). doi:10.1172/jci.insight.147610
17. Jin X, Wang Y, Xu J, et al. Plasma cell-free DNA promise monitoring and tissue injury assessment of COVID-19. Mol Genet Genom. 2023;298(4):823–836. doi:10.1007/s00438-023-02014-4
18. Chen D, Pan S, Xie E, et al. Development and evaluation of a duplex real-time PCR assay with a novel internal standard for precise quantification of plasma DNA. Ann Lab Med. 2017;37(1):18–27. doi:10.3343/alm.2017.37.1.18
19. General Office of the National Health Commission PGD, State Administration of Traditional Chinese Medicine, People’s Republic of China. Diagnosis and treatment Plan for novel coronavirus infection (trial version 10). China Med. 2023;02:161–166.
20. Do TV, Manabe T, Vu GV. Clinical characteristics and mortality risk among critically ill patients with COVID-19 owing to the B.1.617.2 (Delta) variant in Vietnam: a retrospective observational study. PLoS One. 2023;18(1):e0279713. doi:10.1371/journal.pone.0279713
21. Wang B, Zhong F, Zhang H, An W, Liao M, Cao Y. Risk factor analysis and nomogram construction for non-survivors among critical patients with COVID-19. Jpn J Infect Dis. 2020;73(6):452–458. doi:10.7883/yoken.JJID.2020.227
22. Li J, Wang L, Liu C, et al. Exploration of prognostic factors for critical COVID-19 patients using a nomogram model. Sci Rep. 2021;11(1):8192. doi:10.1038/s41598-021-87373-x
23. Zhang JJ, Dong X, Cao YY, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75(7):1730–1741. doi:10.1111/all.14238
24. Cheng L, Bai WH, Yang JJ, Chou P, Ning WS. Construction and validation of mortality risk nomograph model for severe/critical patients with COVID-19. Diagnostics. 2022;12(10):2562.
25. Ben Fredj S, Ghammem R, Zammit N, et al. Risk factors for severe Covid-19 breakthrough infections: an observational longitudinal study. BMC Infect Dis. 2022;22(1):894. doi:10.1186/s12879-022-07859-5
26. Tu C, Wang G, Geng Y, Guo N, Cui N, Liu J. Establishment of a clinical nomogram model to predict the progression of COVID-19 to severe disease. Ther Clin Risk Manag. 2021;17:553–561. doi:10.2147/TCRM.S308961
27. Fiolet T, Kherabi Y, MacDonald CJ, Ghosn J, Peiffer-Smadja N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: a narrative review. Clin Microbiol Infect. 2022;28(2):202–221. doi:10.1016/j.cmi.2021.10.005
28. Moghadas SM, Vilches TN, Zhang K, et al. The impact of vaccination on COVID-19 outbreaks in the United States. medRxiv 2021. doi:10.1101/2020.11.27.20240051
29. Zhou Y, Chi J, Lv W, Wang Y. Obesity and diabetes as high-risk factors for severe coronavirus disease 2019 (Covid-19). Diabetes Metab Res Rev. 2021;37(2):e3377. doi:10.1002/dmrr.3377
30. Scialo F, Daniele A, Amato F, Pastore L, Matera MG, Cazzola M. ACE2: the major cell entry receptor for SARS-CoV-2. Lung. 2020;198(6):867–877. doi:10.1007/s00408-020-00408-4
31. Zhang JJ, Dong X, Liu GH, Gao YD. Risk and protective factors for COVID-19 morbidity, severity, and mortality. Clin Rev Allergy Immunol. 2023;64(1):90–107. doi:10.1007/s12016-022-08921-5
32. Ye Q, Wang B, Mao J. The pathogenesis and treatment of the “Cytokine Storm” in COVID-19. J Infect. 2020;80(6):607–613. doi:10.1016/j.jinf.2020.03.037
33. Ranucci R. Cell-free DNA: applications in different diseases. Methods Mol Biol. 2019;1909:3–12.
34. Papoutsi E, Giannakoulis VG, Xourgia E, Routsi C, Kotanidou A, Siempos II. Effect of timing of intubation on clinical outcomes of critically ill patients with COVID-19: a systematic review and meta-analysis of non-randomized cohort studies. Crit Care. 2021;25(1):121. doi:10.1186/s13054-021-03540-6
35. Russotto V, Rahmani LS, Parotto M, Bellani G, Laffey JG. Tracheal intubation in the critically ill patient. Eur J Anaesthesiol. 2022;39(5):463–472. doi:10.1097/EJA.0000000000001627
36. McKay B, Meyers M, Rivard L, Stankewicz H, Stoltzfus JC, Rammohan G. Comparison of early and late intubation in COVID-19 and its effect on mortality. Int J Environ Res Public Health. 2022;19(5):3075. doi:10.3390/ijerph19053075
37. Zhang H, Zhang Y, Wu J, et al. Risks and features of secondary infections in severe and critical ill COVID-19 patients. Emerg Microb Infect. 2020;9(1):1958–1964. doi:10.1080/22221751.2020.1812437
38. Blumlein D, Griffiths I. Shock: aetiology, pathophysiology and management. Br J Nurs. 2022;31(8):422–428. doi:10.12968/bjon.2022.31.8.422
39. Standl T, Annecke T, Cascorbi I, Heller AR, Sabashnikov A, Teske W. The nomenclature, definition and distinction of types of shock. Dtsch Arztebl Int. 2018;115(45):757–768. doi:10.3238/arztebl.2018.0757
40. Shao M, Li X, Liu F, Tian T, Luo J, Yang Y. Acute kidney injury is associated with severe infection and fatality in patients with COVID-19: a systematic review and meta-analysis of 40 studies and 24,527 patients. Pharmacol Res. 2020;161:105107. doi:10.1016/j.phrs.2020.105107
41. Wang X, Lu J, Song Z, Zhou Y, Liu T, Zhang D. From past to future: bibliometric analysis of global research productivity on nomogram (2000–2021). Front Public Health. 2022;10:997713. doi:10.3389/fpubh.2022.997713
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
