Back to Journals » Cancer Management and Research » Volume 14
Clinical Characteristics of Malignant Pulmonary Sclerosing Pneumocytoma Based on a Study of 46 Cases Worldwide
Authors Zhang W , Cui D, Liu Y, Shi K, Gao X, Qian R
Received 2 June 2022
Accepted for publication 3 August 2022
Published 13 August 2022 Volume 2022:14 Pages 2459—2467
DOI https://doi.org/10.2147/CMAR.S377161
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Bilikere Dwarakanath
Weidong Zhang,1 Dong Cui,1 Yaqian Liu,2 Kefeng Shi,1 Xia Gao,3 Rulin Qian1
1Department of Thoracic Surgery, Henan Provincial Chest Hospital, Zhengzhou City, Henan Province, People’s Republic of China; 2Medical Records and Statistics Room, Henan Provincial Chest Hospital, Zhengzhou City, Henan Province, People’s Republic of China; 3Department of Cardiovascular Surgery, Henan Provincial Chest Hospital, Zhengzhou City, Henan Province, People’s Republic of China
Correspondence: Rulin Qian; Xia Gao, Henan Provincial Chest Hospital, Room 1, Weiwu Road, Zhengzhou City, Henan Province, 450003, People’s Republic of China, Email [email protected]; [email protected]
Objective: To analyze the clinical characteristics of patients with malignant pulmonary sclerosing pneumocytoma (PSP) with metastasis, recurrence, and growth and to improve clinicians’ understanding of PSP in patients with malignant tumor characteristics.
Methods: A total of 46 PSP patients with malignant tumor characteristics were identified in the literature search and compared with 38 patients with benign PSP diagnosed and treated in our hospital in the past 5 years. We explored the pathogenesis, clinical symptoms, diagnostic methods, treatment strategies and prognosis of PSP patients with malignant tumor.
Results: The characteristics of young age (≤ 41 years old), larger tumor (≥ 36mm), lymph node metastasis and distribution in East Asians are indicative of PSP with malignant potential. Such patients should undergo segmental resection or lobectomy, combined with necessary lymph node dissection or biopsy. All patients with PSP should have an entire course of follow-up management, because they may have an adverse prognosis such as recurrence, growth, metastasis, and even death.
Conclusion: PSP has the potential for malignancy. Anatomical lobectomy or segmental resection combined with lymph node dissection should be performed in PSP with some specific characteristics. Inappropriate diagnosis and treatment may lead to poor prognosis in PSP patients.
Keywords: pulmonary sclerosing pneumocytoma, malignant tumor, metastasis, recurrence, growth, treatment and diagnosis
Introduction
Liebow and Hubbell first reported pulmonary sclerosing hemangioma (PSH) in 1956.1 In 2004, the World Health Organization (WHO) proposed that PSH is an intermediate tumor with malignant potential in its histological classification, which is different from that of general benign tumors.2 In 2021, WHO officially renamed primary PSH as PSP and classified it as a pulmonary adenoma.3 PSP is more common in middle-aged female patients in Asia, and its overall incidence is relatively low.4 However, the incidence rate has gradually increased in recent years. Most previous studies5–7 have considered PSP as a single, benign tumor. Most of the PSPs develop slowly and do not progress for many years. Most patients have no obvious symptoms on medical examination. Recently, we found a case of PSP with lymph node metastasis, extrapulmonary metastasis, multiple and growing.8 This patient died of respiratory and circulatory failure caused by PSP. Therefore, by searching the literature of various countries, the authors found that PSP is not a simple benign and harmless nodule, but it may be accompanied by lymph node metastasis, multiple tumors, growth, recurrence, and eventual mortality. But so far, there is no literature report on the systematic study of the clinical characteristics of the type of PSP with malignant characteristics, so we explored the clinical characteristics of PSP patients with malignant tumor characteristics.
In this study, we searched the literature on PSP patients with malignant tumor characteristics (metastasis, recurrence, and growth). There was a total of 40 PSP patients with lymph node or extrapulmonary metastasis,8–48 8 PSP patients with gradually increasing tumor size at follow up, and 6 PSP patients who had relapse at the original location or other locations after the original lesions were removed. We retrieved the medical records of patients with benign PSP who had undergone surgery for PSP in our hospital in the past 5 years for comparative analysis of the clinical characteristics of the these patients and those reported in the literature with malignant tumor characteristics with the goal of to find more appropriate methods of diagnosis and treatment.
Materials and Methods
We used “Pulmonary sclerosing hemangioma, metastasis”, “Pulmonary sclerosing pneumocytoma, metastasis”, “Pulmonary sclerosing hemangioma, recurrence”, and “Pulmonary sclerosing pneumocytoma, recurrence” as the key words in searching the CNKI (China National Knowledge Infrastructure), Wanfang, Google Scholar, and PubMed databases to retrieve a total of 46 patients with malignant tumor characteristics, of which 40 had metastatic characteristics (including 3 cases accompanied by recurrence and 5 cases with a gradual increase in size), and 3 cases with PSP lumps that gradually increased, and 3 cases of PSP relapse. The characteristics of the cases are shown in Tables 1–2.
 |
Table 1 Comparison of Patients’ Characteristics with Malignant and Benign PS |
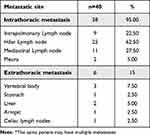 |
Table 2 Metastatic Characteristics of PSP Patients with Metastatic* |
At the same time, 38 cases of benign PSP in patients from our hospital were used as the control group. By analyzing their general characteristics, symptoms, treatment methods, tumor characteristics, and other information, we compared the differences between benign and malignant PSP.
Data presentation: Numerical variables with a normal distribution are displayed as mean ± standard deviation, and those of non-normal distribution are displayed as median (interquartile range). Categorical variables are displayed as frequency (percentage).
Results and Discussion
In 1986, Tanaka et al9 inadvertently found metastasis of hilar lymph nodes in a patient who was diagnosed as having PSP. This discovery revealed that PSP may have malignant characteristics and is not always a benign tumor. Since then, researchers from various countries have gradually reported more PSP patients with lymph node metastasis, extrapulmonary metastasis, gradual tumor growth, and recurrence. The fact that PSP may have certain malignant characteristics has been recognized by an increasing number of researchers, and in 2004 WHO recognized PSP as an intermediate tumor with certain malignant potential, not a simple benign tumor. So we explored the clinical characteristics of PSP patients with malignant tumor characteristics.
General Characteristics
Table 1 shows the increasing number of PSP patients with malignant characteristics being reported since 2000, among which there are more reported cases in 2000–2005 and 2015–2020, accounting for a total of 67.39%. This kind of tumor is mainly distributed in East Asia, mainly in China, Japan, and South Korea (82.61%). It is rarely reported in Europe and the United States, but the reasons for this distribution are still unknown. It may be related to living environment, genetic susceptibility, and so on. This type of tumor occurs more in female patients (75.56%), and the age range of patients with PSP nodules was 10–73 years old (median [IQR]: 41 (25,57) years; mean: 41.87 years); the age distribution of each age group did not show significant differences. Compared with the benign PSP group, patients with malignant PSP were younger [mean (years)41.87: 50.63], and among PSP patients younger than 41 years old, there were significantly more patients with malignant PSP than with benign PSP. On average (50.00%) PSP was detected upon through medical examination and patients did not show any symptoms. Relatively speaking, the symptoms of cough (42.11%), coughing blood (13.16%), breathing difficulties (15.79%) and chest pain (5.26%) are some of the common symptoms of patients with PSP malignant tendencies. More patients in the malignant PSP group showed symptoms than in the benign group, 50.00% and 42.11%, respectively, so the index of suspicion for possible malignant potential should be high in patients showing such symptoms.
Imaging Features
According to the analysis of tumors with malignant characteristics in 46 PSP patients (Table 1), the range of the maximum diameter of PSP tumors was 10–226 mm (median [IQR]: 36 mm [25,65]; mean ± SD: 53.62 ± 46.95 mm), while the range in the benign PSP group was 7–95 mm (median [IQR]: 25.5 mm [20,35.5] mm; mean ± SD: 30.58 ± 18.6 mm). Tumors with diameter greater than 36 mm were found significantly more often in the malignant PSP group than in the benign PSP group (50% and 21.05% respectively, p <0.05), Therefore, when the diameter of PSP exceeds 36 mm, the possibility of malignant PSP should be considered and the treatment strategy should be adjusted accordingly. Because malignant PSP tumors are larger than benign PSP tumors, this kind of tumor may grow faster and have a greater rate of metastasis. Adachi et al28 also holds a similar view. In PSP patients with malignant tumor characteristics, tumors can occur in any lobe of the lungs, multiple lobes of the lungs, or even one side of the chest cavity. PSP patients with tumors in cross-pulmonary lobes had only malignant tumors, accounting for about 20.00% of the malignancies, with more on the left side than the right side (13.33%, 2.22%, respectively, p = 0.02). In 40 PSP patients with metastases, 25 cases described whether there are calcification on the chest CT, of which 20.00% (n = 25) of the patients had calcification, indicating that calcification is not a unique feature of benign tumors, and tumors showing calcification may still affect lymph nodes or produce extrapulmonary metastasis (Table 1).
Pathologic Diagnosis
In 2021, WHO classified PSP as a type of “lung adenoma” and defined it as
a tumor of lung cell origin, which is composed of two kinds of cuboidal surface cells and rounded cells with either eosinophilic or clear cytoplasm. Histology includes papillary, solid, sclerosing, and haemorrhagic areas
that is, the so-called “two cell types, four patterns”, and both kinds of cells have the potential to differentiate into heterotypic cells.3 In this group of patients, 82.22% of the patients were diagnosed as having PSP by postoperative macropathology, the diagnosis rate of preoperative puncture pathology was lower (15.55%), and the intraoperative rapid freezing pathology diagnosis rate was even lower, only 2.22%. Because of the variety of PSP pathological tissues, it is generally difficult to make a definitive diagnosis only by puncture biopsy because of the small sample of material.40 Ordinary hematoxylin and eosin (H&E) staining shows papillary structure, fibrosis, and sclerotic background in PSP and in similar tumors such as lung adenocarcinoma and adenoma; these are difficult to distinguish morphologically,49 and intraoperative frozen sections often do not provide a differential diagnosis or may even misdiagnose PSP (Table 1).
Pathogenesis
As shown in Table 2, among the 40 PSP patients with metastasis, 36 had intrathoracic lymph node metastasis, and the most common metastatic site was the hilar lymph nodes (62.50%). There can also be extrapulmonary metastases, such as to bone, liver, stomach, and distant lymph nodes, of which bone metastases are more common (accounting for 50% of extrapulmonary metastases). During follow up, 5 of 8 patients with enlarged PSP were found to have lymph node or extrapulmonary metastasis. In 6 cases of recurrent PSP, 3 cases had lymph node metastasis, and the site of recurrence was not in the original site, but in other lobes of the lung and even outside the lung.
Pokharel et al31 found that the metastatic lymph nodes in PSP patients are mostly composed of round cells. Sun et al50 believes that round cells may be derived from epithelial-mesenchymal transition (EMT) of surface cells, and EMT may be closely related to tumor metastasis. Matrix metalloproteinase 9 (MMP-9) is a protease that leads to the degeneration of extracellular matrix. It also plays a very important role in tumor development and metastasis.51 Suzuki et al24 found that the expression of MMP-9 is higher in metastatic PSP tumors, which may be related to metastasis and dissemination.
Some researchers have even studied malignant PSP at the genetic level. Jiang et al52 performed Sanger sequencing and next-generation sequencing (NGS) in a patient with multiple PSP and found BRAF V600E mutation and AKT1-E17K pathway activation. He suggested that BRAF V600E mutation and AKT1-E17K pathway activation may be related to PSP carcinogenesis. Zhang et al8 and Fan et al53 through somatic mutation sequencing and pathway analysis also showed that the activation of AKT1 may be involved in the occurrence and development of PSP. Many researchers have discovered the activation of the AKT1-p.E17K pathway in their patients and believe that this activation may be related to the occurrence and metastasis of PSP. However, because of the lack of research on the malignant transformation of PSP, the specific mechanism of PSP transformation is still unclear.
Treatment and Prognosis
At present, the main treatment methods of for PSP are follow-up observation, surgery, and local treatment (radiotherapy, radiofrequency ablation, etc.). Some researchers48 believe that PSP is a benign disease process, even if there are multiple and huge cases and that it does not need surgical resection, and it should just be followed and observed because it will not affect the long-term survival of the patient.22,23 However, as shown by the data in Table 1, surgery is the main treatment for PSP. Local resection (including wedge resection, tumor enucleation, and tumor biopsy) is carried out in most PSP patients, but for those with malignant tumor potential, pulmonary lobectomy (or segmentectomy) combined with lymph node dissection is the main treatment (64.10%). Local treatments such as radiotherapy and radiofrequency ablation can be used for treating metastatic and recurrent tumors. Six of the 8 PSP with growth patients were observed only, and the increase was found after 6–492 months (median [IQR]: 42 months (34.75–120)), and then surgical resection was performed again. One of the patients died after gradual enlargement of the tumor during observation,8 and one patient who was not followed up in a timely manner underwent pneumonectomy because the tumor size increased.46 Some patients even had extrapulmonary metastases.30,35 The reasons that Vaideeswar,22 Chien et al,23 He et al48 and other investigators advocate only the observation of PSP, the authors believe that the observation time for PSP tumors is not long enough, and the PSP patients with tumors with malignant potential cannot be identified in time. The inappropriate observation may cause PSP to progress to malignant lesions, enlargement, local and even distant metastasis, eventually resulting in fatal outcomes. Therefore, the authors believe that lung masses that are considered or diagnosed as PSP should be surgically treated early. The surgical method can be wedge-shaped resection for PSP without malignant potential. However, the authors recommend retaining sufficient margins and do not recommend simple tumor enucleation, which may cause residual tumors and may lead to recurrence at the original site of the tumor. For PSP patients with malignant potential, lobectomy or segmental pneumonectomy combined with lymph node dissection (or biopsy) is recommended. This treatment method can evaluate whether there is lymph node metastasis while removing the tumor. For PSP patients with recurrence or metastasis, it is recommended resecting the foci of recurrence or metastasis again. If the patient does not tolerate surgical treatment, nonsurgical local treatment methods can be selected, such as stereotactic body radiotherapy (SBRT) or radiofrequency ablation, among others. For patients with a newly discovered small PSP and PSP patients after surgical resection, follow-up strategies should be adhered to, and chest CT examination should be conducted every year to detect tumor metastasis and recurrence in time. And the treatment strategy can refer to Figure 1.
 |
Figure 1 PSP diagnosis and treatment process (reference). |
This study has some limitations. The sample size of patients with malignant PSP was even smaller, and the distribution was scattered. The authors could analyze the characteristics of PSP patients with malignant only through globally reported data. Although we draw some conclusions, there is still a lack of multicenter clinical research verification. Despite these shortcomings, the authors believe that our research results can still provide reference for clinicians and provide research ideas for follow up.
Conclusions
We recognize that PSP has the potential for malignancy, and PSP without follow up and treatment may also be fatal. Using punch biopsy and other small tissue pathology procedures makes it difficult to definitively diagnose PSP, and most tumors need to be diagnosed in large tissue specimens after surgical resection. The characteristics of young age, larger tumor (≥ 36 mm), lymph node metastasis, and East Asian residence are clues to the diagnosis of PSP with malignant potential (Figure 2). Such patients should undergo anatomical lobectomy or segmental pneumonectomy combined with necessary lymph node dissection or biopsy. Patients with PSP should have an entire course of follow-up management, because there may be adverse events such as recurrence, growth, metastasis, and even death. In the future, genetic testing may become one of the methods to predict the malignant potential of PSP. The mechanism of PSP metastasis and malignant transformation is unknown and still needs further study.
 |
Figure 2 Clinical characteristics of malignant PSP and Benign PSP. Abbreviations: M, month; Y, year; LLL, left lower lobe; RLL, right lower lobe. |
Ethics Statement
This study was conducted in accordance with the principles of the Declaration of Helsinki, and the study protocol was approved by the Ethics Committee of the Henan Provincial Chest Hospital. Written informed consent has been provided by our hospital patients to have the case details. And another part of patients’ information in this article comes from the references.
Disclosure
The authors declare no competing interests.
References
1. Liebow AA, Hubbell DS. Sclerosing hemangioma (histiocytoma, xanthoma) of the lung. Cancer. 1956;9(1):53–75. doi:10.1002/1097-0142(195601/02)9:1<53::AID-CNCR2820090104>3.0.CO;2-U
2. Travis WD, Brambilla E, Müller-Hermelink HK, et al. Pathology and Genetics: Tumours of the Lung, Pleura, Thymus and Heart. Lyon: IARC; 2004.
3. WHO Classification of Tumours Editorial Board. WHO classification of tumours.
4. Chen B, Gao J, Chen H, et al. Pulmonary sclerosing hemangioma: a unique epithelial neoplasm of the lung (report of 26 cases). World J Surg Oncol. 2013;11(1):85. doi:10.1186/1477-7819-11-85
5. Hu AM, Zhao D, Zheng H, et al. Preoperative diagnosis in 46 cases of pulmonary sclerosing hemangioma. Chin Med J. 2016;129(11):1377–1378. doi:10.4103/0366-6999.182839
6. Khoo AC, Hamzah F, Ong CK. Incidental sclerosing pneumocytoma detected on bone scintigraphy. Clin Nucl Med. 2017;42(1):e77–e79. doi:10.1097/RLU.0000000000001371
7. Zhou J, Covinsky MH. Sclerosing pneumocytoma: a carcinoma mimicker. a case report and literature review. Ann Clin Lab Sci. 2017;47(1):103–105.
8. Zhang W, Liu Y, Chai Y, et al. Case report: rare pulmonary sclerosing pneumocytoma: large, multiple, metastatic, and fatal. Front Med. 2021;8:661032. doi:10.3389/fmed.2021.661032
9. Tanaka I, Inoue M, Matsui Y, et al. A case of pneumocytoma (so-called sclerosing hemangioma) with lymph node metastasis. Jpn J Clin Oncol. 1986;16(1):77–86.
10. Devouassoux-Shisheboran M, Hayashi T, Linnoila RI, et al. A clinicopathologic study of 100 cases of pulmonary sclerosing hemangioma with immunohistochemical studies: TTF-1 is expressed in both round and surface cells, suggesting an origin from primitive respiratory epithelium. Am J Surg Pathol. 2000;24(7):906–916. doi:10.1097/00000478-200007000-00002
11. Chan AC, Chan JK. Pulmonary sclerosing hemangioma consistently expresses thyroid transcription factor-1 (TTF-1): a new clue to its histogenesis. Am J Surg Pathol. 2000;24(11):1531–1536. doi:10.1097/00000478-200011000-00009
12. Yano M, Yamakawa Y, Kiriyama M, et al. Sclerosing hemangioma with metastases to multiple nodal stations. Ann Thorac Surg. 2002;73(3):981–983. doi:10.1016/S0003-4975(01)03122-8
13. Nicholson AG, Magkou C, Snead D, et al. Unusual sclerosing haemangiomas and sclerosing haemangioma-like lesions, and the value of TTF-1 in making the diagnosis. Histopathol. 2002;41(5):404–413. doi:10.1046/j.1365-2559.2002.01522.x
14. Miyagawa-Hayashino A, Tazelaar HD, Langel DJ, et al. Pulmonary sclerosing hemangioma with lymph node metastases: report of 4 cases. Arch Pathol Lab Med. 2003;127(3):321–325. doi:10.5858/2003-127-0321-PSHWLN
15. Kim KH, Sul HJ, Kang DY. Sclerosing hemangioma with lymph node metastasis. Yonsei Med J. 2003;44(1):150–154. doi:10.3349/ymj.2003.44.1.150
16. Chan NG, Melega DE, Inculet RI, et al. Pulmonary sclerosing hemangioma with lymph node metastases. Can Respir J. 2003;10(7):391–392. doi:10.1155/2003/534147
17. Kim GY, Kim J, Choi YS, et al. Sixteen cases of sclerosing hemangioma of the lung including unusual presentations. J Korean Med Sci. 2004;19(3):352–358. doi:10.3346/jkms.2004.19.3.352
18. Wang L, Chen B, Ye ZC. Pulmonary sclerosing hemangioma with lymph node metastasis: a case report. Mod Med Imageol. 2005;14(5):240. Chinese.
19. Katakura H, Sato M, Tanaka F, et al. Pulmonary sclerosing hemangioma with metastasis to the mediastinal lymph node. Ann Thorac Surg. 2005;80(6):2351–2353. doi:10.1016/j.athoracsur.2004.06.099
20. Li J, Lu Q, Jie J. Mediastinal lymph node and pleura metastasis of pulmonary sclerosing hemangioma: case report. Chin J Med Imaging Technol. 2006;22(6):815. Chinese.
21. Jiang ZN, Zhu T, Jing M, et al. Pulmonary sclerosing hemangioma with lymph node metastasis: a case report. Chin J Pathol. 2007;36(4):282. Chinese.
22. Vaideeswar P. Sclerosing hemangioma with lymph nodal metastases. Indian J Pathol Microbiol. 2009;52(3):392–394. doi:10.4103/0377-4929.55004
23. Chien NC, Lin CW, Tzeng JE. Sclerosing haemangioma with lymph node metastasis. Respirology. 2009;14(4):614–616. doi:10.1111/j.1440-1843.2009.01523.x
24. Suzuki H, Saitoh Y, Koh E, et al. Pulmonary sclerosing hemangioma with pleural dissemination: report of a case. Surg Today. 2011;41(2):258–261. doi:10.1007/s00595-009-4220-5
25. Zhou Y Pulmonary sclerosing hemangioma with Hilar lymph node metastasis: case report and related literature review. Zhejiang University, 2013.
26. Bae YS, Ro JY, Shim HS, et al. Pulmonary sclerosing haemangioma with metastatic spread to stomach. Histopathol. 2012;60(7):1162–1164. doi:10.1111/j.1365-2559.2012.04213.x
27. Kita H, Shiraishi Y, Katsuragi N, et al. リンパ節転移をきたした肺硬化性血管腫. [Pulmonary sclerosing hemangioma with lymph node metastasis]. Kyobu Geka. 2013;66(13):1141–1144. Japanese.
28. Adachi Y, Tsuta K, Hirano R, et al. Pulmonary sclerosing hemangioma with lymph node metastasis: a case report and literature review. Oncol Lett. 2014;7(4):997–1000. doi:10.3892/ol.2014.1831
29. Xu HM, Zhang G. A rare case of pulmonary sclerosing hemangioma with lymph node metastasis and review of the literature. Int J Clin Exp Pathol. 2015;8(7):8619–8623.
30. Kim MK, Jang SJ, Kim YH, et al. Bone metastasis in pulmonary sclerosing hemangioma. Korean J Intern Med. 2015;30(6):928–930. doi:10.3904/kjim.2015.30.6.928
31. Pokharel S, Dhillon SS, Ylagan L, et al. Sclerosing pneumocytoma with lymph node metastasis. J Thorac Oncol. 2016;11(10):1802–1804. doi:10.1016/j.jtho.2016.06.005
32. Fayers RW, Lim TS, Havlat MF. Pulmonary sclerosing pneumocytoma (sclerosing haemangioma): radical radiation therapy. J Med Imaging Radiat Oncol. 2016;60(5):693–695. doi:10.1111/1754-9485.12477
33. Su XL, Qi F, Hu H, et al. Pulmonary sclerosing hemangioma with lymph node metastasis: a case report and literature review. J Bengbu Med Coll. 2016;41(7):923–924. Chinese.
34. Soo IX, Sittampalam K, Lim CH. Pulmonary sclerosing pneumocytoma with mediastinal lymph node metastasis. Asian Cardiovasc Thorac Ann. 2017;25(7–8):547–549. doi:10.1177/0218492317727668
35. Zhan XY, Wang JQ, Zhou JX, et al. Pulmonary sclerosing hemangioma with liver metastasis: report of one case and literature review. Chin J Hepat Surg. 2017;1:50–53. Chinese.
36. Wang T, Dong JN. One case: rare diffuse sclerosing pneumocytoma located in full left lung. J Pract Radiol. 2018;34(6):984–985. Chinese.
37. Wang X, Zhang L, Wang Y, et al. Sclerosing pneumocytoma with metastasis to the mediastinal and regional lymph nodes. Indian J Pathol Microbiol. 2018;61(3):407–409. doi:10.4103/IJPM.IJPM_98_17
38. Sakai T, Miyoshi T, Umemura S, et al. Large pulmonary sclerosing pneumocytoma with massive necrosis and vascular invasion: a case report. Oxf Med Case Rep. 2019;2019(7):omz066. doi:10.1093/omcr/omz066
39. Lim B, Jeon W, Han SW, et al. A case of pulmonary sclerosing pneumocytoma with multiple lung and bone metastasis. Am J Respir Crit Care Med. 2019;199:A6950.
40. Lin L, Xu J, Feng Y, et al. Sclerosing pulmonary cell tumor with lymph node metastasis: a case report. J Clin Exp Pathol. 2020;36(1):116–117. Chinese.
41. Kocaman G, Yenigün MB, Ersöz CC, et al. Pulmonary sclerosing pneumocytoma with mediastinal lymph node metastasis: a case report. Gen Thorac Cardiovasc Surg. 2021;69(1):142–146. doi:10.1007/s11748-020-01431-1
42. Gao Q, Zhou J, Zheng Y, et al. Clinical and histopathological features of pulmonary sclerosing pneumocytoma with dense spindle stromal cells and lymph node metastasis. Histopathol. 2020;77(5):718. doi:10.1111/his.14159
43. Odaka E, Shiba M, Mitsunaga S, et al. A resected case of recurrent pulmonary sclerosing hemangioma. Haigann. 1993;33(2):281–287. Japanese. doi:10.2482/haigan.33.281
44. Iyoda A, Hiroshima K, Shiba M, et al. Clinicopathological analysis of pulmonary sclerosing hemangioma. Ann Thorac Surg. 2004;78(6):1928–1931. doi:10.1016/j.athoracsur.2004.05.069
45. Wei S, Tian J, Song X, et al. Recurrence of pulmonary sclerosing hemangioma. Thorac Cardiovasc Surg. 2008;56(2):120–122. doi:10.1055/s-2007-989280
46. Shibata R, Mukai M, Okada Y, et al. A case of sclerosing hemangioma of the lung presenting as a gigantic tumor occupying the left thoracic cavity. Virchows Arch. 2003;442(4):409–411. doi:10.1007/s00428-003-0777-3
47. Hanaoka J, Ohuchi M, Inoue S, et al. Bilateral multiple pulmonary sclerosing hemangioma. Jpn J Thorac Cardiovasc Surg. 2005;53(3):157–161. doi:10.1007/s11748-005-0024-8
48. He C, Fang H, Liu Y, et al. Pulmonary sclerosing hemangioma: report of two cases. World J Surg Oncol. 2012;10:182. doi:10.1186/1477-7819-10-182
49. Yang CH, Lee LY. Pulmonary sclerosing pneumocytoma remains a diagnostic challenge using frozen sections: a clinicopathological analysis of 59 cases. Histopathology. 2018;72(3):500–508. doi:10.1111/his.13391
50. Sun Y, Zhou LX, Zhao M, et al. 孙宇,周立新,赵敏,等. 肺硬化性血管瘤组织起源的探讨[J]. 中华病理学杂志. [Histogenesis of pulmonary sclerosing hemangioma]. Zhonghua Bing Li Xue Za Zhi. 2012;41(4):239–242. Chinese. doi:10.3760/cma.j.issn.0529-5807.2012.04.006
51. Cho SJ, Jin LJ, Kim BY, et al. Increased expression of matrix metalloproteinase 9 and tubulin-alpha in pulmonary sclerosing hemangioma. Oncol Rep. 2007;18(5):1139–1144.
52. Jiang G, Zhang M, Tan Q, et al. Identification of the BRAF V600E mutation in a patient with sclerosing pneumocytoma: a case report. Lung Cancer. 2019;137:52–55. doi:10.1016/j.lungcan.2019.09.004
53. Fan X, Lin L, Wang J, et al. Genome profile in an extremely rare case of pulmonary sclerosing pneumocytoma presenting with diffusely-scattered nodules in the right lung. Cancer Biol Ther. 2018;19(1):13–19. doi:10.1080/15384047.2017.1360443
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
