Back to Journals » Infection and Drug Resistance » Volume 17
Clinical Characteristics of Chlamydia psittaci Infection Diagnosed by Metagenomic Next-Generation Sequencing: A Retrospective Multi-Center Study in Fujian, China
Authors Liu K , Wu L, Chen G, Zeng D, Zhong Q, Luo L, Song B, Ying X, Ni F, Yu L, Xu L, Lin X, Chen X, Zou X, Xiao J, Hu Y
Received 10 October 2023
Accepted for publication 31 January 2024
Published 21 February 2024 Volume 2024:17 Pages 697—708
DOI https://doi.org/10.2147/IDR.S443953
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Kaixiong Liu,1– 3,* Luling Wu,4,5,* Gongping Chen,1– 3,* Dunhuan Zeng,1– 3 Qiwei Zhong,6 Li Luo,1,2 Bin Song,7 Xiuhong Ying,1,2 Fayu Ni,8 Lifang Yu,8 Liyu Xu,9 Xin Lin,9 Xiaoyan Chen,10 Xin Zou,11 Jianhong Xiao,7 Yuekai Hu5,12,13
1Department of Respiratory and Critical Care medicine, National Regional Medical Center, Binhai Campus of the First Affiliated Hospital, Fujian Medical University, Fuzhou, People’s Republic of China; 2Department of Respiratory and Critical Care Medicine, First Affiliated Hospital of Fujian Medical University, Fuzhou, People’s Republic of China; 3Institute of Respiratory Disease, Fujian Medical University, Fuzhou, People’s Republic of China; 4Institute of Antibiotics, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; 5Department of Infectious Diseases, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; 6Department of Respiratory and Critical Care Medicine, Minnan Hospital, the First Affiliated Hospital of Fujian Medical University, Quau zhou, People’s Republic of China; 7Department of Respiratory and Critical Care Medicine, Mindong Hospital Affiliated to Fujian Medical University, Ningde, People’s Republic of China; 8Department of Respiratory, Fuqing General Hospital Affiliated to Fujian Medical University, Fuzhou, People’s Republic of China; 9Department of Respiratory and Critical Care Medicine, Fuzhou First Hospital of Fujian Medical University, Fuzhou, People’s Republic of China; 10Department of Emergency Medicine, Zhangzhou Affiliated Hospital of Fujian Medical University, Zhangzhou, People’s Republic of China; 11Department of Respiratory and Critical Care Medicine, LongYan First Hospital Affiliated to Fujian Medical University, Longyan, People’s Republic of China; 12Department of Infectious Diseases, First Affiliated Hospital of Fujian Medical University, Fuzhou, People’s Republic of China; 13Department of Infectious Diseases, National Regional Medical Center, Binhai Campus of the First Affiliated Hospital, Fujian Medical University, Fuzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yuekai Hu; Jianhong Xiao, Email [email protected]; [email protected]
Objective: This study aimed to describe and compare the epidemiological, demographic, clinical, laboratory and radiological characteristics as well as the complications, treatments, and outcomes of these patients.
Methods: We retrospectively investigated clinical data of patients with C. psittaci infection (psittacosis) in eight Grade IIIA hospitals of Fujian. Metagenomic next-generation sequencing (mNGS) was used identify C. psittaci in clinical samples of all included patients.
Results: A total of 74 patients (39 severe/35 non-severe) was diagnosed with psittacosis, 25 (33.8%) of whom had history of poultry exposure. Common symptoms included high fever (98% [37/74]), fatigue (52.7% [39/74]), and dyspnea (51.4% [38/74]). Common manifestations in imaging included consolidation (89.2%), pleural effusion (77.0%), and air bronchogram (66.2%). Common complications included acute respiratory distress syndrome (55.4% [41/74]), type I respiratory failure (52.7% [39/74]), acute liver injury (41.9% [31/74]), and secondary infection (27.0% [20/74]). The in-hospital mortality rate was 8.11% (6/74).
Conclusion: C. psittaci infection is represents an underestimated cause of CAP. For SCAP patients with poultry and bird contact history, specimens were encouraged to be sended for mNGS test in time. C. psittaci infection can lead to severe, multiple system involvement, and several complications. mNGS facilitate timely diagnosis of C. psittaci infection.
Keywords: Chlamydia psittaci, metagenomic next-generation sequencing, community-acquired pneumonia, bronchoalveolar lavage fluid
Introduction
Community-acquired pneumonia (CAP) is a severe infection of the lower respiratory tract that frequently results in admission to the intensive care unit (ICU),1 and severe CAP (SCAP) is associated with particularly high mortality rates, reaching 23–47%.2 SCAP, which represents 15–28% of all CAP cases, is commonly caused by atypical pathogens, such as Legionella pneumophila, Chlamydia pneumoniae, or Chlamydia psittaci.3 C.psittaci is a zoonotic intracellular bacterial pathogens that can infect a broad range of animal hosts and occasionally, humans.4 C. psittaci infection in humans, also known as psittacosis, is usually believed to be an uncommon and occurs sporadically worldwide, typically presenting as CAP.5 C. psittaci has been reported as the causative agent in 1.03% of all CAP cases, and 7.3–7.5% of all SCAP, constituting a public health risk.6–9
Disease severity ranged from mild to severe, and severe cases can present with fulminant sepsis, acute respiratory distress syndrome (ARDS), multiple organ dysfunction syndrome (MODS) and even death.10 Psittacosis-related mortality can range from 0% to 20% among different geographic regions and clinics,6–8 and C. psittaci is classified as a biothreat agent by the United States Centers for Disease Control and Prevention (CDC) due to its potentially life-threatening symptoms (https://www.cdc.gov/nndss). Since its symptoms are relatively non-specific and current testing methods are limited, psittacosis is easily under-diagnosed, misdiagnosed, or diagnosed late. Moreover, standard tests in CAP patients do not typically include C. psittaci infection.11 Currently, there are culture-based methods, PCR-based assays, and various serological methods available to test for C. psittaci infection.12 However, serological tests for C. psittaci are prone to cross-reaction with other chlamydial species, false negatives in the early acute phase, and delays between symptom onset and laboratory-based diagnosis in the interval before convalescent samples are available. These cumulative factors have subsequently hindered efforts to determine its precise incidence and prevalence, resulting in underestimation of psittacosis incidence globally.13,14
Metagenomic next-generation sequencing (mNGS) has emerged as an efficient, high throughput method for simultaneous identification of different pathogens, including bacteria, fungi, viruses, eukaryotic parasites, and novel pathogens in clinic, and has led to increased detection of rare and unexpected pathogens.15 Thus, mNGS can provide a relatively rapid diagnosis to expedite antibiotic treatments and improve patient prognosis. mNGS has been used in the confirmed diagnosis of C. psittaci infection in recent years, which limited to a few case reports and a small case series.16–18 mNGS seemed superior to the traditional methods in diagnosis of C. psittaci infection. Till now, there are no reports of CP colonization in humans. Moreover, CP is not common microbial contaminants present in the clinical sample. Positive mNGS results using specimens from sputum, blood, or BALF can serve as diagnostic criteria of C. psittaci infection. Furthermore, there are no detail data in China concerning on C. psittaci infection. The objective of this study was to describe clinical, laboratory, and radiological characteristics, treatment, and outcomes of patients with C. psittaci infection diagnosed by mNGS and to compare the clinical features between severe ill and non-severe patients.
Methods
Study Design and Setting
This retrospective study was performed by collecting data from medical records of confirmed C. psittaci infection patients referred to six hospital between April 1, 2021 and March 30, 2022. C. psittaci infection was confirmed by mNGS in serial samples of sputum, endotracheal aspirate (ETA), broncho-alveolar lavage fluid (BALF), or serum. The study was approved by the ethical review board of each participating clinical center. Written informed consent was waived for this retrospective study. The present study was performed in accordance with the Helsinki Declaration. Specimens were placed in a sterile sputum container, stored at −20°C, and then sent to Jieyi Genomics Institute (Hangzhou, China) for detection. The workflow of mNGS for sample collection and processing includes nucleic acid extraction, library construction, sequencing, and sequence data analysis, as previously described.16 Severe pneumonia was diagnosed according to guidelines of the American Thoracic Society/Infectious Disease Society of America.11 Demographics data, clinical symptoms and signs, laboratory tests, radiological characteristics, treatments, and outcomes data were retrieved.
Radiological Assessment
Two radiologists independently interpreted all chest CT scans and were blinded to the clinical information of each patient. The major CT indications were described using international standard nomenclature defined by the Fleischner Society glossary.19
Statistical Analysis
Categorical data was presented as counts and percentages, and continuous data were expressed as mean ± SD if the data were normally distributed, or expressed as median with interquartile range (IQR) values. Means for continuous variables were compared using independent group Students t tests when the data were normally distributed and the Mann–Whitney test in cases of non-normal distribution. Proportions for categorical variables were compared using the χ2 test. A P value less than 0.05 was considered statistically significant. All statistical analyses were done with SPSS version 20.
Results
Demographic Data of Included Patients
In total, 74 patients with C. psittaci infection diagnosed by mNGS were enrolled in this study. The full demographic and clinical characteristics of the patients are presented in Table 1. All cases were community acquired. Of these, 39 patients required ICU admission, and were therefore classified as severe cases. The large majority of our sample were obtained from the respiratory system, with 71 (96.0%) from BALF, four from serum (5.4%), and three from cerebrospinal fluid (CSF, 8.4%). The mean turnaround time for mNGS ranged from 24 to 48 h after receipt by sequencing facility.
 |
Table 1 Demographics and Clinical Characteristics of Patients with C. psittaci Infection |
The median age of these patients (51 males and 23 females) was 64.5 (55.7, 74.0) years. Sex distribution and age did not differ significantly between the severe and non-severe groups (p = 0.951 and p = 0.729, respectively). Forty (54.1%) had coexisting conditions, including hypertension (27.0%), diabetes (18.9%), and malignancy (5.4%). Underlying diseases did not significantly differ between the two groups (p = 0.150). There was a distinct seasonality to the cases with 33/74 (44.6%) presenting in the 3-month period from October to December (Figure 1). The monthly distribution of onset peaked in November and December, with relatively few cases occurring in warmer months (Figure 1).
 |
Figure 1 Cumulative hospital admissions of psittacosis per month. |
Sixty-six (89.2%) patients lived in rural areas, and 46.0% of the patients had a history of environmental exposure. Severe patients had more contact history than non-severe patients (61.5% vs 28.6%, p = 0.004). History of avian exposure was similar between the two severity groups (10.3% vs.14.3%, p = 0.727). Seven patients worked as either poultry slaughterers or breeders. Ten patients had slaughtered live poultry for cooking. Eleven patients had raised ducks or chickens at home privately. Nine patients had raised birds (pet parrots [n = 4], pigeon [n = 3], Garrulax canorus [n = 1], peacock [n = 1]).
Disease Severity
Duration from onset of symptoms to admission ranged from 1 to 15 days, with a median of 5.5 days. The median time from hospitalization to confirmed diagnosis was 4.0 days. The duration from onset of symptoms to confirmed diagnosis and hospitalization to confirmed diagnosis was similar (9.0 days vs 10.0 days, p = 0.064, and 4.0 days vs 5.0 days,p = 0.155). Severe patients had higher CURB65, sepsis-related organ failure assessment (SOFA), and APACHE II than non-severe patients (all p<0.05).
Clinical Symptoms and Signs
The most common symptoms on admission were high fever (64/74 [86.5%]), productive cough (50.00% [37/74]) and dyspnea (51.4% [38/74]); less common symptoms included dry cough (11/74 [28%]), headache (13.5% [10/74]), haemoptysis (6.8% [5/74]), vomit (4.1% [3/74]), and diarrhea (6.8% [5/74]) (Table 1). Compared with non-severe patients, severe patients were more likely to report dyspnea (79.5% [31/39] vs 20.0% [7/35] in severe vs non-severe respectively; p<0.0001), and delirium (13/39 [33.3%] vs 2/35 [5.7%]; severe vs non-severe respectively; p = 0.003) at presentation. Relative bradycardia was found in 38 patients (51.4% [38/74]). Relative bradycardia and dry rale did not differ between groups (all p>0.05).
Laboratory Testing
Laboratory findings between the two groups were shown in Table 2. The levels of creatinine, urea nitrogen, procalcitonin, D-dimer on admission were significantly higher in the severe group compared with the non-severe group (all p<0.05), while lymphocyte, platelet counts, and albumin were all lower upon admission in the severe group than in the non-severe group (all p<0.05). Other laboratory parameters, including white blood cell counts, neutrophil counts, and lactate dehydrogenase levels were notsignificantly different between groups (all p>0.05). Lymphocytopenia was identified in around 60% of the total cohort and in 81.5% of the severe patients, and 29.1% of all patients had thrombocytopenia. Lymphocytopenia, proteinuria and hematuresis were more common in the severe group compared with the non-severe group (all p<0.05).
 |
Table 2 Laboratory Findings and Chest CT Findings Upon Hospital Admission with C. psittaci Infection |
CT Findings on Admission
On admission, abnormalities in chest CT images were detected among all patients (Table 2). In the full cohort, 64.9% (48/74) of patients presented with bilateral lung involvement while 74.3% (55/74) presented with multi-lobe involvement. Multiple lobular involvement was more common in severe patients than in non-severe patients (84.6% [33/39] vs 62.9% [22/35], p = 0.032). Subpleural lesions were more common than central lung lesions (63.5% vs 12.2%). The most common findings of chest CT images were consolidation (89.2% [66/74]), pleural effusion (77.0% [57/74]), and air bronchogram (66.2% [49/74]) (Figures 2 and 3). In contrast, ground-glass opacity (GGO, 20.2% [15/74]) and pericardial effusion (6.8% [5/74]) were less common in CT imaging of the full cohort. Bilateral pleural effusion was more common in severe patients than in non-severe patients (56.4 [22/39]% vs 28.6% [10/35]; p = 0.016).
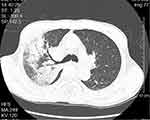 |
Figure 2 Chest imaging of a 67-yr-old man with C. psittaci infection show multiple consolidations in the right upper and lower lobe, with air bronchogram in the upper lobe. |
Complications
During the hospital stay, 81.2% (69/74) of patients presented with common complications involving multiple vital organs, including ARDS (55.4% [41/74]), followed by type I respiratory failure (52.7% [39/74]), acute liver injury (41.9% [31/74]), secondary infection (27.03% [20/74]), anemia (25.7% [19/74]), acute myocardial injury (18/74 [24.3%]), and acute heart failure (21.6% [16/74]) (Table 3). The less common complications included acute or chronic heart failure (6.76% [5/74]), rhabdomyolysis (6.76% [5/74]), diffuse intravascular coagulation (6.76% [5/74]), and gastrointestinal bleeding (4.05% [3/74]). 16.2% (12/74), 18.9% (14/74). The median time from admission to ARDS in severe patients was 24.0 (7.5, 42.0) hours. 24.3% [18/74] of all patients progressed to multi-organ dysfunction (MODS). Time from admission to MODS was 36.0 (24.0, 48.0) hours.
 |
Table 3 Complications, Treatments and Outcomes of Patients with C. Psittaci Infection |
Treatments
Most patients were treated with doxycycline (73.0%) or moxifloxacin (73.0%). Forty-three patients (58.1%) did not receive any initial targeted treatment with activity against C. psittaci. Compared with the severe group, more patients received broad-spectrum antibiotic treatment than in the non-severe group (61.5% vs 34.3%; p=0.019). More severe patients than non-severe patients received combination therapy (64.1% vs 37.1%; p = 0.035). 31.1% patients (23/74) received high-flow nasal cannula therapy and 39.2% (29/74) received non-invasive ventilation. Compared with non-severe patients, severe patients were more likely to receive mechanical ventilation, either invasively or non-invasively. Seventeen of the severe patients (43.6%) received invasive mechanical ventilation, one of whom received extracorporeal membrane oxygenation (ECMO) as a rescue therapy, while six died during the ICU stay. The duration of invasive mechanical ventilation in severe patients was 212.0 (166.5, 240.0) hours. Four patients received CRRT as salvage therapy.
Outcomes
The in-hospital mortality of patients was 8.11% (6/74) (Table 3). No patients in the non-severe group died during their hospital stay. The average length of hospital stay for severe patients was significantly longer than for non-severe patients (17 days vs 13 days, p = 0.001). The average length of ICU stay for severe patients was 7.0 (0.0, 10.0) days.
Discussion
To our knowledge, the present study represents the largest case series in China to date describing the demographic, clinical, laboratory, and radiological characteristics as well as the complications, treatments, and outcomes of these patients with C. psittaci infection. We found that C. psittaci infection does not appear to be rare in China. C. psittaci infection is an important emerging etiology of SCAP, but is not well described in epidemiologic studies of CAP. Our study also compared clinical characteristics between severe and non-severe patients to facilitate accurate diagnosis and treatment.
C. psittaci has been reported in relatively few case studies of CAP. Dumke et al developed a real-time qPCR assay to specifically detect members of family Chlamydiaceae in pharyngeal swabs of CAP patients, and found C. psittaci in 1.4% of CAP cases.20 Sun and co-workers used mNGS to detect etiologies of SCAP in Beijing, China and reported that C. psittaci is responsible for ~9.0% (4 of 44) of SCAP.8 Similarly, Wu and colleagues et al found a prevalence of 7.3% (24 of 329) C. psittaci infection in mNGS-based diagnosis of BALF samples in a multicenter, prospective study of 329 adult SCAP patients.9 A nationwide, multicenter study of SCAP etiology in China that employed a combination of traditional culture-based assays, antigen tests, PCR assays, and mNGS diagnostics revealed that C. psittaci infection contributed to 6.8% (15/222) of the SCAP cases.10 mNGS can facilitate C. psittaci detection in the relatively easily acquired BALF samples of CAP patients.9,10 In this study, Psittacosis was detected in serum samples of three patients in the severe group, presumably due to high bacterial load, though possibly attributable to detection of circulating bacterial DNA fragments. Other studies reported C. psittaci in stool and CSF specimens detected by PCR, suggesting that these specimens could also facilitate mNGS-based diagnosis.13
Most cases of the reported C. psittaci infection were from southern China.10 Behavioural and environmental factors, such as altitude, temperature, and developed poultry breeding industry contribute to the higher prevalence of psittacosis in southern China than that in northern China, and thus warrant further study. Fujian province has a population of 39.41 million, and the number of cases reported from this province annually is higher than the total number reported from several countries, including Japan and the United State.13,14 However, it remains unknown whether C. psittaci infection is endemic to Fujian or if a high genetic susceptibility among Han Chinese contributes to this rate. The epidemiology of C. psittaci infection in our study follows seasonal clustering and more than three quarters of the patients came from rural areas. It is speculated that the temperature is related to the pathogen growth. It should also be noted that males comprised a predominant proportion of these cases. Although the reason is unclear why psittacosis patients in this study were more likely to be older, it is reasonable to speculate that older people have a greater likelihood of entering close contact with poultry in rural China. C. psittaci can be found in many species of poultry or other birds, sheep, and horses; and in the United States and Japan, domesticated psittacine birds (eg, parrots and cockatoos) have been identified as the most common source of psittacosis.21 C. psittaci typically enters hosts through the respiratory tract via inhalation of aerosolised bacteria in bird secretions, droppings, or feathers.4 These studies suggest that the live poultry in markets and small breeding operations prevalent throughout rural China likely serve as the largest reservoirs for psittacosis. Thus, we regarded contact with poultry as the greatest risk factor for C. psittaci infection in our study, and especially in severe cases. Aggressive intervention to limit further animal-to-person transmission in live poultry markets warrants careful consideration. Many of the cases in previous reports involved a history of avian contact, particularly with psittacines and racing pigeons. Diagnosis and treatment of SCAP should include a thorough enquiry into the patients poultry and avian exposure history.
Notably, a greater proportion of the psittacosis patient cohort in our study was identified as severe, which aligned well with previous studies.13,17 Indeed, another study from China reported mortality rates as high as 20% among adult patients with severe C. psittaci infection.10 Disparities in fatality rates among studies are potentially attributable to ethnicity, pathogen genotype, and pathogen exposure level. We found that abrupt onset of symptoms was frequent in this study cohort, with fever representing the most common symptom, followed by dyspnea, and cough, which aligned with other reports.17,18 Some patients displayed non-specific flu-like symptom, including pharyngeal congestion or sore throat. Dyspnea was significantly higher in the severe group than in the non-severe group, which is likely a manifestation of impaired cardiopulmonary function. Unlike typical bacterial pneumonia in which the clinical findings are confined to the lungs, multi-system symptoms involving gastrointestinal and neurological presentations were not uncommon in this series. Psittacosis is often mistaken for gastroenteritis or meningitis in the absence of respiratory symptoms, and without exposure history. In addition, no association has yet been established between symptoms of C. psittaci infection and pathogen genotype. Various genotypes are known to exhibit differences in host preference and virulence characteristics, which can overlap, thereby confounding identification. In this cohort, lobar consolidation accompanied by air bronchogram in chest CT scans was the main finding, which agreed well with findings by Qu and Su et al.10,17 Complications were frequent among severe patients in this study. The pathophysiology leading to multi-organ injury in C. psittaci infection is poorly understood. ARDS was the most common complication among severe cases. C. psittaci infects pulmonary epithelial cells where it then proliferates, leading to alveolar and endothelial injury.22,23 Damage to the alveolar-capillary barrier, inflammation, and the accumulation of detritus and protein-rich fluid in alveolae can cause ARDS. Compromising the alveolar-capillary membrane can allow pathogen into the bloodstream, resulting in sepsis.22,23 In severe cases, C. psittaci has been shown to infect the kidneys, liver, and central nervous system,19,24 and the detection of C. psittaci DNA in CSF suggested that this pathogen could directly invade the central nervous system,13 indicating encephalitis. Our series recorded one case C. psittaci in cerebrospinal fluid. It is possible that the acute kidney injury observed in our study may have been related to direct effects of C. psittaci, hypoxia, and shock. Cardiac injury has been associated with other respiratory diseases such as avian flu and COVID-19, and is potentially lethal if it develops into fulminant myocarditis. We observed cardiac injury in almost 70% of severe patients in our study, in sharp contrast with previous studies that reported relatively few cases of C. psittaci endocarditis and myocarditis. The pathogenesis of cardiac injury and exacerbated congestive heart failure associated with C. psittaci infection remains unclear. Co-infection was common in our study, especially in severe cases, and could possibly reflect impaired immunity and barrier function, and significant lymphocyte reduction, although this phenomenon has not been described in other studies.
CAP management guidelines suggest that severe patients should be treated with a combination therapy that includes quinolone or on admission, and severe cases in this study were given the most effective antibiotic based on clinical suspicion without waiting for laboratory confirmation. However, empirical treatment with doxycycline, macrolide, and quinolone antibiotics, which covers this rare, should be considered for an avian or poultry source of infection indicated by contact history. Given the high mortality of psittacosis, we suggest that severe patients receive double or triple drug combination therapies. There is a prominent lack of data concerning drug resistance among Chlamydiae species, and randomized, controlled clinical trials could validate or substantially improve interventions for severe patients. However, no significant conclusions can be drawn from this study on the efficacy of particular therapeutic interventions. Future work should also explore the use of corticosteroid therapies in severe cases, in light of the role of inflammation in exacerbating pulmonary dysfunction. Corticosteroids were shown to reduce mortality for severe COVID-19 patients, but should be administered cautiously in treatment of severe C. psittaci infection due to potential side effects.
Some limitations of the study are listed below: First, data were collected retrospectively and recall bias could have influenced data. Because there are many confounding factors due to retrospective study conducted in different hospitals, we did not perform risk factor analysis in current study. Second, clinical follow-up data were not obtained from patients after discharge, and future work will assess the long-term effects or outcomes of C. psittaci infection. Third, the institutions included in this study are major hospitals in Fujian may have introduced bias in group selection, so caution should be exercised in the interpretation of our results between severe and non-severe cases possibly related to selection bias. Fourth, verification with serological tests and/or cultures should be included in future work to support the mNGS-based diagnoses.
Conclusion
Our study provides physicians with a better understanding of the demographic, clinical, laboratory and radiological characteristics of C. psittaci infection, as well as possible complications, treatment strategies, and outcomes of patients with severe infection. These findings are especially relevant for clinicians in China, where the prevalence appears higher than that reported in other countries. Clinicians should be aware of such disease entity in CAP. Early detection of C. psittaci infection is critical to informing clinical interventions and appropriately targeted antibiotic. We also propose mNGS as an effective and quick diagnostic method that can improve accuracy and reduce delays in identifying psittacosis. Further study of the pathogenesis of psittacosis will improve its management, epidemic control, and pandemic preparedness.
Data Sharing Statement
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.
Ethics Statement
The ethics committees of the First Affiliated Hospital of Fujian Medical University, Mindong Hospital, Fuqing General Hospital, Fuzhou First Hospital, Zhangzhou Affiliated Hospital of Fujian Medical University and LongYan First Hospital jointly approved the study. Because it is a retrospective study, the ethics Committees approved it without informed consent. All research data are anonymous. This study follows the Helsinki Declaration.
Funding
This work was supported by grants from the Fujian Provincial Health Technology Project (2020GGA053), Joint Funds for the Innovation of Science and Technology of Fujian Province (No.2060304) and the Natural Sciences Foundation of Fujian Province (2021J01228).
Disclosure
The authors report no potential conflicts of interest in this work.
References
1. Torres A, Chalmers JD, Dela Cruz CS, et al. Challenges in severe community-acquired pneumonia: a point-of-view review. Intensive Care Med. 2019;45(2):159–171. doi:10.1007/s00134-019-05519-y
2. Cilloniz C, Dominedo C, Garcia-Vidal C, Torres A. Community-acquired pneumonia as an emergency condition. Curr Opin Crit Care. 2018;24(6):531–539. doi:10.1097/MCC.0000000000000550
3. Cillóniz C, Torres A, Niederman M, et al. Community-acquired pneumonia related to intracellular pathogens. Intensive Care Med. 2016;42(9):1374–1386. doi:10.1007/s00134-016-4394-4
4. Radomski N, Einenkel R, Müller A. Chlamydia-host cell interaction not only from a bird’s eye view: some lessons from Chlamydia psittaci. FEBS Lett. 2016;590(21):3920–3940. doi:10.1002/1873-3468.12295
5. Beeckman DS, Vanrompay DC. Zoonotic Chlamydophila psittaci infections from a clinical perspective. Clin Microbiol Infect. 2009;15:11–17. doi:10.1111/j.1469-0691.2008.02669.x
6. Hogerwerf L, Gier DE, Baan B. Chlamydia psittaci (psittacosis) as a cause of community-acquired pneumonia: a systematic review and meta-analysis. Epidemiol Infect. 2017;145(15):3096–3105. doi:10.1017/S0950268817002060
7. Spoorenberg SM, Bos WJ, van Hannen EJ, et al. Chlamydia psittaci: a relevant cause of community-acquired pneumonia in two Dutch hospitals. Neth J Med. 2016;74(2):75–81.
8. Sun T, Wu X, Cai Y, et al. Metagenomic next-generation sequencing for pathogenic diagnosis and antibiotic management of severe community-acquired pneumonia in immunocompromised adults. Front Cell Infect Microbiol. 2021;11:661589. doi:10.3389/fcimb.2021.661589
9. Wu X, Li Y, Zhang M, et al. Etiology of severe community-acquired pneumonia in adults based on metagenomic next-generation sequencing: a prospective multicenter study. Infect Dis Ther. 2020;9(4):1003–1015. doi:10.1007/s40121-020-00353-y
10. Qu J, Zhang J, Chen Y, et al. Aetiology of severe community acquired pneumonia in adults identified by combined detection methods: a multi-centre prospective study in China. Emerg Microbes Infect. 2022;11(1):556–566. doi:10.1080/22221751.2022.2035194
11. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and Treatment of Adults with Community-acquired Pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200(7):e45–e67. doi:10.1164/rccm.201908-1581ST
12. Nieuwenhuizen AA, Dijkstra F, Notermans DW, van der Hoek W. Laboratory methods for case finding in human psittacosis outbreaks: a systematic review. BMC Infect Dis. 2018;18(1):442. doi:10.1186/s12879-018-3317-0
13. McGovern OL, Kobayashi M, Shaw KA, et al. Use of real-time PCR for Chlamydia psittaci detection in human specimens during an outbreak of Psittacosis - Georgia and Virginia, 2018. MMWR Morb Mortal Wkly Rep. 2021;70(14):505–509. doi:10.15585/mmwr.mm7014a1
14. Kozuki E, Arima Y, Matsui T, et al. Human psittacosis in Japan: notification trends and differences in infection source and age distribution by gender, 2007 to 2016. Ann Epidemiol. 2020;44:60–63. doi:10.1016/j.annepidem.2020.03.001
15. Simner PJ, Miller S, Carroll KC. Understanding the promises and hurdles of metagenomic next-generation sequencing as a diagnostic tool for infectious diseases. Clin Infect Dis. 2018;66(5):778–788. doi:10.1093/cid/cix881
16. Fischer N, Rohde H, Indenbirken D, et al. Rapid metagenomic diagnostics for suspected outbreak of severe pneumonia. Emerg Infect Dis. 2014;20(6):1072–1075. doi:10.3201/eid2006.131526
17. Zhang Z, Zhou H, Cao H, et al. Human-to-human transmission of Chlamydia psittaci in China, 2020: an epidemiological and aetiological investigation. Lancet Microbe. 2022;3(7):e512–e520. doi:10.1016/S2666-5247(22)00064-7
18. Su S, Su X, Zhou L, et al. Severe Chlamydia psittaci pneumonia: clinical characteristics and risk factors. Ann Palliat Med. 2021;10(7):8051–8060. doi:10.1080/22221751.2021.1948358
19. Zhang H, Zhan D, Chen D, et al. Next-generation sequencing diagnosis of severe pneumonia from fulminant psittacosis with multiple organ failure: a case report and literature review. Ann Transl Med. 2020;8(6):401. doi:10.21037/atm.2020.03.17
20. Dumke R, Schnee C, Pletz MW, et al. Mycoplasma pneumoniae and Chlamydia spp. infection in community-acquired pneumonia, Germany, 2011-2012. Emerg Infect Dis. 2015;21(3):426–434. doi:10.3201/eid2103.140927
21. Balsamo G, Maxted AM, Midla JW, et al. Compendium of measures to control Chlamydia psittaci infection among humans (Psittacosis) and pet birds (Avian Chlamydiosis), 2017. J Avian Med Surg. 2017;31(3):262–282. doi:10.1647/217-265
22. Branley JM, Weston KM, England J, Dwyer DE, Sorrell TC. Clinical features of endemic community-acquired psittacosis. New Microbes New Infect. 2014;2(1):7–12. doi:10.1002/2052-2975.29
23. Knittler MR, Berndt A, Böcker S, Jiang Y. Chlamydia psittaci: new insights into genomic diversity, clinical pathology, host-pathogen interaction and anti-bacterial immunity. Int J Med Microbiol. 2014;304(7):877–893. doi:10.1016/j.ijmm.2014
24. Wu HH, Feng LF, Fang SY. Application of metagenomic next-generation sequencing in the diagnosis of severe pneumonia caused by Chlamydia psittaci. BMC Pulm Med. 2021;21(1):300. doi:10.1186/s12890-021-01673-6
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.