Back to Journals » Infection and Drug Resistance » Volume 16
Clinical Characteristics, Diagnosis, and Management of Aseptic Meningitis Induced by Trimethoprim-Sulfamethoxazole
Authors Fan Z, He Y, Sun W, Li Z, Zhu M, Wang C
Received 11 June 2023
Accepted for publication 30 August 2023
Published 5 September 2023 Volume 2023:16 Pages 5825—5832
DOI https://doi.org/10.2147/IDR.S425464
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Zhiqiang Fan,1 Yang He,1 Wei Sun,2 Zuojun Li,2 Min Zhu,3 Chunjiang Wang2
1Department of Pharmacy, The First Hospital of Hunan University of Chinese Medicine, Changsha, Hunan, 410007, People’s Republic of China; 2Department of Pharmacy, The Third Xiangya Hospital, Central South University, Changsha, Hunan, 410013, People’s Republic of China; 3Department of Ophthalmology, Central South University, Changsha, Hunan, 410013, People’s Republic of China
Correspondence: Chunjiang Wang, Department of Pharmacy, The Third Xiangya Hospital, Central South University, No. 138 Tongzipo Road, YueLu District, Changsha, Hunan, 410013, People’s Republic of China, Email [email protected]
Objective: Trimethoprim sulfamethoxazole (TMP-SMX) is related to aseptic meningitis. However, a detailed description of its phenotype is lacking, which easily leads to misdiagnosis. The purpose of this article is to explore the clinical characteristics of TMP-SMX-induced aseptic meningitis (TSIAM).
Methods: We collected literature related to TSIAM published before July 31, 2023, by searching Chinese and English databases. Data were extracted and analyzed descriptively.
Results: The 55 patients were mostly female (60.0%), with a median age of 43 years (range: 2.5– 90 years). The first onset time was from a few minutes to 3 months after administration, and the time of reonset was within 12 hours. Fever (98.2%), headache (78.2%), altered mental status (42.3%), nausea and vomiting (41.8%), and neck pain (34.5%) were the most common symptoms. In severe cases, patients presented with low blood pressure, seizures, unconsciousness, or coma. Typical cerebrospinal fluid analysis showed elevated white blood cell counts, with polymorphonuclear leukocytes predominating, elevated protein levels, and normal glucose levels. Brain imaging usually showed no abnormalities. Symptoms resolved rapidly after the discontinuation of TMP-SMX, within a median time of 2 days (range: 1, 60). Readministration of TMP-SMX led to another relapse of aseptic meningitis. Aseptic meningitis usually culminated in a full recovery, although one patient experienced permanent paraplegia.
Conclusion: Clinicians should be aware that aseptic meningitis is a rare adverse effect of TMP-SMX. TMP-SMX should be discontinued in patients with TSIAM to reduce unnecessary testing and treatment, and readministration of TMP-SMX should be avoided.
Keywords: aseptic meningitis, trimethoprim-sulfamethoxazole, drug-induced aseptic meningitis, headache, trimethoprim
Introduction
Aseptic meningitis is defined as an acute community-acquired syndrome with leukocytosis in cerebrospinal fluid (CSF) and negative Gram staining and culture, without a parameningeal focus or a systemic illness.1 Enteroviruses are the most common etiologies of aseptic meningitis, while other less common etiologies include autoimmune diseases, leukemia, vaccines and drugs.2 Drug-induced aseptic meningitis (DIAM) is a rare disease that occurs mainly in women and patients with autoimmune disorders. The most commonly involved drugs are nonsteroidal anti-inflammatory drugs (NSAIDs), antibiotics, intravenous immunoglobulins, monoclonal antibodies and intrathecal drugs.3–5
Trimethoprim-sulfamethoxazole (TMP-SMX), a combination of sulfamethoxazole (SMX) and trimethoprim (TMP) in a ratio of 1:5, is approved for urinary tract infections, otitis media, shigellosis, and Pneumocystis carinii pneumonia.6 TMP-SMX, currently available as an oral and intravenous preparation, is widely distributed in sputum, cerebrospinal fluid, prostatic fluid, and bile after absorption. The serum half-lives of TMP and SMX are 8 to 10 hours and 10 hours, respectively. This half-life can be extended to 20 to 30 hours or more in the case of impaired renal function.3 The most common adverse effects of TMP-SMX are gastrointestinal reactions, anaphylaxis, hematotoxicity, and nephrotoxicity.6 Aseptic meningitis is a rare complication of TMP-SMX. However, the clinical characteristics of TMP-SMX-induced aseptic meningitis (TSIAM) have not been clearly characterized. The purpose of this study was to explore the clinical characteristics and general rules of TSIAM, increase clinical awareness, and provide a reference for the rational use of TMP-SMX, avoidance of adverse reactions and treatment with this drug.
Methods
Search Strategy and Data Sources
Case reports, case series, original studies, clinical trials and reviews of TMP-SMX-related aseptic encephalitis were searched through PubMed/Medline, Embase, Cochrane Library, China Biomedical Literature Database, CNKI, Wanfang, Chinese Science and Technology Journal database and other databases. The retrieval period was from database establishment to July 31, 2023. The search was conducted by combining subject terms and keywords, including trimethoprim - sulfamethoxazole, trimethoprim, sulfamethoxazole, cotrimoxazole, aseptic meningitis, meningitis, encephalitis, meningoencephalitis, and neurologic toxicities.
Inclusion and Exclusion Criteria
Case reports and case series were included. Reviews, duplicate literature, animal studies, clinical studies and studies involving patients without a CSF analysis were excluded.
Data Extraction
According to the self-designed table, we extracted the authors, publication years, sex, age, medical history, usage and dosage, concomitant medications, onset time, number of episodes, clinical symptoms and signs, laboratory examination, cerebrospinal fluid examination, imaging examination, electroencephalograph, treatment and outcomes.
Data Analysis
Descriptive analysis was conducted using SPSS version 20.0 (IBM, New York, US). Continuous data are presented herein as the median value (minimum, maximum). Count data are presented as n (%).
Results
Basic Information
After the independent literature screening by two authors, a total of 46 studies were finally included in this study according to the inclusion and exclusion criteria (Figure 1). Table 1 summarizes the basic information of the 55 patients. Of the 55 patients, 33 (60%) were women with a median age of 43 years (range 2.5–90). Forty-eight patients (87.3%) developed aseptic meningitis to TMP-SMX; 6 patients (10.9%), to TMP; and 1 patient (1.8%), to SMX. TMP-SMX was mainly used for urinary tract infection (34 cases, 61.8%), Pneumocystis carinii pneumonia (PCP) prophylaxis (8 cases, 14.5%) and skin and soft tissue infection (6 cases, 10.9%). Ten patients (18.2%) were diagnosed with autoimmune diseases, and 6 patients (10.9%) had human immunodeficiency virus (HIV). Six patients (10.9%) used other drugs simultaneously. Prior to this admission, 22 patients (40.0%) had been exposed to TMP-SMX, 19 patients (34.5%) had meningitis symptoms, and 3 patients (5.5%) did not have meningitis. The first onset of meningitis occurred within 12 h in 27 patients (50.9%), 1–3 d in 12 patients (22.6%), 4–10 d in 9 patients (17.0%), and 14–90 d in 5 patients (9.4%). Meningitis occurred within 12 hours after the reintroduction of TMP-SMX in 28 patients.
 |
Table 1 Basic Information of 55 Patients with TSIAM |
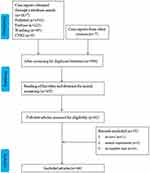 |
Figure 1 Flow chart of the study selection process for reported cases of trimethoprim-sulfamethoxazole-induced aseptic meningitis. |
Clinical Signs and Symptoms
The clinical symptoms in 55 cases of meningitis are summarized in Table 2. Twenty-four patients (43.6%) had one episode of symptoms, and 31 patients (56.4%) had more than two episodes of symptoms, with a maximum of five episodes. Fever (54 cases, 98.2%) was the most common symptom, followed by headache (43 cases, 78.2%), altered mental status (42.3%), nausea and vomiting (23 cases, 41.8%), neck pain (19 cases, 34.5%), and confusion (15 cases, 27.3%). Nineteen patients (34.5%) experienced high fever, up to 41.4 °C. Other symptoms and signs included photophobia, myalgia, lethargy, seizure, nuchal rigidity (8 cases, 14.5%), conjunctival injection, neck stiffness, mental status changes, hypotension, allergy (7 cases, 12.7%), rigors, and chills (5 cases, 9.1%).
 |
Table 2 The Clinical Symptoms and Laboratory Tests of 55 Patients with TSIAM |
Laboratory Tests
The peripheral blood cell count showed that 22 (40.0%) of the 36 patients had normal white blood cell counts. C-reactive protein increased in 4 (7.3%) of 6 patients.
Cerebrospinal Fluid Analysis
The results of the CSF analysis are summarized in Table 3. Of the 50 patients, 47 (85.5%) had normal glucose levels, and 3 (5.5%) had elevated glucose levels. Of the 53 patients, 46 patients (83.4%) had elevated protein levels, with a median level of 114 mg/dl (range 34, 1144). In 46 patients, 41 patients (74.5%) had leukocytosis, with a median level of 140.5/mm3 (range 0, 4300). Thirty-four patients (61.8%) had elevated polymorphonuclear leukocytes (PMNs), with a median level of 77% (0, 100). The red blood cell count was increased in 18 patients (32.7%) and normal in 4 patients (7.3%). The cerebrospinal fluid was cloudy in 3 (5.5%) of the eight patients reported. Lumbar puncture documented a normal opening pressure in 9 patients (16.4%) and an opening pressure of 250 mmH2O in one patient (1.8%).
 |
Table 3 Cerebrospinal Fluid, Imaging Examination and Electroencephalogram Examination of 55 Patients with TSIAM |
Electroencephalogram
Six patients (10.9%) underwent an electroencephalogram (EEG) examination, of whom four patients (7.3%) showed encephalitis, epidural activity, global dysfunction and continuous left greater than right frontal slowing.
Imaging Examination
The imaging examinations of 55 patients are summarized in Table 3. Computed tomography (CT) examination of 31 patients showed normal density (30 cases, 56.4%) and hypodensity throughout the cerebral white matter (1 case, 1.8%). Magnetic resonance imaging (MRI) of 10 patients showed white matter abnormalities in 2 patients (3.6%).
Treatment and Prognosis
All 55 patients finally stopped using TMP-SMX, and 41 patients (74.5%) were given empirical antibiotic treatment. Fifty-three patients (96.4%) recovered completely at a median time of 2 days (range 1, 60), 1 patient (1.8%) did not report their outcome, and 1 patient (1.8%) had permanent sequelae of nervous system paralysis.
Discussion
Aseptic meningitis is a rare disease with an uncertain incidence. Many cases remain undetected due to limited diagnostic methods. Drug-induced aseptic meningitis (DIAM) is an exclusive diagnosis.6 TSIAM is independent of age, with the youngest reported patient being 2.5 years old and the oldest being 90 years old.7,8 Most patients usually develop symptoms within 24 hours of first exposure to TMP-SMX, but one patient was reported to have been taking TMP/SMX for 3 months before symptoms developed.9 The time until meningitis occurrence after re-exposure to TMP-SMX was shorter than that after the first exposure to TMP-SMX.
TSIAM is more common in women than in men. This may be because autoimmune diseases are more common in women, and it may be that women are also more susceptible to DIAM.10 Another possibility is that women have significantly more urinary tract infections than men, and TMP-SMX is the first line of treatment.11 DIAM is more likely to occur in patients with autoimmune conditions, mainly SLE.5,12 It is estimated that up to 60% of SLE patients have inflammation-related central nervous system symptoms at some stage of their disease that predispose them to DIAM.3 SLE is a typical immune complex-mediated disease that may be related to the specific binding of IgG antibodies to drugs and their metabolites in CSF.3 In addition, it may be that alterations in adhesion molecules play a role in directing leucocytes to the CNS, resulting in DIAM.
TSIAM can present with symptoms similar to those in other types of meningitis, including fever, headache, nausea, vomiting, altered mental status and meningeal symptoms, although many other rare manifestations may also occur. Notably, both TMP and SMX in TMP-SMX have the potential to induce aseptic meningitis. In patients with a history of TSIAM, TMP-SMX and its component drugs should be avoided whenever possible. For the diagnosis of TSIAM, the available methods are limited. It may be unethical to rechallenge patients with TMP-SMX. CSF analysis in TSIAM is variable. There are 100–2000 white blood cells per microliter, with PMN predominance. It is not clear whether the white blood cell count in aseptic meningitis is related to the type or severity of the drug. Lymphocytes and eosinophils are also present in a small number of patients. Proteins in the CSF are usually elevated, while the glucose levels remain normal. The results of CSF analysis, as well as the associated time for symptoms to resolve after discontinuation, suggest a diagnosis of TSIAM. Previous exposure to TMP-SMX resulting in similar symptoms can further increase the diagnostic accuracy.
The pathophysiology of TSIAM has not been fully elucidated. One hypothesis is that the lipophilicity and low molecular weight of TMP-SMX allow it to cross the blood brain barrier and may also cause direct chemical stimulation of the meninges.13 Another hypothesis is type III (immune complex-mediated) or type IV (delayed) hypersensitivity to the meninges.14 Sulfonamide drugs and intermediates combine with proteins or peptides to form pro-hapten, which is recognized by T lymphocytes. In addition to hypersensitivity, the p-i concept may also play a role in TSIAM, which involves the direct recognition of drugs or metabolites by T-cell receptors.15
There are no clear guidelines for drug-induced aseptic encephalitis. Many patients with meningitis are initially treated with empiric antimicrobial agents. Symptoms of DIAM usually resolve soon after discontinuation of the drug. In patients with TSIAM, symptoms usually resolve within 2 to 3 days, consistent with the serum half-lives of TMP and SMX.6 One patient with systemic lupus erythematosus had neurological sequelae of permanent paralysis because it may provoke recurrent CNS relapses of their autoimmune disease.16 Ultimately, 96% of patients made a complete recovery.
Limitations
There are some limitations in this study. The sample size was small, and we excluded patients who did not have their CSF examined. The data are mainly based on case reports and case series and are incomplete in many case reports. Examples include previous allergies, TMP dose, degree of fever, liver and kidney function, and follow-up. Factors such as simultaneous treatment with multiple drugs and the presence of underlying diseases cannot be ruled out. Further clinical trial data are needed to determine the clinical characteristics and risk factors for TSIAM.
Conclusion
Clinicians and pharmacists should have a better understanding of TSIAM and its severity and should conduct early identification and timely discontinuation of TMP-SMX to reduce morbidity. More prospective studies are needed to support the establishment of guidelines to help with the early diagnosis of aseptic encephalitis without subjecting patients to a series of costly and invasive tests.
Ethical Considerations
This study did not require an ethical board approval because the study was a retrospective study and did not involve sensitive personal information.
Funding
This study was supported by Natural Science Foundation of Hunan Province (2023JJ40499), Scientific Research Fund Project of Hunan University of Chinese Medicine (2022XYLH004).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Shukla B, Aguilera EA, Salazar L, et al. Aseptic meningitis in adults and children: diagnostic and management challenges. J Clin Virol. 2017;94:110–114. doi:10.1016/j.jcv.2017.07.016
2. Agabawi S. Trimethoprim-sulfamethoxazole-induced aseptic meningitis: a rare presentation of commonly used antibiotic. Case Rep Infect Dis. 2019;2019:4289502. doi:10.1155/2019/4289502
3. Yelehe-Okouma M, Czmil-Garon J, Pape E, Petitpain N, Gillet P. Drug-induced aseptic meningitis: a mini-review. Fundam Clin Pharmacol. 2018;32(3):252–260. doi:10.1111/fcp.12349
4. Bihan K, Weiss N, Théophile H, Funck-Brentano C, Lebrun-Vignes B. Drug-induced aseptic meningitis: 329 cases from the French pharmacovigilance database analysis. Br J Clin Pharmacol. 2019;85(11):2540–2546. doi:10.1111/bcp.14073
5. Jolles S, Sewell WA, Leighton C. Drug-induced aseptic meningitis: diagnosis and management. Drug Saf. 2000;22(3):215–226. doi:10.2165/00002018-200022030-00005
6. Smilack JD. Trimethoprim-sulfamethoxazole. Mayo Clin Proc. 1999;74(7):730–734. PMID: 10405706. doi:10.4065/74.7.730
7. Masters PA, O’Bryan TA, Zurlo J, Miller DQ, Joshi N. Trimethoprim-sulfamethoxazole revisited. Arch Intern Med. 2003;163(4):402–410. doi:10.1001/archinte.163.4.402
8. van Asperdt JAA, De Moor RA. Aseptic meningitis, hepatitis and cholestasis induced by trimethoprim/sulfamethoxazole: a case report. BMC Pediatr. 2021;21(1):345. doi:10.1186/s12887-021-02820-y
9. Andrade A, Hilmas E, Walter C. A rare occurrence of trimethoprim/sulfamethoxazole (TMP/SMX)-induced aseptic meningitis in an older woman. J Am Geriatr Soc. 2000;48(11):1537–1538. doi:10.1111/jgs.2000.48.11.1537
10. Therrien R. Possible trimethoprim/sulfamethoxazole-induced aseptic meningitis. Ann Pharmacother. 2004;38(11):1863–1867. doi:10.1345/aph.1D581
11. Fairweather D, Rose NR. Women and autoimmune diseases. Emerg Infect Dis. 2004;10(11):2005–2011. PMID: 15550215; PMCID: PMC3328995. doi:10.3201/eid1011.040367
12. Tuleja E, Bourgarit A, Abuaf N, Le Beller C, Séréni D. Rifampin-induced severe aseptic meningitis. Rev Med Interne. 2010;31(9):e1–e3. French. doi:10.1016/j.revmed.2009.07.022
13. Elmedani S, Albayati A, Udongwo N, Odak M, Khawaja S. Trimethoprim-sulfamethoxazole-induced aseptic meningitis: a new approach. Cureus. 2021;13(6):e15869. doi:10.7759/cureus.15869
14. Bruner KE, Coop CA. White KM: trimethoprim-sulfamethoxazole induced aseptic meningitis-not just another sulfa allergy. Ann Allergy Asthma Immunol. 2014;113(5):520–526. doi:10.1016/j.anai.2014.08.006
15. Dorn JM, Alpern M, McNulty C, Volcheck GW. Sulfonamide Drug Allergy. Curr Allergy Asthma Rep. 2018;18(7):38. doi:10.1007/s11882-018-0791-9
16. River Y, Averbuch-Heller L, Weinberger M, et al. Antibiotic induced meningitis. J Neurol Neurosurg Psychiatry. 1994;57(6):705–708. doi:10.1136/jnnp.57.6.705
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
