Back to Journals » Infection and Drug Resistance » Volume 16
Clinical Characteristics Associated with Poor Prognosis of Acquired Immunodeficiency Syndrome Patients Complicated with Disseminated Talaromycosis marneffei
Received 9 August 2023
Accepted for publication 26 October 2023
Published 7 November 2023 Volume 2023:16 Pages 7097—7108
DOI https://doi.org/10.2147/IDR.S434695
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Lianpeng Wu,1– 3 Yong Pan,1,2 Ke Xu1– 3
1Department of Clinical Laboratory Medicine, The Ding Li Clinical College of Wenzhou Medical University, Wenzhou, 325000, People’s Republic of China; 2Department of Clinical Laboratory Medicine, Wenzhou Central Hospital, Wenzhou, 325000, People’s Republic of China; 3Key Laboratory of Diagnosis and Treatment of New and Recurrent Infectious Diseases of Wenzhou, Wenzhou, 325000, People’s Republic of China
Correspondence: Ke Xu, Wenzhou Central Hospital, Key Laboratory of Diagnosis and Treatment of New and Recurrent Infectious Diseases of Wenzhou, The Ding Li Clinical College of Wenzhou Medical University, No. 252 Baili East Road, Lucheng District, Wenzhou, 325000, People’s Republic of China, Tel +86-13566290303, Email [email protected]
Purpose: To analyze the clinical characteristics of AIDS with dTSM, especially in patients with poor prognosis.
Patients and Methods: One hundred and seventy AIDS patients were enrolled in this single-center retrospective study. The epidemiological characteristics, clinical manifestations, laboratory tests, imaging examination, and treatment outcome were collected. Logistic regression analysis was used to estimate the risk of mortality in AIDS patients with dTSM. The predictive value was evaluated using the receiver operating characteristic (ROC) curve.
Results: From 2015 to 2022, the incidence of AIDS with dTSM in the Wenzhou region increased yearly, mainly in young adults. The mortality rate was 16.47%. The most common clinical manifestations were lymph-node enlargement (92.35%) and fever (78.24%). Multivariate logistic regression analysis showed that procalcitonin (PCT), blood urea nitrogen (BUN), shock, and antiretroviral therapy (ART) were the risk factors for poor outcomes. The model comprised four risk factors and showed an excellent prediction performance, with an AUC of 0.987 in the training cohort (95% CI: 0.946– 0.999) and 0.976 in the validation cohort (95% CI: 0.887– 0.999).
Conclusion: This study suggested that PCT, BUN, shock, and ART were associated with the prognosis and outcome of AIDS with dTSM and had a specific predictive value.
Keywords: acquired immunodeficiency syndrome, Talaromycosis marneffei, clinical characteristics, risk factor, prediction model, poor prognosis
Introduction
Talaromycosis marneffei (TSM) is an invasive fungal disease caused by infection with Talaromyces marneffei (formerly Penicillium marneffei). The regional source of infection was concentrated in Southeast Asia.1 Talaromyces marneffei is an opportunistic pathogenic fungus in the genus Penicillium. The lung is the earliest organ involved and may be infected through inhalation of airborne spores, with most patients presenting with extensive disseminated damage.2 Disseminated TSM (dTSM) is mainly endemic to southern China. Most dTSM cases are reported in Guangdong and Guangxi Provinces.3
Immunodeficiency or immunocompromised hosts are extremely susceptible to infection, such as patients with acquired immunodeficiency syndrome (AIDS).4 Once AIDS patients are infected with Talaromyces marneffei, it is easy to form dTSM with the involvement of multiple organs. The etiology and pathogenesis of dTSM are complex, and the onset is insidious and lacks specific clinical manifestations. In addition, the disease develops rapidly. If left untreated, the infection is associated with a high fatality rate.5 It is necessary to analyze the potential risk factors for a poor prognosis. In this study, we retrospectively analyzed the clinical characteristics of AIDS patients with dTSM and the risk factor of the poor prognosis in our hospital over the past seven years. In addition, using the logistic regression analysis, we established a nomogram prediction model to predict the risk of poor prognosis. We aim to prove clinically beneficial for optimizing therapy for AIDS patients complicated with dTSM infection.
Materials and Methods
Study Subjects
From October 2015 to July 2022, 170 inpatients diagnosed with AIDS complicated with dTSM admitted to the Department of Infectious Diseases of Wenzhou Central Hospital were selected as the research subjects. The diagnosis of AIDS followed the diagnostic criteria of the Chinese Guidelines for the Diagnosis and Treatment of HIV/AIDS (2021 Edition).6 It was confirmed by the Western blot test of the Wenzhou Center for Disease Control and Prevention. The diagnosis of dTSM was based on positive blood or bone marrow culture and identification of Talaromyces marneffei. This study has been approved by the hospital medical ethics (batch No. L2022-01-094) and used retrospective and anonymous data collection methods, which did not involve patient privacy. All experiments were carried out in compliance with relevant laws and guidelines and with the ethical standards of the Declaration of Helsinki.
Data Collection
Medical records of 170 AIDS patients with dTSM were reviewed. The clinical information was collected retrospectively, including the basic information (age, sex, habitual residence, HIV infection route, occupation, and admission time), baseline clinical characteristics (fever, superficial lymphadenopathy, anemia, serous cavity effusion, cough and sputum, fatigue and poor appetite, chills, splenomegaly, rash), laboratory tests and CT imaging examination, comorbidities, treatment regimens, prognosis and outcome.
Statistical Analysis
Statistical analysis was performed using SPSS software (version 22.0, IBM, New York, USA) and R software (version 4.1.5, R Foundation for Statistical Computing, Vienna, Austria). Kolmogorov–Smirnov statistics were used for the normality test. Normal distribution measurement data were expressed as mean ± standard deviation, and the comparison between groups was performed by t-test. Non-normal distribution data was represented by M (P25, P75), and comparisons were analyzed with the Mann–Whitney U-test. Count data were expressed as percentages, and the chi-squared test was used for intergroup comparison. Univariate and multivariate logistic regression analyses were performed to identify independent predictors for poor outcomes. The R software rms package was used to establish a nomogram prediction model. Internal validation was done using Bootstrap. The calibration curve of the prediction model was founded to evaluate the difference between the predicted and actual probability. The concordance index (C-index) and receiver operating characteristic curve (ROC) analysis were applied to evaluate the discrimination performance of the nomogram. Hosmer-Lemeshow goodness of fit test was used to assess the model fit. The test level was defined as α=0.05, and the difference was statistically significant at P<0.05.
Results
Subject Characteristics and Clinical Manifestations
A total of 170 AIDS patients complicated with dTSM were included in this study. The median age is 37 (30–47) years old, 149 males and 21 females, of which 121 cases (71.18%) were young adults (18–45 years old). The habitual residents of the patients were all in Wenzhou City. The main route of HIV infection was sexual transmission (61.54% by heterosexual contact and 30.77% by homosexual contact). In addition, most of the patients were unemployed (90.00%). According to the annual distribution of admission time, the incidence of AIDS patients complicated with dTSM rose gradually yearly.
The most common clinical manifestations of AIDS patients complicated with dTSM were lymph-node enlargement (92.35%) and fever (78.24%) (Table 1). In addition, 146 of 170 cases had co-infection disease. The main co-infection types were oral candidiasis (33.53%), pulmonary infection (31.76%), cytomegalovirus infection (24.12%), and Pneumocystis jirovecii pneumonia (21.18%) (Table 1). Chest computed tomography (CT) scan revealed that 168 cases had pulmonary abnormalities, including mediastinal/hilar lymph node enlargement, the patchy or punctate shadow in lung fields, pericardial effusion, and pleural effusion. Abdominal CT scan revealed that 138 cases had abdominal abnormalities, and the main types were retroperitoneal and inguinal lymph node enlargement, splenomegaly, pelvic effusion, and ascites effusion.
 |
Table 1 The Demographics Clinical Manifestations of AIDS Patients Complicated with dTSM |
Clinical and Laboratory Characteristics Comparison Between the Patients with Different Outcomes
Among the 170 patients, 142 were improved and discharged, 28 died (The mortality rate was 16.47%). One hundred and sixty-five cases received antifungal therapy (of which 141 improved, and 24 died), mainly including amphotericin B combined with itraconazole (68/165, 41.21%), amphotericin B combined with voriconazole (46/165, 24.85%), voriconazole alone (35/165, 21.21%). One hundred and thirty-nine cases received antiretroviral therapy (ART) (of which 132 improved, and 7 died). Specifically, of the 24 patients who died after receiving antifungal therapy, 17 did not receive ART. There was no significant difference in gender between the survival group and the death group. The age of the patients in the death group was older than that in the survival group (P < 0.05, Table 1). The proportion of dyspnea and shock in the death group was significantly higher than in the survival group (Table 1). The other clinical manifestations were not significantly different between the two groups. The death group’s D-dimer, PCT, AST, BUN, LDH, and CRP were significantly higher than those in the survival group. In contrast, platelet and ALB in the death group were significantly lower than in the survival group (Table 1). The proportion of tuberculosis in the death group was higher than in the survival group, and the difference was statistically significant. However, there was no significant difference in other co-infection diseases (Table 1). In the death group, the patients had a lower proportion of received antifungal therapy and ART than the survival group (85.71.00% vs 99.30% for antifungal therapy, P < 0.01; 25.00% vs 92.96% for ART, P < 0.001, Table 1). In addition, patients who received amphotericin B combined with itraconazole had a higher survival rate than the patients who received amphotericin B combined with voriconazole (97.1% vs 82.6%, P < 0.05) or voriconazole alone (97.1% vs 80%, P < 0.05). The median length of hospital stay for the death group was significantly shorter than that for the survival patients (P < 0.001, Table 1).
Risk Factors Analysis for Mortality in AIDS Patients with dTSM in Uni- and Multivariate Analysis
The Age, dyspnea, shock, antifungal therapy, ART, tuberculosis, platelet, D-dimer, PCT, AST, ALB, BUN, LDH, and CRP levels of the AIDS patients complicated with dTSM were analyzed by logistic test for risk factors for death. Univariate analysis showed that Age, dyspnea, shock, antifungal therapy status, ART status, tuberculosis, platelet, D-dimer, PCT, ALB, AST, BUN, and LDH levels were the risk factors for mortality in AIDS patients complicated with dTSM (Table 2).
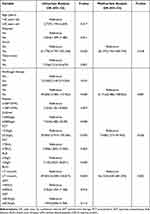 |
Table 2 Logistic Regression Analysis of the Risk Factors for Death from AIDS Patients with dTSM |
Multivariate logistic regression analysis was performed to evaluate the risk factors for mortality while adjusting for confounding variables. The results showed that shock (OR=28.197; 95% CI:1.764–450.744; P<0.05), ART (OR=61.716; 95% CI:5.086–748.822; P<0.01), higher level of BUN (≥7.1mmol/L; OR=56.153; 95% CI: 4.630–681.094; P<0.01) and higher level of PCT (≥2.0ug/L; OR=7.648; 95% CI:1.273–45.950; P<0.05) exhibited a statistically significant association with fatal outcome.
Prediction Model Construction and Evaluation
To prevent overfitting, we performed a Spearman correlation analysis with the four risk factors (PCT, BUN, shock, and ART). None of the four risk factors was found to be significantly highly correlated (all R < 0.80) (Figure 1). Therefore, four risk factors were used to establish a nomogram of the risk of fatal outcome (Figure 2). The C-index of the nomogram prediction model was as high as 0.981 (95% CI: 0.959–1.000) after bootstrap analyses with 1000 resamplings. The calibration curve showed that the predicted probability of the nomogram model was consistent with the actual probability (Figure 3).
 |
Figure 1 Spearman correlation coefficient of four risk factors. Abbreviations: PCT, procalcitonin; BUN, blood urea nitrogen; ART, antiretroviral therapy. |
 |
Figure 2 Nomogram model for predicting death in acquired immunodeficiency syndrome (AIDS) patients complicated with disseminated Talaromycosis marneffei (dTSM). |
 |
Figure 3 Correction curve of nomogram model for predicting death in acquired immunodeficiency syndrome (AIDS) patients complicated with disseminated Talaromycosis marneffei (dTSM). |
Logistic regression was trained with the four risk factors to predict the outcome of AIDS patients with dTSM. To obtain an accurate prediction model, we randomly divided the patients into two cohorts: the training cohort (n=120) and the validation cohort (n=50). We found that our prediction model showed an excellent prediction performance, with an AUC of 0.987 in the training cohort (95% CI: 0.946–0.999, Figure 4a) and 0.976 in the validation cohort (95% CI: 0.887–0.999, Figure 4b). In the training cohort, the model predicted poor outcome with a sensitivity of 95.0% and a specificity of 99.0%. In the validation cohort, the model achieves a sensitivity of 87.5% and a specificity of 100.0%. The best diagnostic cut-off value was set to 0.464 according to the Youden-index (Youden-index=0.94). When the predictive value of the model is more than 0.464, it is considered that the patient may present with a poor prognosis.
 |
Figure 4 Receiver operating characteristic curve (ROC) of the prediction model developed in the training (a) and validation (b) cohorts. |
Discussion
Talaromyces marneffei was first found in the liver of bamboo rats at the Pasteur Institute of South Vietnam in 1956 and first reported in human infection in the United States in 1973. It was not until 1988 that the first case of AIDS combined with TSM infection was reported.7 With the epidemic of AIDS, the number of TSM cases reported worldwide has gradually increased, which has become the third opportunistic infection disease of AIDS patients.8 This study showed that the number of AIDS patients with dTSM in Wenzhou increased yearly from 2015 to 2022, the same conclusion as Lai’s study in Fujian, China.9 The distribution of TSM shows regional characteristics. It is prone to occur in areas with warm and humid climates. Specific air humidity can promote the growth of Talaromyces marneffei and the exposure/release of spores, thus increasing the risk of human infection.10 Wenzhou is located on the southeast coast of China, and the environmental humidity is relatively high from April to June each year. June is the typical plum rain season, and the environment is humid. In this study, the proportion of AIDS patients with dTSM admitted from April to June was the highest, consistent with the environmental humidity changes. The median age of the patients in this study was 37 (30–47) years old, mainly young adults. Male patients accounted for most of the patients, related to the high prevalence of HIV in young adults and the high incidence of HIV infection in men who have sex with men (MSM).
The immune systems of AIDS patients are compromised. Once AIDS patients are infected with Talaromyces marneffei, it is easy to form dTSM. After engulfing Talaromyces marneffei, the macrophages massively proliferate and then cause systemic disseminated infection through the blood and lymphatic system circulation. Talaromyces marneffei mainly invades blood vessels, the monocyte-phagocyte system, and the reticuloendothelial system, causing the involvement of multiple organs and systems.5 The clinical manifestations of AIDS patients with dTSM are often complex and diverse, such as fever, fatigue, cough, flesh loss, rash, anemia, hepatosplenomegaly, lymphadenopathy, or serous membrane fluid.11 The results of this study were also similar. “Small volcano” verrucous rash is a relatively typical clinical feature of TSM, which has referential significance for clinical diagnosis. Ying et al showed that 44.5% of AIDS patients with dTSM had characteristic rash,12 which the percentage was 42.94% in this study. AIDS patients with dTSM are prone to be complicated with other opportunistic infections under the dual effects of HIV attacking the immune system and Talaromyces marneffei involving multiple organ systems. In this study, oral candidiasis, pulmonary infection, and cytomegalovirus infection were the main co-infection types, which made the clinical manifestations of AIDS patients with dTSM more complicated. Therefore, no matter whether AIDS patients have a characteristic rash or not, dTSM infection should be highly suspected when they have clinical symptoms of multi-organ and multi-system involvement.
The current guidelines recommend diagnostic methods available for TSM are relatively time-consuming. In recent years, there is an increasing number of researches which reported the novel diagnostic techniques in order to improve the early diagnosis of TSM.13–16 Clinically, AIDS patients with CD4 T lymphocytes less than 50 cells/μL are classified as advanced AIDS patients.17 In this study, 81.18% of patients had CD4 T lymphocytes less than 50 cells /μL, indicating that dTSM infection mainly occurred in advanced AIDS. The same view has been put forward in Pruksaphon’s report,18 suggesting that patients with advanced AIDS should be actively screened for TSM. The results of the blood routine test showed that ALT, AST, BUN, and LDH were increased to different extents, and ALB was significantly decreased, suggesting that the patient was prone to liver, kidney, and heart function damage. The significant increase in PCT and C-reactive protein indicated that the patient may have a significant inflammatory response. Based on the imaging assay results, the lung was the most frequently involved organ in AIDS patients with dTSM, similar to Shen’s report.19 However, the proportion of each pulmonary abnormality was different. A possible explanation might be the two studies’ different sample sizes and case composition.
AIDS patients complicated with dTSM developed rapidly. Previous studies showed that the mortality of AIDS patients with dTSM was about 10–20%,7 and the mortality in this study was 16.47%. Most of the patients received antifungal therapy and achieved good results. In particular, patients who treated with amphotericin B combined with itraconazole had better survival. In addition, 17 of 24 died patients received antifungal therapy but did not receive ART. Therefore, initiating ART and antifungal therapy is vital to inhibit HIV replication and restore patients’ immune function to reduce mortality. Furthermore, this study comprehensively analyzed the general condition, clinical features, laboratory and imaging examination results, comorbidities, and treatment regimens of the patients and identified that serum PCT (≥ 2.0ng/mL), serum BUN (≥ 7.1mmol/L), shock, and failure to initiate ART were the risk factors for death in AIDS patients with dTSM. However, Klus et al20 have found that intravenous drug use, higher respiratory rate, higher absolute lymphocyte count, a concurrent respiratory infection, or central nervous system infection were the independent predictors of death due to HIV. Sun et al21 showed that low CD4 and CD8 cell counts were the risk factors for poor prognosis of HIV patients with Talaromyces marneffei infection. One possible reason for the discrepancy is the difference in the sample size, severity of the disease, and analysis indicators of cases included in these two studies. BUN is one of the indicators of renal function. The level of serum BUN significantly increased and was associated with the poor prognosis in AIDS patients complicated with dTSM. The results are mainly attributed to the following reasons. Talaromyces marneffei mainly invades the mononuclear phagocytic system, which can cause renal damage. Amphotericin B, the primary drug used in antifungal therapy, has more significant toxicity and side effects, which is easy to cause BUN elevation.22 Some ART drugs, such as Tenofovir Disoproxil Fumarate, have particular damage to renal function.23 PCT is one of the indicators for the diagnosis of sepsis, which is positively correlated with the severity of the disease. AIDS patients with dTSM are likely to be complicated with sepsis, so patients with a high level of PCT have a poor prognosis. Wei et al24 confirmed that the high level of PCT is one of the factors of poor prognosis in patients with TSM. The shock was a life-threatening abnormal pathophysiological state. The cause of shock in AIDS patients with dTSM may be related to the toxins produced by Talaromyces marneffei. More and more research has confirmed that septic shock is a risk factor for the death of AIDS patients.25–28 Among HIV patients infected with Talaromyces marneffei in Beijing Ditan Hospital, septic shock accounted for 10.2% of the total death causes, ranking fourth.29 A prediction result of hospitalization mortality of HIV/AIDS patients with Talaromyces marneffei infection in Guangxi showed that septic shock was the most important predictor of patient death.25 These results suggested that clinicians should be familiar with the early symptoms and signs of shock and treat the primary diseases actively to prevent the occurrence of shock. If the shock has developed, timely treatment should be taken to curb the development of the disease and improve the prognosis of patients. Previous studies have confirmed that Talaromyces marneffei clearance mainly depends on macrophages. During the clearance process, IFN-γ secreted by T lymphocytes activated the macrophages, which can destroy intracellular spores and inhibit the growth of Talaromyces marneffei.30 Several studies31,32 have shown that timely initiation of ART can significantly reduce the mortality of patients. Influential ART can not only inhibit the replication of HIV and promote the reconstruction of immune function but also indirectly enhance the effect of antifungal therapy. Therefore, ART should be started as soon as possible for eligible AIDS patients to reduce mortality.
In recent years, more and more models have been used to evaluate the incidence and prognosis of diseases. The nomogram prediction model can numerically, graphically, and visualize the results of multivariate logistic regression analysis, which can predict the probability of an event accurately and individually.33 This study successfully constructed a nomogram model based on screening the risk factors of death in AIDS patients with dTSM, which is convenient for clinicians to understand and remember. Regarding model validation, the Bootstrap method was used for internal verification, which showed that the calibration curve fitted the ideal curve better. The predicted probability was in good agreement with the actual probability, and the ROC curve also proved that the model had high prediction accuracy, indicating that the prediction model had good clinical application value. Clinicians can take preventive measures in advance according to the risk predictive value of the patients.
There are some limitations of this study. First of all, the sample size of this study was limited. Some factors, such as the co-infections types, were not retained in the multivariate model because of the limited cases. Second, this study was a single-center retrospective study. More population in areas with a high incidence of AIDS with dTSM should be included in the extrapolation of the results. In the future, prospective, multicenter, and large sample studies are needed to mine more indicators incorporated into the prediction model and make it more accurate and practical.
Conclusion
In summary, this study suggested that the serum PCT, serum BUN, shock, and initiate antiviral therapy were associated with the outcome of AIDS with dTSM and had a particular predictive value for poor outcomes of patients, which is helpful for clinicians to optimize the treatment plan and reduce the mortality rate of the AIDS patients complicated with dTSM.
Data Sharing Statement
All data generated or analyzed during this study are available to the corresponding author upon reasonable request.
Ethics Approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Wenzhou Central Hospital (batch No. L2022-01-094) and used retrospective and anonymous data collection methods, which did not involve patient privacy.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or all these areas; have drafted, revised, or critically reviewed the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Key Laboratory of Diagnosis and Treatment of New and Recurrent Infectious Diseases of Wenzhou (grant. No. 2021HZSY0067).
Disclosure
The authors have no relevant financial or non-financial interests to disclose for this work.
References
1. Le T, Kinh NV, Cuc NTK, et al. A trial of itraconazole or amphotericin B for HIV-associated talaromycosis. N Engl J Med. 2017;376(24):2329–2340. doi:10.3760/cma.j.issn.0253-2727.2019.01.010
2. Li Y, Chen H, Li S, et al. LncSSBP1 functions as a negative regulator of IL-6 through interaction with hnRNPK in bronchial epithelial cells infected with talaromyces marneffei. Front Immunol. 2019;10:2977. doi:10.3389/fimmu.2019.02977
3. Chen J, Zhang R, Shen Y, et al. Clinical characteristics and prognosis of penicilliosis among human immunodeficiency virus-infected patients in eastern China. Am J Trop Med Hyg. 2017;96(6):1350–1354. doi:10.4269/ajtmh.16-0521
4. Dong RJ, Zhang YG, Zhu L, et al. Innate immunity acts as the major regulator in Talaromyces marneffei coinfected AIDS patients: cytokine profile surveillance during initial 6-month antifungal therapy. Open Forum Infect Dis. 2019;6(6):ofz205. doi:10.1093/ofid/ofz205
5. Peng L, Shi YB, Zheng L, et al. Clinical features of patients with talaromycosis marneffei and microbiological characteristics of the causative strains. J Clin Lab Anal. 2022;36(11):e24737. doi:10.1002/jcla.24737
6. AIDS and Hepatitis C Professional Group, Society of Infectious Diseases, Chinese Medical Association. Chinese guidelines for diagnosis and treatment of HIV/AIDS (2021 edition). Zhonghua Nei Ke Za Zhi. 2021;60(12):1106–1128. doi:10.3760/cma.j.cn112138-20211006-00676
7. Huang W, Li T, Zhou C, et al. Voriconazole versus amphotericin B as induction therapy for Talaromycosis in HIV/AIDS patients: a retrospective study. Mycopathologia. 2021;186(2):269–276. doi:10.1007/s11046-021-00533-5
8. Panapruksachat S, Iwatani S, Oura T, et al. Identification and functional characterization of Penicillium marneffei pleiotropic drug resistance transporters ABC1 and ABC2. Med Mycol. 2016;54(5):478–491. doi:10.1093/mmy/myv117
9. Lai J, Liu Y, Ye H, et al. Talaromyces marneffei is the persistent overwhelming bloodstream infection pathogen among HIV inpatients in Fujian, China. Infect Drug Resist. 2022;15:5207–5214. doi:10.2147/IDR.S379100
10. Le T, Wolbers M, Chi NH, et al. Epidemiology, seasonality, and predictors of outcome of AIDS-associated Penicillium marneffei infection in Ho Chi Minh City. Viet Nam Clin Infect Dis. 2011;52(7):945–952. doi:10.1093/cid/cir028
11. Hu Y, Zhang J, Li X, et al. Penicillium marneffei infection: an emerging disease in mainland China. Mycopathologia. 2013;175(1–2):57–67. doi:10.1007/s11046-012-9577-0
12. Ying RS, Le T, Cai WP, et al. Clinical epidemiology and outcome of HIV-associated talaromycosis in Guangdong, China, during 2011–2017. HIV Med. 2020;21(11):729–738. doi:10.1111/hiv.13024
13. Shu F, Pruksaphon K, Nosanchuk JD, et al. Evaluation of the yeast phase-specific monoclonal antibody 4D1 and Galanthus nivalis agglutinin sandwich ELISA to detect Talaromyces marneffei antigen in human urine. Front Cell Infect Microbiol. 2023;13:1163868. doi:10.3389/fcimb.2023.1163868
14. Chen X, Ou X, Wang H, et al. Talaromyces marneffei Mp1p antigen detection may play an important role in the early diagnosis of Talaromycosis in patients with acquired immunodeficiency syndrome. Mycopathologia. 2022;187(2–3):205–215. doi:10.1007/s11046-022-00618-9
15. Pruksaphon K, Intaramat A, Simsiriwong P, et al. An inexpensive point-of-care immunochromatographic test for Talaromyces marneffei infection based on the yeast phase specific monoclonal antibody 4D1 and Galanthus nivalis agglutinin. PLoS Negl Trop Dis. 2021;15(5):e0009058. doi:10.1371/journal.pntd.0009058
16. Thu NTM, Chan JFW, Ly VT, et al. Superiority of a novel Mp1p antigen detection enzyme immunoassay compared to standard BACTEC blood culture in the diagnosis of Talaromycosis. Clin Infect Dis. 2021;73(2):e330–e336. doi:10.1093/cid/ciaa826
17. Naidoo K, Rampersad S, Karim SA. Improving survival with tuberculosis & HIV treatment integration: a mini-review. Indian J Med Res. 2019;150(2):131–138. doi:10.4103/ijmr.IJMR_660_19
18. Pruksaphon K, Intaramat A, Ratanabanangkoon K, et al. Diagnostic laboratory immunology for talaromycosis (penicilliosis): review from the bench-top techniques to the point-of-care testing. Diagn Microbiol Infect Dis. 2020;96(3):114959. doi:10.1016/j.diagmicrobio.2019.114959
19. Shen Q, Sheng L, Zhang J, et al. Analysis of clinical characteristics and prognosis of talaromycosis (with or without human immunodeficiency virus) from a non-endemic area: a retrospective study. Infection. 2022;50(1):169–178. doi:10.1007/s15010-021-01679-6
20. Klus J, Ly VT, Chan C, et al. Prognosis and treatment effects of HIV-associated talaromycosis in a real-world patient cohort. Med Mycol. 2021;59(4):392–399. doi:10.1093/mmy/myab005
21. Sun J, Sun W, Tang Y, et al. Clinical characteristics and risk factors for poor prognosis among HIV patients with Talaromyces marneffei bloodstream infection. BMC Infect Dis. 2021;21(1):514. doi:10.1186/s12879-021-06232-2
22. Tragiannidis A, Gkampeta A, Vousvouki M, et al. Antifungal agents and the kidney: pharmacokinetics, clinical nephrotoxicity, and interactions. Expert Opin Drug Saf. 2021;20(9):1061–1074. doi:10.1080/14740338.2021.1922667
23. Romo FT, Aziz M, Livak B, et al. Renal function recovery and HIV viral suppression following Tenofovir discontinuation for renal impairment. J AIDS Clin Res. 2014;5(11). doi:10.4172/2155-6113.1000379
24. Wei HY, Liang WJ, Li B, et al. Clinical characteristics and risk factors of Talaromyces marneffei infection in human immunodeficiency virus-negative patients: a retrospective observational study. World J Emerg Med. 2021;12(4):281–286. doi:10.5847/wjem.j.1920-8642.2021.04.005
25. Shi M, Lin J, Wei W, et al. Machine learning-based in-hospital mortality prediction of HIV/AIDS patients with Talaromyces marneffei infection in Guangxi, China. PLoS Negl Trop Dis. 2022;16(5):e0010388. doi:10.1371/journal.pntd.0010388
26. Nascimento L, Improta-Caria AC, Brites C. Mortality in hospitalized HIV-infected patients in a referral center in Bahia, Brazil. Braz J Infect Dis. 2022;26(6):102716. doi:10.1016/j.bjid.2022.102716
27. Pacholec M, Sami F, Newell K, et al. Fatal disseminated Mycobacterium haemophilum infection involving the central nervous system in a renal transplant recipient. J Clin Tuberc Other Mycobact Dis. 2020;21:100197. doi:10.1016/j.jctube.2020.100197
28. Sandler BJ, Davis KA, Schuster KM. Symptomatic human immunodeficiency virus-infected patients have poorer outcomes following emergency general surgery: a study of the nationwide inpatient sample. J Trauma Acute Care Surg. 2019;86(3):479–488. doi:10.1097/TA.0000000000002161
29. Xiao J, Du S, Tian Y, et al. Causes of death among patients infected with HIV at a tertiary care hospital in China: an observational cohort study. AIDS Res Hum Retroviruses. 2016;32(8):782–790. doi:10.1089/aid.2015.0271
30. Wu J, Lu AD, Zhang LP, et al. Study of clinical outcome and prognosis in pediatric core binding factor-acute myeloid leukemia. Zhonghua Xue Ye Xue Za Zhi. 2019;40(1):52–57.
31. Zheng J, Gui X, Cao Q, et al. A clinical study of acquired immunodeficiency syndrome associated Penicillium marneffei infection from a non-endemic area in China. PLoS One. 2015;10(6):e0130376. doi:10.1371/journal.pone.0130376
32. Qin Y, Zhou Y, Liu S, et al. HIV-associated talaromycosis: does timing of antiretroviral therapy matter? J Infect. 2022;84(3):410–417. doi:10.1016/j.jinf.2021.12.032
33. Pang CG, Yang XG, Zhao YL, et al. A novel tool for predicting the survival of endoprosthesis used for reconstruction of the knee following tumor resection: a retrospective cohort study. BMC Cancer. 2021;21(1):986. doi:10.1186/s12885-021-08710-x
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
