Back to Journals » Infection and Drug Resistance » Volume 15
Clinical Characteristics and the Effect of Timing for Metagenomic Next-Generation Sequencing in Critically Ill Patients with Sepsis
Authors He D , Liu M, Chen Q, Liu Y, Tang Y, Shen F, Wang D, Liu X
Received 17 September 2022
Accepted for publication 6 December 2022
Published 14 December 2022 Volume 2022:15 Pages 7377—7387
DOI https://doi.org/10.2147/IDR.S390256
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Dehua He, Ming Liu, Qimin Chen, Ying Liu, Yan Tang, Feng Shen, Difen Wang, Xu Liu
Department of Critical Care Medicine, the Affiliated Hospital of Guizhou Medical University, Guiyang, People’s Republic of China
Correspondence: Xu Liu, Department of Critical Care Medicine, the Affiliated Hospital of Guizhou Medical University, No. 28, Guiyi Street, Yunyan District, Guiyang, Guizhou, 550004, People’s Republic of China, Tel +86-851-86771459, Email [email protected]
Background: Metagenomic next-generation sequencing (mNGS) has a good performance for the identification of pathogens in infectious diseases, but few studies on the clinical characteristics of mNGS and the effect of timing for mNGS in critically ill patients with sepsis.
Methods: We retrospectively included all patients diagnosed with sepsis after admission to the intensive care unit (ICU) of a university-affiliated hospital between Aug 1, 2019 and Apr 1, 2021. During the study period, pathogens for all enrolled subjects were obtained by mNGS. We analyzed the composition and positive rate of different samples type for mNGS. And then we used the univariable and multivariable logistic regression to explore the risk factors associated with all-cause mortality at 28 days.
Results: A total of 87 patients were included and 87 samples were analyzed among these patients. The most common sample for mNGS was bronchoalveolar lavage fluid (BALF), about 84% (73/87). The positive rate of pathogens identification by mNGS was higher than conventional culture (92% vs 36%, p < 0.001). In addition to the pathogens detected by conventional culture, mNGS can detect more viruses and fungi. Based on the mNGS report, clinicians made adjustments to the antibiotic regimen for 72% patients. The multivariate binary logistic regression analysis suggested that age (OR, 1.036; 95% CI, 1.005– 1.067; p = 0.021) and the sequential organ failure assessment (SOFA) score on the day of mNGS sampling were independent risk factors of death at 28 days (OR, 1.204; 95% CI, 1.038– 1.397; p = 0.014).
Conclusion: In critically ill patients with sepsis, the most common sample type for mNGS was BALF, and the positive rate of mNGS is higher than conventional cultures, especially in viruses and fungi. Meanwhile, mNGS can guide clinicians in adjusting antibiotic regimens. Age and the SOFA score on the day of mNGS sampling were independent risk factors for death.
Keywords: sepsis, metagenomic next-generation sequencing, critical care, timing, outcome
Introduction
As a common cause of intensive care unit (ICU) admission, sepsis is life-threatening organ dysfunction caused by a dysregulated immune response to infection.1,2 Sepsis causes 20% of all deaths globally and has been recognized as a global health priority by the World Health Organization (WHO).3 Early diagnosis and identification of the underlying microbial pathogens of sepsis are critical to the timely selection of appropriate antibiotic therapy, which affects survival in sepsis.4,5 The EPIC III study showed that the proportion of patients with suspected or confirmed infection in the ICU ranged from 43% to 60%, but only 65% of these patients had positive microbiological cultures.6 The conventional pathogenic detection methods, such as culture and polymerase chain reaction (PCR, eg, direct PCR, multiplex PCR), have a low positive rate, limited variety of diagnostic microorganisms, and may take a long time.7 With conventional pathogenic detection methods, about 50% hospitalized with pneumonia in the ICU have no clear pathogens, more than 40% in sepsis, and approximately 50% in the central nervous system infection.8–10 The failure to obtain a targeted and timely pathogen result may delay precision antimicrobial treatment, leading to unnecessary broad-spectrum antibiotic usage, inducing multi-drug resistance, and increasing mortality and health-care costs.11,12 Thus, we need a more sensitive, faster and more accurate method to identify the pathogen of sepsis.
Next-generation sequencing (NGS), also termed high-throughput or massively parallel sequencing, is a genre of technologies that allows for thousands to billions of DNA fragments to be simultaneously and independently sequenced. The applications of NGS in clinical microbiological testing are manifold and include metagenomic next-generation sequencing (mNGS), which allows for an unbiased approach to the detection of pathogens.7 Numerous published case reports and clinical studies have shown that mNGS has been successfully applied in various types of specimens, such as cerebrospinal fluid (CSF), respiratory secretions or bronchoalveolar lavage fluid (BALF), plasma, and whole blood.13–16 For the identification of pathogens in patients with complex, rare and severe infections, mNGS is more sensitive and less affected by prior antibiotic exposure.11 Limin Sun et al also confirmed that mNGS has the advantages of rapid and high positive rate in the detection of pathogens in patients with severe sepsis.17
However, many publications about the application of mNGS in critical sepsis are case reports or limited to a single infection site, for instance, Guan et al only studied patients with central nervous system viral infections.16,18–20 Relatively few studies have specifically concentrated on the application for critical sepsis, especially for the delivery rate and positive rate of mNGS in different infection sites, and the effect of timing for mNGS. To describe the clinical characteristics of mNGS, furthermore, to explore the effect of timing for mNGS in critical sepsis, we conducted this study.
Materials and Methods
Study Design and Participants
We did this single-centered, retrospective, observational study in the ICU, the Affiliated Hospital of Guizhou Medical University (Guizhou, China). From August 1, 2019, to April 1, 2021, all patients admitted to the research ICU due to confirmed or suspected infection and life-threatening organ dysfunction will be screened for eligibility. Organ dysfunction was identified as an acute change in total sequential organ failure assessment (SOFA) score ≥2 points consequent to the infection.21 The inclusion criteria were as follows: (1) age 18 years old or older; (2) meeting sepsis 3.0 criteria; (3) sending mNGS for pathogens during ICU stay. The exclusion criterion was a diagnosis of brain death before ICU admission. Because of the national health insurance policy, the mNGS test is not covered by health insurance. Therefore, the attending physician will recommend mNGS to the patient or authorized representatives only if the patient meets the following conditions: (1) antibiotics had been used before being transferred to the ICU, but the etiology was unknown and the condition was serious (required high doses of vasoactive drugs and/or high levels of respiratory support); (2) after transferred to the ICU, the empirical anti-infection effect was not dissatisfied, the etiology was unknown, and the condition had not improved; (3) infection in the immunocompromised, but the etiology was unknown. All enrolled patient files were subsequently reviewed by the study team.
Metagenomic Next-Generation Sequencing and Analysis
Samples of infection sites or peripheral blood samples were collected according to standard protocol. Blood samples or BALF were at least 5 mL, and 1–3mL from sputum, CSF or other body fluids was collected. Before nucleic acid extraction, all samples were inactivated by 56°C water bath for 30 minutes as the infection was suspected.22 Different types of samples underwent different pretreatment. Nucleic acid extraction took place using a TIANamp Micro DNA Kit (DP316, Tiangen Biotech Co., Beijing, China). The extracted sample DNA was ultrasonically fragmented into fragments, end-repaired, aptamer ligated and PCR amplified to construct DNA libraries.11 DNA libraries were quality-controlled for DNA samples using an Agilent 2100 Bioanalyzer (Agilent Technologies, USA) and a Qubit 2.0 fluorometer (Invitrogen, USA). Double-stranded DNA libraries that pass quality control were subjected to DNA degradation and cyclization for conversion to single-stranded circular DNA. Then, DNA nanoballs (DNA nanoball, DNB) were generated by a rolling loop amplification technique. The Qubit 2.0 fluorometer (ThermoFisher, USA) was used for quality control of DNB. The qualified DNBs were loaded onto the sequencing chip and then sequenced by the high-throughput sequencing system BGISEQ-50 platform (BGI, Shenzhen, Guangdong, China) for SE50 bp with 20 M reads of sequencing data. High-quality sequencing data were generated, followed by filtering of low-quality, short segments (length <35 bp).23 Data on human reference genome sequences were eliminated by BWA sequencing analysis software (http://bio-bwa.sourceforge.net/). The data filtered after the above steps were compared with four microbial genome databases of bacteria (6350 species), fungi (1064 species), viruses (4945 species) and parasites (234 species) to obtain the number of sequences that could match to a certain pathogen. The classification reference databases were downloaded from the National Center of Biotechnology Information (NCBI) (ftp://ftp.ncbi.nlm.nih.gov/genomes/). The possible pathogens were determined based on the high or low sequence number and other clinical tests.
Criteria for a Positive mNGS Result
A wide variety of microorganisms can be detected by mNGS, but how to identify infection or colonization and how to interpret mNGS reports is one of the current problems. The infectious pathogen was determined if it met any of the following thresholds:24–26 (1) the relative abundance of bacteria (Mycobacterium tuberculosis excluded) and fungi was greater than 30% at the genus level; (2) at least one unique read from Mycobacterium tuberculosis complex; (3) positive virus detection was considered when the stringent map read number (SMRN) was no less than 3; (4) when the pathogen was detected by traditional pathogen detection methods and the mNGS reads number was more than 50; (5) for pathogen with unique reads less than 50, it still can be diagnosed as an infectious pathogen with the consistent clinical situation.
Data Collection
Reviewing clinical electronic medical records, nursing records, and laboratory values, we collected data on age, sex, chronic medical histories (chronic cardiac disease, chronic pulmonary disease, chronic neurologic disorder, cerebrovascular disease, diabetes, immunocompromised), date and time (ICU admission/discharge, sampling for mNGS and microbiology report of mNGS), shock or not, infection site (lung, blood, abdomen, central nervous system), life support (mechanical ventilation, renal replacement therapy), laboratory values on admission/sampling. The ratio of arterial oxygen partial pressure to fractional inspired oxygen (P/F) and SOFA score (ICU admission and the day of mNGS sampling) were calculated.
Outcomes
The primary outcome was all-cause mortality at 28 days. The secondary outcomes were the clinical characteristics of patients with mNGS, including baseline characteristics associated with all-cause mortality at 28 days, and variables on the day of sampling associated with all-cause mortality at 28 days. Finally, an exploratory analysis included associations between all risk factors and 28-day mortality.
Statistical Analysis
Since most of our data were not normally distributed, as evidenced by the Shapiro–Wilk test and the Kolmogorov–Smirnov test, we expressed continuous variables as the median and interquartile range (IQR), while categorical data were numbers with percentages. The Wilcoxon rank sum test was used for the comparison of continuous variables, while the chi-square test was used for the comparison of categorical variables. The independent risk factors of the prognostic indicators were determined by binary logical regression analysis as odds ratios including 95% confidence intervals (CI) and shown by forest plot. The Receiver Operating Characteristic (ROC) curve and the area under the ROC curve (ROC-AUC) were also calculated to evaluate the predictive ability of the binary logistic regression model. All the analyses were computed at a two-sided α level of 0.05 with SPSS software, version 23.0.
Results
Patient Characteristics
Among the 697 patients admitted to the ICU with a diagnosis of sepsis, 88 received mNGS but one was excluded because of diagnosed brain death before ICU admission (Figure 1). Of the included 87 patients, 38 patients died and the all-cause mortality at 28 days was 44%. Baseline characteristics for all 87 patients are described in Table 1. For all patients included in this study, the median age was 60 (IQR 47–74) years and the main site of infections was lung (84%). Except for age and the P/F ratio at ICU admission, there were no significant differences in baseline characteristics between survivors and non-survivors.
 |
Table 1 Demographic, Clinical, and Laboratory Findings of Patients at ICU Admission |
Clinical Characteristics of mNGS
A total of 87 samples, including 73 for BALF, 7 for cerebrospinal fluid, 3 for whole blood, and 4 for abdominal drainage or ascites, were sent for identification of pathogens by culture and mNGS. The positive rates of mNGS and culture for different types of samples are shown in Figure 2. The positive rates of mNGS and culture were significantly different within BALF (97% vs 45%, p < 0.001), CSF (43% vs 0%, p = 0.001) and total samples (92% vs 36%, p < 0.001). Due to the small sample size, there was no statistical difference in the positivity rate between the two methods in whole blood and abdominal drainage or ascites. The positive rate of mNGS is higher than culture, and in addition to detecting pathogens detected by culture, mNGS also can detect more viruses and fungi. The most frequent pathogens for bacteria/fungi/viruses by mNGS were Klebsiella pneumonia/Pneumocystis jirovecii/Human pesvirus (Figure 3). The pathogens detected by conventional culture are mainly common, single bacteria, such as Klebsiella pneumoniae and Acinetobacter baumannii, and the positive rate in viruses and fungi is extremely low.
 |
Figure 3 Distribution of identified pathogens in mNGS and culture. Abbreviation: mNGS, metagenomic next-generation sequencing. |
Based on the pathogenic reports of mNGS, clinicians increased the type of antibiotic used for 28% patients, changed the type of antibiotic for 25% patients, decreased the type of antibiotic used for 19% patients, and only 28% patients did not require an antibiotic regimen adjustment (Figure 4). In other words, empiric antibiotics only covered the causative organisms in 47% (decreased or unadjusted antibiotics) patients with sepsis.
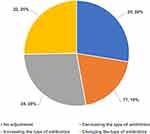 |
Figure 4 Adjusting antibiotic regimens based on mNGS reports. Abbreviation: mNGS, metagenomic next-generation sequencing. |
Risk Factors for 28-Day Mortality at ICU Admission and on the Day of Sampling
In univariable analysis, age and the P/F ratio at ICU admission were associated with 28-day mortality (Table 1). Compared with survivors, non-survivors had a higher age 69 (IQR 50–75) vs 56 (IQR 42–67) years and a lower P/F ratio at ICU admission 134 (IQR 98–184) vs 170 (IQR 120–237) mmHg. On the day of mNGS sampling, platelet, lymphocyte and the SOFA score were associated with 28-day mortality (Table 2). Lymphocytes and platelets were lower in non-survivors compared to survivors. And the difference with ICU admission was that the SOFA score increased in the non-survivors and became statistically significant compared with survivors 10 (IQR 7–14) vs 6 (IQR 5–10) on the day of mNGS sampling.
 |
Table 2 Variables on the Day of Sampling Associated with All-Cause Mortality at 28 Days |
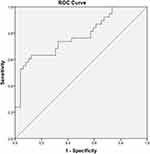
Variables that were associated (p < 0.05) with 28-day mortality were included in the multivariate binary logistic regression analysis. The independent risk factors of death at 28-day included age (OR, 1.036; 95% CI, 1.005–1.067; p = 0.021) and the SOFA score on the day of mNGS sampling (OR, 1.204; 95% CI, 1.038–1.397; p = 0.014) (Figure 5). Furthermore, the ROC curve was constructed to evaluate the performance of the binary logistic regression model for predicting death at 28-day and the AUC was 0.780 (95% CI: 0.680–0.879; p < 0.001, the sensitivity of 60.5%, and specificity of 89.8%) (Figure 6).
Discussion
To our knowledge, few studies describe the clinical characteristics of mNGS and evaluate the effect of timing for mNGS in critically septic patients. Our major findings include as follows. Firstly, BAlF is the most common type of sample for mNGS in the ICU. And the positive rate of pathogen identification by mNGS was 92%, higher than conventional culture, especially for viruses and fungi. Secondly, empirical antibiotics only cover about half of the pathogens in patients with sepsis, and mNGS reports can guide clinicians adjust antibiotic regimens. Thirdly, the binary logistic regression model showed that age and the SOFA score on the day of mNGS sampling are independent risk factors for death at 28-day.
For the sample distribution of mNGS, BALF constitutes the main part, which maybe include two reasons. One is that pneumonia is a frequent cause of ICU admission, and the other one is that BALF can improve the quality of samples and increase the reliability of results. The procedure of BALF allows for the performance of directed bronchoalveolar lavage in specific areas of the lung with radiologic abnormalities.27 Some studies have shown that BALF for mNGS has remarkable advantages in detecting opportunistic pathogens, especially in patients with immunosuppression.28–30 In our study, 70% (14/20) patients with immunosuppression were diagnosed with pneumocystis jirovecii pneumonia but the conventional culture all were negative.
Consistent with a previous study, our data suggest that the positive rate of mNGS is less likely to be affected by prior antibiotic exposure.11 All patients in our study received antibiotic treatment before mNGS and the positive rate is up to 92% by mNGS. mNGS detected significantly more potential pathogens than culture. In this study, the pathogens detected by the conventional culture were mainly single, common bacteria and the positive rate of viruses and fungi was lower than mNGS. The application of empirical antibiotics is dependent on the clinician’s experience and varies widely among individuals.31 As shown in our results, the empirical antibiotic application is appropriate for only about half of the patients with sepsis. In the absence of an etiologic report, it can be difficult and confusing for clinicians to make further adjustments to antibiotics. The faster and more sensitive nature of mNGS, as well as the broader pathogen spectrum, can guide clinicians in selecting the appropriate antibiotic regimen in a timely manner.
For the best timing of mNGS in critically ill patients with sepsis, there is currently no unified and accurate recommendation. Qian et al called for timely mNGS diagnosis of etiology to improve the prognosis of patients, especially for critically ill patients.23 However, the evidence is limited. Our study can be used as a supplement. mNGS does not replace current conventional identification methods of pathogens, only be used as an adjunct to these methods.32 Considering economic costs, we chose mNGS as the second choice when traditional culture is negative under most circumstances. However, it may lead to postpone the applications of mNGS and result in delayed or inadequate treatment because of this clinical practice. As shown in our data, the SOFA score at ICU admission has no statistical difference between survivors and non-survivors. But it is higher than survivors on the day of mNGS sampling in non-survivors and associated with 28-day mortality. The median period from ICU admission to mNGS sampling is 56 (IQR 19–129) hours, which may lead to prolonged inappropriate antibiotic therapy. In addition, for our hospital, the laboratory for mNGS is located in another city, sample transport about 24 hours and finally it costs about 48–72 hours to get the mNGS report. All these factors may cause inappropriate treatment and deterioration of the disease.
Similar to the findings of Bin Du et al, age is an independent risk factor of death in patients with sepsis that increasing age is associated with increased sepsis-related mortality.33 However, the mortality in this study is higher, which is different from previous research results.20,34 The main reason for this difference was the severity of the patients, with those included in this study being more severe than those in previous studies. In our study, the direct admission patients were critically ill patients transferred from other hospitals, and the main reason for the transfer of these patients was the poor response to early treatment. The median SOFA score especially in non-survivors is 8 (IQR 5–11) at ICU admission among our patients, which is higher than the previous study and the absolute difference is 4 on the day of mNGS sampling.34
There are several limitations in this study. First, the small sample size limits the power of statistical analysis. Therefore, the findings are exploratory and further validation is necessary, such as a larger cohort study or potentially randomly controlled trials are needed. Second, to the nature of the retrospective study, not all laboratory tests are done in all patients, therefore missing data may limit the power of some of the association analyses. For example, some patients died after ICU or hospital discharge, we could not get the main cause of death. Third, the delivery rate of mNGS is low in our sepsis patients. Many medical treatments are restricted by the local economic level, for now, the cost of mNGS is still expensive for some low-middle-income families. Therefore, it should be careful when our results are considered as a reference to other hospital settings.
Conclusions
In critically ill patients with sepsis, the most common sample type for mNGS was BALF, and the positive rate of mNGS is higher than conventional cultures, especially in viruses and fungi. Meanwhile, mNGS can guide clinicians in adjusting antibiotic regimens. Age and the SOFA score on the day of mNGS sampling were independent risk factors for death. A longer period from ICU admission to mNGS sampling may delay pathogen identification and inappropriate antibiotic therapy, and increase risk of death in critically ill patients with sepsis.
Abbreviations
mNGS, metagenomic next-generation sequencing; ICU, intensive care unit; BALF, bronchoalveolar lavage fluid; SOFA, sequential organ failure assessment; PCR, polymerase chain reaction; CSF, cerebrospinal fluid; P/F, The ratio of arterial oxygen partial pressure to fractional inspired oxygen; DNB, DNA nanoball; SMRN, stringent map read number; IQR, interquartile range; CI, confidence intervals; ROC, Receiver Operating Characteristic; ROC-AUC, the area under the ROC curve.
Ethics Approval and Informed Consent
The study was conducted in accordance with the Declaration of Helsinki. The study was approved by the ethics committee of the Affiliated Hospital of Guizhou Medical University (2020115K). The registration number of the clinical study is ChiCTR2100043089. Informed consent was waived because this was a retrospective study. We obtained patient data from the Medical Records and analysed the data anonymously.
Acknowledgments
We thank all the participants in this study.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This study was supported by the funds from the National key research and development plan (2018YFC2001904), the National Science Foundation of China (Grant Nos. 81701958 and 81960357), Guizhou Provincial Science and Technology Projects (Qian Ke He Jichu[2020]1Y330, Qian Ke He Jichu-ZK[2022]Yiban 370), National Key Clinical Specialty Construction Project of China (2011-170), Key Clinical Discipline Construction Project of Guizhou Province (2011-52) and Special Aid Fund for High-level Personnel in Guizhou Province (TZJF-2011-25).
Disclosure
The authors declare no conflicts of interest.
References
1. Li A, Ling L, Qin H, et al. Epidemiology, management, and outcomes of sepsis in ICUs among countries of differing national wealth across Asia. Am J Respir Crit Care Med. 2022;206(9):1107–1116. doi:10.1164/rccm.202112-2743OC
2. Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;47(11):1181–1247. doi:10.1007/s00134-021-06506-y
3. Reinhart K, Daniels R, Kissoon N, Machado FR, Schachter RD, Finfer S. Recognizing sepsis as a global health priority - A WHO resolution. N Engl J Med. 2017;377(5):414–417. doi:10.1056/NEJMp1707170
4. Paul M, Shani V, Muchtar E, Kariv G, Robenshtok E, Leibovici L. Systematic review and meta-analysis of the efficacy of appropriate empiric antibiotic therapy for sepsis. Antimicrob Agents Chemother. 2010;54(11):4851–4863. doi:10.1128/AAC.00627-10
5. Ferrer R, Martin-Loeches I, Phillips G, et al. Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: results from a guideline-based performance improvement program. Crit Care Med. 2014;42(8):1749–1755. doi:10.1097/CCM.0000000000000330
6. Vincent JL, Sakr Y, Singer M, et al. Prevalence and outcomes of infection among patients in intensive care units in 2017. JAMA. 2020;323(15):1478–1487. doi:10.1001/jama.2020.2717
7. Gu W, Miller S, Chiu CY. Clinical metagenomic next-generation sequencing for pathogen detection. Annu Rev Pathol. 2019;14:319–338. doi:10.1146/annurev-pathmechdis-012418-012751
8. Cookson W, Cox MJ, Moffatt MF. New opportunities for managing acute and chronic lung infections. Nat Rev Microbiol. 2018;16(2):111–120. doi:10.1038/nrmicro.2017.122
9. Baykara N, Akalın H, Arslantaş MK, et al. Epidemiology of sepsis in intensive care units in Turkey: a multicenter, point-prevalence study. Crit Care. 2018;22(1):93. doi:10.1186/s13054-018-2013-1
10. Geng S, Mei Q, Zhu C, et al. Metagenomic next-generation sequencing technology for detection of pathogens in blood of critically ill patients. Int J Infect Dis. 2021;103:81–87. doi:10.1016/j.ijid.2020.11.166
11. Miao Q, Ma Y, Wang Q, et al. Microbiological diagnostic performance of metagenomic next-generation sequencing when applied to clinical practice. Clin Infect Dis. 2018;67(suppl_2):S231–s240. doi:10.1093/cid/ciy693
12. Liu VX, Fielding-Singh V, Greene JD, et al. The timing of early antibiotics and hospital mortality in sepsis. Am J Respir Crit Care Med. 2017;196(7):856–863. doi:10.1164/rccm.201609-1848OC
13. Wilson MR, Naccache SN, Samayoa E, et al. Actionable diagnosis of neuroleptospirosis by next-generation sequencing. N Engl J Med. 2014;370(25):2408–2417. doi:10.1056/NEJMoa1401268
14. Langelier C, Zinter MS, Kalantar K, et al. Metagenomic sequencing detects respiratory pathogens in hematopoietic cellular transplant patients. Am J Respir Crit Care Med. 2018;197(4):524–528. doi:10.1164/rccm.201706-1097LE
15. Grumaz S, Stevens P, Grumaz C, et al. Next-generation sequencing diagnostics of bacteremia in septic patients. Genome Med. 2016;8(1):73. doi:10.1186/s13073-016-0326-8
16. Gosiewski T, Ludwig-Galezowska AH, Huminska K, et al. Comprehensive detection and identification of bacterial DNA in the blood of patients with sepsis and healthy volunteers using next-generation sequencing method - The observation of DNAemia. Eur J Clin Microbiol Infect Dis. 2017;36(2):329–336. doi:10.1007/s10096-016-2805-7
17. Sun L, Zhang S, Yang Z, et al. Clinical application and influencing factor analysis of Metagenomic Next-Generation Sequencing (mNGS) in ICU patients with sepsis. Front Cell Infect Microbiol. 2022;12:905132. doi:10.3389/fcimb.2022.905132
18. Long Y, Zhang Y, Gong Y, et al. Diagnosis of sepsis with cell-free DNA by next-generation sequencing technology in ICU patients. Arch Med Res. 2016;47(5):365–371. doi:10.1016/j.arcmed.2016.08.004
19. Guan H, Shen A, Lv X, et al. Detection of virus in CSF from the cases with meningoencephalitis by next-generation sequencing. J Neurovirol. 2016;22(2):240–245. doi:10.1007/s13365-015-0390-7
20. Xie Y, Du J, Jin W, et al. Next generation sequencing for diagnosis of severe pneumonia: China, 2010–2018. J Infect. 2019;78(2):158–169. doi:10.1016/j.jinf.2018.09.004
21. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–810. doi:10.1001/jama.2016.0287
22. Lu H, Ma L, Zhang H, et al. The comparison of metagenomic next-generation sequencing with conventional microbiological tests for identification of pathogens and antibiotic resistance genes in infectious diseases. Infect Drug Resist. 2022;15:6115–6128. doi:10.2147/IDR.S370964
23. Qian YY, Wang HY, Zhou Y, et al. Improving pulmonary infection diagnosis with metagenomic next generation sequencing. Front Cell Infect Microbiol. 2020;10:567615. doi:10.3389/fcimb.2020.567615
24. Huang J, Jiang E, Yang D, et al. Metagenomic next-generation sequencing versus traditional pathogen detection in the diagnosis of peripheral pulmonary infectious lesions. Infect Drug Resist. 2020;13:567–576. doi:10.2147/IDR.S235182
25. Mohammad NS, Nazli R, Zafar H, Fatima S. Effects of lipid based Multiple Micronutrients Supplement on the birth outcome of underweight pre-eclamptic women: a randomized clinical trial. Pak J Med Sci. 2022;38(1):219–226. doi:10.12669/pjms.38.1.4396
26. Liang Y, Dong T, Li M, et al. Clinical diagnosis and etiology of patients with Chlamydia psittaci pneumonia based on metagenomic next-generation sequencing. Front Cell Infect Microbiol. 2022;12:1006117. doi:10.3389/fcimb.2022.1006117
27. Brownback KR, Thomas LA, Simpson SQ. Role of bronchoalveolar lavage in the diagnosis of pulmonary infiltrates in immunocompromised patients. Curr Opin Infect Dis. 2014;27(4):322–328. doi:10.1097/QCO.0000000000000072
28. Pan T, Tan R, Qu H, et al. Next-generation sequencing of the BALF in the diagnosis of community-acquired pneumonia in immunocompromised patients. J Infect. 2019;79(1):61–74. doi:10.1016/j.jinf.2018.11.005
29. Parize P, Muth E, Richaud C, et al. Untargeted next-generation sequencing-based first-line diagnosis of infection in immunocompromised adults: a multicentre, blinded, prospective study. Clin Microbiol Infect. 2017;23(8):574.e571–574.e576. doi:10.1016/j.cmi.2017.02.006
30. Azoulay E, Russell L, Van de Louw A, et al. Diagnosis of severe respiratory infections in immunocompromised patients. Intensive Care Med. 2020;46(2):298–314. doi:10.1007/s00134-019-05906-5
31. Goodman KE, Baghdadi JD, Magder LS, et al. Patterns, predictors, and inter-center variability in empiric gram-negative antibiotic use across 928 U.S. hospitals. Clin Infect Dis. 2022. doi:10.1093/cid/ciac504
32. Han D, Li Z, Li R, Tan P, Zhang R, Li J. mNGS in clinical microbiology laboratories: on the road to maturity. Crit Rev Microbiol. 2019;45(5–6):668–685. doi:10.1080/1040841X.2019.1681933
33. Weng L, Zeng XY, Yin P, et al. Sepsis-related mortality in China: a descriptive analysis. Intensive Care Med. 2018;44(7):1071–1080. doi:10.1007/s00134-018-5203-z
34. Yang L, Haidar G, Zia H, et al. Metagenomic identification of severe pneumonia pathogens in mechanically-ventilated patients: a feasibility and clinical validity study. Respir Res. 2019;20(1):265. doi:10.1186/s12931-019-1218-4
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.