Back to Journals » Drug Design, Development and Therapy » Volume 16
Clinical Characteristics and Risk Factors of Lung Injury Induced by Nab-Paclitaxel
Authors Yoshioka K, Abe M , Shiko Y, Koshikawa K, Kawasaki Y, Iwasawa S, Terada J, Tsushima K, Tatsumi K , Suzuki T
Received 12 November 2021
Accepted for publication 14 February 2022
Published 22 March 2022 Volume 2022:16 Pages 759—767
DOI https://doi.org/10.2147/DDDT.S342283
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Manfred Ogris
Keiichiro Yoshioka,1 Mitsuhiro Abe,1 Yuki Shiko,2 Ken Koshikawa,1 Yohei Kawasaki,2 Shunichiro Iwasawa,1 Jiro Terada,1,3 Kenji Tsushima,3 Koichiro Tatsumi,1 Takuji Suzuki1
1Department of Respirology, Graduate School of Medicine, Chiba University, Chiba, Japan; 2Biostatistics Section, Clinical Research Center, Chiba University Hospital, Chiba, Japan; 3Department of Pulmonary Medicine, International University of Health and Welfare, School of Medicine, Chiba, Japan
Correspondence: Mitsuhiro Abe, Department of Respirology, Graduate School of Medicine, Chiba University, 1-8-1 Inohana Chuo-ku, Chiba, 260-8670, Japan, Tel +81 43 222 2576, Fax +81 43 226 2176, Email [email protected]
Background/Aim: Nab-paclitaxel (Nab-PTX) has been widely used to treat several advanced cancers. Nab-PTX can cause drug-induced lung injury (DILI); however, its clinical and radiographic features have not been clarified. We aimed to assess the clinical characteristics of Nab-PTX-induced lung injury and identify its associated risk factors.
Patients and Methods: We retrospectively investigated 304 patients who received Nab-PTX at Chiba University Hospital between November 2010 and November 2017. We obtained information regarding the clinical course, laboratory findings, and chest computed tomography findings from their medical records.
Results: Forty-one patients (13%) developed DILI. Grade 1 lung injury occurred in 27 patients (8.8%), grade 2, 8 patients (2.6%); grade 3, 3 patients (0.9%); grade 4, 1 (0.3%); and grade 5, 2 (0.6%). Multivariate analysis revealed that age > 56 years (odds ratio [OR]: 3.0), pre-existing interstitial lung changes (OR: 3.2), and combined drugs with gemcitabine (OR: 2.7) were independent risk factors for DILI owing to Nab-PTX administration.
Conclusion: Nab-PTX-induced lung injury is not rare; however, most cases are asymptomatic (grade 1). Older age, pre-existing interstitial lung changes, and combined drugs with gemcitabine could increase the incidence of Nab-PTX-induced lung injury; such patients should be treated with greater care.
Keywords: drug-induced lung injury, gemcitabine, multivariate analysis, nab-paclitaxel, risk factor
Introduction
Drug-induced lung injury (DILI) occurs when drug administration results in inflammation and, eventually, fibrosis in the lung interstitium.1 The mechanism of DILI is primarily classified into two types:2 cytotoxic pulmonary injury or immune-mediated pulmonary injury. The pathogenesis of cytotoxic pulmonary injury may include direct injury to pneumocytes or the alveolar capillary endothelium with subsequent release of cytokines and recruitment of inflammatory cells.2 All drugs have the possibility to induce DILI; however, the frequency of reported DILI is biased.3,4
Nab-paclitaxel (Nab-PTX) is an anticancer drug formulated to allow paclitaxel (PTX) to be dissolved in physiological saline by binding to human serum albumin.5 Moreover, it is no longer necessary to administer steroids or antihistamines to prevent hypersensitivity to the solvent necessary for administration; thus, Nab-PTX can be administered to patients with alcohol hypersensitivity. Nab-PTX is used worldwide for the management of many solid cancers, such as non-small cell lung cancer (NSCLC), gastric cancer, breast cancer, ovarian cancer, and pancreatic cancer. However, although relatively rare, DILI following the administration of PTX or Nab-PTX is a notable adverse effect. According to ABSOLUTE, a Phase III clinical trial that verified the noninferiority of Nab-PTX (weekly or every 3 weeks) to PTX therapy for gastric cancer, the incidence rate of DILI was 0.8% (2/244).6
In general, patients with interstitial pneumonia have been excluded from most prospective clinical trials on NSCLC; however, one Phase II trial of carboplatin plus Nab-PTX every 3 weeks was performed in advanced NSCLC patients with idiopathic pulmonary fibrosis (IPF).7 Of the 18 patients (6 of whom had confirmed IPF) enrolled in that trial, only 1 (5.6%) with IPF experienced acute exacerbation. It is evident that the safety and efficacy of chemotherapy in such patients have not been established; however, Nab-PTX is relatively safe for patients with lung cancer associated with interstitial pneumonia.
With the onset of DILI, patients are required to adhere to notable therapeutic restrictions. However, the clinical characteristics of DILI owing to Nab-PTX and the factors involved in the onset have not been undefined. Therefore, the present study aimed to evaluate the clinical features of Nab-PTX-induced lung injury and determine its associated risk factors.
Patients and Methods
Patients
A total of 409 patients received Nab-PTX at Chiba University Hospital according to the patient medical records between November 2010 and November 2017. Among them, four patients received Nab-PTX only once owing to non- lung injury related adverse effects. However, 101 patients did not undergo chest computed tomography (CT) examination during the administration cycle owing to no obvious signs of pulmonary side effects. Since these patients were not suitable for assessment in the adverse effect studies, they were excluded from this study.
After the exclusion of these participants, the clinical course, laboratory data, and radiographic findings in 304 patients (154 men, 150 women; mean age 62 years [29–82 years]) were retrospectively reviewed (Figure 1).
Diagnosis of Nab-PTX-Induced Lung Injury
The definition of drug-induced lung injury due to Nab-PTX was new pulmonary lesions in the patients’ lungs that developed during or within 30 days following the last Nab-PTX administration, and other lung diseases with obvious causes (infection, pulmonary congestion, etc.) were excluded based on the clinical manifestations (clinical course, radiological findings, laboratory findings, microbiological findings, etc.).1 Ultimately, the decision was made by two pulmonologists (MA and KY).
Chest CT Findings
CT findings obtained before Nab-PTX administration were evaluated to determine the pre-existing interstitial lung changes, interstitial pneumonia, and interstitial lung abnormalities (ILA). The multidisciplinary position paper by the Fleischner Society was referred to for the definitions, subcategories, and assessment of the ILA.8 Early ILAs are common incidental findings on CT, particularly in older individuals, although it is suggested that the presence of ILAs is an independent predictor of mortality. With any respiratory sign or symptom or functional impairment, ILAs are likely to represent mild interstitial lung disease (ILD). In the present study, the definition of ILAs was purely radiological and based on the identification of a CT abnormality, without any respiratory sign or symptom.
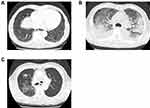
CT findings of DILI were classified based on the ATS guideline of 20139 as nonspecific idiopathic pneumonia (NSIP), diffuse alveolar damage (DAD), organizing pneumonia (OP), chronic exacerbation of existing lung interstitial lesions, and unclassifiable (Figure 2). Predominant lung abnormalities were selected as CT findings of DILI.
Two respiratory medicine specialists (MA and KY) independently evaluated the CT images of each patient before and post DILI onset. A final diagnosis of DILI was made based on a consensus according to two respiratory medicine specialists (MA and KY) (clinical experience of 12 and 8 years, respectively).
Evaluated Parameters
Data from 304 patients concerning the background, laboratory findings, and chest CT findings were retrieved from their medical records (Table 1). The existence of interstitial changes in the lung prior to DILI was carefully evaluated according to the radiological features. The information on the underlying disease, presence of pulmonary metastasis, and combined drugs was extracted. Laboratory findings included the estimated glomerular filtration rate (eGFR), lactate dehydrogenase (LDH) levels, white blood cell (WBC) and eosinophil counts, and Krebs von den Lungen-6 (KL-6; normal range, <500 U/mL) levels.
 |
Table 1 Patients’ Characteristics Prior to Nab-PTX |
Statistical Analysis
The mean and standard deviation (SD) of continuous data and the number and percentage of categorical data were calculated. Risk factors for DILI onset were investigated using the univariate and multivariate logistic regression analyses; the odds ratio (OR) and associated 95% confidence intervals (CIs) were calculated. In the multivariate analysis, stepwise selection method was performed (p ≤ 0.05 included and p > 0.05 removed). All the statistical analyses were performed using the JMP Pro 13.2.0 (Japanese version, SAS institute Inc. Cary, NC, USA). A 2-sided test result of p < 0.05 was considered statistically significant.
Results
Patient Characteristics
The patient characteristics before Nab-PTX administration are shown in Table 1. Forty-one (13%; 28 men and 13 women) of the 304 patients were diagnosed with DILI. The underlying disease was pancreatic cancer in 158 patients, breast cancer in 70 patients, lung cancer in 54 patients, gastric cancer in 17 patients, and other cancers in 5 patients. Ten out of these 41 patients had not undergone CT examination following the discontinuation of Nab-PTX.
In the 41 patients who developed DILI, 13 (32%) were pre-diagnosed as interstitial pneumonia and 6 (15%) as ILA in chest CT images. However, 39 (15%) and 17 (6.5%) patients had pre-existing interstitial pneumonia and ILA, respectively, from the total 263 patients who did not experience DILI. This suggests that pre-existing lung interstitial changes are findings that warrant the clinician’s attention from the standpoint of DILI.
The dosing method and the dosage of Nab-PTX varied based on the type of cancer (Supplementary Table 1). All patients with pancreatic cancer received Nab-PTX in combination with gemcitabine (GEM).
Characteristics of the DILI Group
The characteristics of the patients diagnosed with DILI are shown in Table 2. The median time from Nab-PTX administration to the onset of DILI was 76 days (range, 14–574 days). The severity of DILI was graded according to the Common Terminology Criteria for Adverse Events (CTCAE) criteria.10 Of the 41 patients with DILI, 27 (66%) exhibited grade 1 injury (asymptomatic), 8 (20%) grade 2 injury (no interference with daily living), 3 (7.3%) grade 3 injury (interference with daily living or requirement of oxygen supplementation), 1 (3.7%) grade 4 (life-threatening pneumonitis, and 2 (7.4%) grade 5 injury (death). At the onset of DILI, the LDH (204 ± 47 IU/L) levels were significantly elevated compared with those before Nab-PTX administration (p = 0.008) (Supplementary Figure 1).
 |
Table 2 Characteristics of the Patients Diagnosed with DILI |
The predominant radiographic images of DILI onset are shown in Table 3. Sixteen patients (39%) exhibited a nonspecific idiopathic pneumonia pattern, 9 (21%) a diffuse alveolar damage pattern, and 6 (15%) an organizing pneumonia pattern. Four patients (10%) were considered to experience a chronic exacerbation of the existing interstitial lung lesions (Figure 2). At the point of image evaluation, two patients continued to receive Nab-PTX in the grade 2 or higher group, although they recovered despite continued Nab-PTX administration.
 |
Table 3 The Radiographic Images of DILI |
Of the 20 patients with grade 1, 17 recovered with only interruption of Nab-PTX (Table 4). Of the 14 patients with grades 2–5, 2 (4.9%) died: 1 (2%) died despite of steroid therapy, and the other one died owing to exacerbation of an underlying disease.
 |
Table 4 Clinical Outcome Following DILI Diagnosis |
Univariate and Multivariable Analyses According to the Risk Profile of the DILI Group
Two logistic regression analyses were performed, the results of which are shown in Table 5. The univariate logistic regression analysis demonstrated that male sex (OR 2.3; 95% CI 1.17–4.79; p = 0.02), age ≥56 years (OR 4.6; 95% CI 1.76–15.6; p = 0.0009), pancreatic cancer as primary disease (OR 2.5; 95% CI 1.26–5.31 p = 0.009), pre-existing interstitial lung changes (OR 3.2; 95% CI 1.61–6.32; p = 0.001), and GEM administration in combination with Nab-PTX (OR 2.5; 95% CI 1.26–5.31; p = 0.009) were significantly correlated with an increased risk of DILI.
 |
Table 5 Results of Univariate and Multivariate Analysis of the Parameters |
Since Nab-PTX has been administered without the consideration of renal function, we did not include renal function in the multivariate analysis. In the present study, we could not explain a decreased risk of DILI associated with an eGFR <66 mL/min (OR 0.31; 95% CI 0.0736–0.908 p = 0.03).
Stepwise variable selection was subsequently performed for the aforementioned parameters. Male, older age, pre-existing interstitial lung changes, pancreatic cancer as underlying disease, and GEM as combination therapy were considered for the multivariate logistic regression analysis. Consequently, older age, pre-existing interstitial lung changes, and pancreatic cancer as underlying disease were selected. The multivariate logistic regression analysis revealed that age (OR 3.0; 95% CI 1.10–9.54; p = 0.03), pre-existing interstitial lung changes (OR 3.2; 95% CI 1.55–6.72; p = 0.003), and GEM-combined therapy (OR 2.7; 95% CI 1.29–5.99; p = 0.008) were independent risk factors for DILI.
Discussion
In this study, we demonstrated that older age, pre-existing interstitial lung changes, and combined drugs with GEM were significant risk factors of Nab-PTX-induced lung injury. Kubo et al reported that advanced age (60 years or older), pre-existing pulmonary lesions (particularly for interstitial pneumonia), history of pulmonary surgery, decreased respiratory function, oxygen inhalation, radiation exposure to the lung, and existing renal impairment are risk factors for DILI.1 To the best of our knowledge, this is the first report describing that the combined use of GEM with Nab-PTX and pre-existing interstitial lung changes are risk factors of DILI following Nab-PTX administration.
Previous reports have identified older age as a risk factor for bleomycin (age >40 years), GEM (age >80 years), eGFR-targeted agents (age >65 years), and amiodarone (every 10-year increase in age)-induced DILI.11,12 Although the mechanism of DILI remains unclear, the decreased ability to repair pulmonary tissue damage with aging could be the reason.
Pre-existing pulmonary fibrosis is a risk factor for DILI.13 Sawada et al reported that the incidence of DILI in patients with rheumatoid arthritis and interstitial pneumonia treated by leflunomide was 5.7%, suggesting that pre-existing ILD was a significant risk factor.14 In our study, the incidence of DILI in patients with pre-diagnosed interstitial pneumonia or ILA was 32% and 15%, respectively (Table 1), and pre-existing interstitial lung changes were found to be significant risk factors (Table 5). However, the mechanisms supporting patients with existing interstitial changes in the lungs being more prone to DILI development remain unclear.
Kashiwada et al performed a retrospective study on the clinical characteristics and risk factors of Nab-PTX-induced lung injury for lung cancer.15 According to this report, 9 of 110 (8.2%) patients with lung cancer treated by Nab-PTX developed pneumonitis, and existing interstitial lung disease; combined drugs with bevacizumab, and a lower dose of Nab-PTX were found to be risk factors. Based on our findings, we consider existing interstitial changes in the lungs to be a risk factor for DILI.
In this study, the combination of GEM with Nab-PTX was a risk factor for the development of DILI. According to MPACT, a Phase 3 trial comparing the efficacy and safety of Nab-PTX plus GEM combination therapy and GEM alone in patients with previously untreated metastatic pancreatic cancer, the incidence of DILI was 4% in the Nab-PTX + GEM group and 1% in the GEM alone group.16 This suggests that DILI may be more likely to occur under the combined use of GEM. The incidence of DILI following the administration of GEM has been reported to be 1–2%,17 and the frequency of DILI appears to be low. Von Hoff et al reported that in mice with human pancreatic cancer xenografts, Nab-PTX alone and in combination with GEM depleted the desmoplastic stroma, while the intra-tumoral concentration of GEM was increased 2.8-fold in mice receiving Nab-PTX plus GEM versus those receiving GEM alone.18 Although this was demonstrated in a mouse model, it is possible that GEM causes DILI owing to the augmented action of Nab-PTX.
Furthermore, in this study, the serum LDH levels were significantly elevated at the onset of DILI. LDH is a cytoplasmic enzyme found in all major organs. Elevated levels of serum LDH are found in many diffuse lung diseases, and it has been reported that serum LDH levels were correlated with the severity of and response to treatment in DILI caused by Chinese herbal medicine.19 We considered that LDH could be used as a marker for the early detection of DILI.
In total, 14% of the patients in the present study were diagnosed with DILI, and the median time from Nab-PTX administration to DILI onset was 76 days. The higher incidence of DILI reported in clinical trials6 was due to the fact that we studied the frequency of DILI in various types of cancer, and not only in gastric cancer. Furthermore, GEM, which is used in combination with Nab-PTX for pancreatic cancer (accounting for the majority of cases in this study population), could increase the pulmonary toxicity of Nab-PTX, as mentioned above. We believe that even the appearance of a small shadow is included in the judgment as DILI. In our study, CTCAE grade 1 patients accounted for 27 of 41 patients (66%). Patients of grade 1 had no clinical symptoms, and these cases were found accidentally following CT examination. There were 14 cases (35%) classified as grade 2 or higher. The incidence in all the patients treated with Nab-PTX was 4.6% (14 in 304 patients). This finding is similar to those reported in clinical trials.
Interestingly, two of the patients who developed DILI and subsequently continued with Nab-PTX following onset recovered from DILI (Table 3). Although this does not imply that the drug can be re-administered safely, it does suggest that in some cases, re-administration is possible even following the onset of DILI.
This study has several limitations. First, it was designed as a retrospective, single-centre study. Second, the diagnosis of DILI was primarily based on conventional CT findings; therefore, if a higher resolution CT had been performed, more shadows could have been detected and the incidence of DILI might have been higher. Third, this study may have included DILI caused by combining drugs with Nab-PTX rather than DILI caused by Nab-PTX.
This is the first retrospective study to identify the factors for DILI in patients who underwent Nab-PTX treatment for various diseases. Further studies are needed to confirm these findings. However, we believe that our study has the potential to inform and caution non-respiratory physicians in particular, thereby ensuring early detection and treatment.
Conclusions
Our data indicate that older age, pre-existing interstitial lung changes, and a combination of GEM and Nab-PTX are the risk factors for Nab-PTX-induced lung injury. Although more than half of the patients exhibited grade 1 DILI, DILI with grades higher than 2 is likely to occur. Pre-existing interstitial lung changes, which are risk factors of DILI, include interstitial pneumonia and interstitial lung abnormalities.
Abbreviations
CI, confidence interval; CT, computed tomography; CTCAE, Common Terminology Criteria for Adverse Events; DILI, drug-induced lung injury; eGFR, estimated glomerular filtration rate; FEV, forced expiratory volume; GEM, gemcitabine; KL-6, Krebs von den Lungen-6; LDH, lactate dehydrogenase; Nab-PTX, nab-paclitaxel; NSCLC, non-small cell lung cancer; OR, odds ratio; VC, vital capacity; WBC, white blood cell.
Data Sharing Statement
The datasets supporting the conclusions of this article are included within the article. The underlying datasets are available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
This single-centre, retrospective study was performed in accordance with the amended Declaration of Helsinki. The study protocol was approved by the Human Ethics Committee of Chiba University Hospital (approval number: 2265). We obtained informed consent with an opt-out option.
Acknowledgments
We would like to thank Editage for English language editing.
Author Contributions
KY, MA, KK, SI, JT, KTs, KTa and TS contributed to the study concept and design. KY examined the enrolled patients in the hospital. KY, MA, YS, and YK performed the statistical analyses. KY wrote the main manuscript text. All authors reviewed the manuscript. All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; drafted the article or revised it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
No funding was received for this study.
Disclosure
SI reports grants and personal fees from ONO PHARMACEUTICAL CO., LTD., personal fees from Chugai Pharmaceutical Co., Ltd., personal fees from AstraZeneca K.K., personal fees from MSD K.K, personal fees from Bristol-Myers Squibb K.K., personal fees from Taiho Pharmaceutical Co., Ltd., personal fees from DAIICHI SANKYO COMPANY, LIMITED., outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Kubo K, Azuma A, Kanazawa M, et al. Consensus statement for the diagnosis and treatment of drug-induced lung injuries. Respir Investig. 2013;51(4):260. PMID: 24238235. doi:10.1016/j.resinv.2013.09.001
2. Matsuno O. Drug-induced interstitial lung disease: mechanisms and best diagnostic approaches. Respir Res. 2012;13(1):39. PMID: 22651223. doi:10.1186/1465-9921-13-39
3. Pavlakis N, Bell DR, Millward MJ, Levi JA. Fatal pulmonary toxicity resulting from treatment with gemcitabine. Cancer. 1997;80(2):286–291. PMID: 9217042. doi:10.1002/(sici)1097-0142(19970715)80:2<286::aid-cncr17>3.0.co;2-q
4. Prat A, Martínez P, Serrano C, Montero M, Andreu J, Cortes J. Acute lung injury associated with docetaxel and bevacizumab. Clin Oncol. 2007;19(10):803–805. PMID: 17889516. doi:10.1016/j.clon.2007.08.010
5. Yardley DA. Nab-paclitaxel mechanisms of action and delivery. J Control Release. 2013;170(3):365–372. PMID: 23770008. doi:10.1016/j.jconrel.2013.05.041
6. Shitara K, Takashima A, Fujitani K, et al. Nab-paclitaxel versus solvent-based paclitaxel in patients with previously treated advanced gastric cancer (absolute): an open-label, randomised, non-inferiority, phase 3 trial. Lancet Gastroenterol Hepatol. 2017;2(4):277–287. PMID: 28404157. doi:10.1016/S2468-1253(16)30219-9
7. Otsubo K, Kishimoto J, Kenmotsu H, et al. Treatment rationale and design for j-sonic: a randomized study of carboplatin plus nab-paclitaxel with or without nintedanib for advanced non–small-cell lung cancer with idiopathic pulmonary fibrosis. Clin Lung Cancer. 2018;19(1):e5–e9. PMID: 28687482. doi:10.1016/j.cllc.2017.06.003
8. Hatabu H, Hunninghake GM, Richeldi L, et al. Interstitial lung abnormalities detected incidentally on ct: a position paper from the Fleischner society. Lancet Resp Med. 2020;8(7):726–737. PMID: 32649920. doi:10.1016/S2213-2600(20)30168-5
9. Travis WD, Costabel U, Hansell DM, et al.; on behalf of the ATS/ERS Committee on Idiopathic Interstitial Pneumonias. An Official American Thoracic Society/European Respiratory Society Statement: update of the International Multidisciplinary Classification of the Idiopathic Interstitial Pneumonias. Am J Respir Crit Care Med. 2013;188(6):733–748. PMID 24032382. doi:10.1164/rccm.201308-1483ST
10. Health UDo, Services H. Common terminology criteria for adverse events (CTCAE) Version 5.0. 2017; 2018.
11. O’sullivan J, Huddart R, Norman A, Nicholls J, Dearnaley D, Horwich A. Predicting the risk of bleomycin lung toxicity in patients with germ-cell tumours. Ann Oncol. 2003;14(1):91–96. PMID: 12488299. doi:10.1093/annonc/mdg020
12. Yamada Y, Shiga T, Matsuda N, Hagiwara N, Kasanuki H. Incidence and predictors of pulmonary toxicity in Japanese patients receiving low-dose amiodarone. Circ J. 2007;71(10):1610–1616. PMID: 17895560. doi:10.1253/circj.71.1610
13. Kudoh S, Kato H, Nishiwaki Y, et al. Interstitial lung disease in Japanese patients with lung cancer: a cohort and nested case-control study. Am J Respir Crit Care Med. 2008;177(12):1348–1357. PMID: 18337594. doi:10.1164/rccm.200710-1501OC
14. Sawada T, Inokuma S, Sato T, et al. Leflunomide-induced interstitial lung disease: prevalence and risk factors in Japanese patients with rheumatoid arthritis. Rheumatology. 2009;48(9):1069–1072. PMID: 19321513. doi:10.1093/rheumatology/kep052
15. Kashiwada T, Saito Y, Terasaki Y, et al. Interstitial lung disease associated with nanoparticle albumin-bound paclitaxel treatment in patients with lung cancer. Jpn J Clin Oncol. 2019;49(2):165–173. PMID: 30508192. doi:10.1093/jjco/hyy180
16. Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691–1703. PMID: 24131140. doi:10.1056/NEJMoa1304369
17. Aapro MS, Martin C, Hatty S. Gemcitabine–a safety review. Anticancer Drugs. 1998;9(3):191–201. PMID: 9625429. doi:10.1097/00001813-199803000-00001
18. Von Hoff DD, Ramanathan RK, Borad MJ, et al. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase i/ii trial. J Clin Oncol. 2011;29(34):4548. PMID: 21969517. doi:10.1200/JCO.2011.36.5742
19. Enomoto Y, Nakamura Y, Enomoto N, Fujisawa T, Inui N, Suda T. Japanese herbal medicine-induced pneumonitis: a review of 73 patients. Respir Investig. 2017;55(2):138–144. PMID: 28274529. doi:10.1016/j.resinv.2016.11.007
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.