Back to Journals » Neuropsychiatric Disease and Treatment » Volume 17
Clinical Characteristics and Risk Factors of Cerebral Hemorrhage in Patients with Occult Malignant Tumors
Authors Zhao Y, Xie H, Pan C, Yao Y, Gong Z, Li Y, Jia Y
Received 24 May 2021
Accepted for publication 1 August 2021
Published 19 August 2021 Volume 2021:17 Pages 2729—2738
DOI https://doi.org/10.2147/NDT.S321571
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
Yi Zhao, Haojie Xie, Chunyang Pan, Yaobing Yao, Zhe Gong, Yanfei Li, Yanjie Jia
Department of Neurology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, People’s Republic of China
Correspondence: Yanjie Jia
Department of Neurology, The First Affiliated Hospital of Zhengzhou University, 1st Jian She East Road, Zhengzhou, 450052, People’s Republic of China
Tel +86-135 13802916
Email [email protected]
Propose: To investigate the clinical characteristics and potential risk factors of the first onset of cerebral hemorrhage in patients with occult malignant tumors.
Patients and methods: In this retrospective study, 23 patients with occult malignant tumors with the first onset of cerebral hemorrhage were enrolled in the tumor group, and 92 patients without occult tumors in the same period were enrolled in the control group. There were no statistical differences in age and sex between both groups by propensity score matching. Collected clinical data included age, sex, smoking history, drinking history, hypertension history, diabetes history, past medical history, routine blood tests, neutrophil-to-lymphocyte ratio (NLR), liver and kidney function, fasting blood glucose level, coagulation function, tumor markers, imaging examinations, National Institute of Health stroke scale (NIHSS) score on admission, modified Rankin Scale (mRS) score 90 days after intracerebral hemorrhage and final mRS score.
Results: Compared with the control group, the tumor group had fewer patients with hypertension (52.2% vs 81.5%, P< 0.05), and the NLR was significantly decreased in the tumor group (2.74 vs 5.46, P< 0.05). The tumor group had a greater number of patients with the bleeding sites located in the lobar regions (43.5% vs.19.6%, P< 0.05) and a higher coagulation dysfunction (52.2% vs 29.3%, P< 0.05) than the control group. Multivariate logistic regression analysis revealed that no history of hypertension (OR: 3.141, 95% CI: 1.107– 8.916), lobar cerebral hemorrhage (OR: 3.465 95% CI:1.172– 10.243), and coagulation dysfunction (OR: 3.176, 95% CI: 1.131– 8.913) were independent predictors of occult tumors, and the receiver operating characteristic (ROC) curve showed that the area under the curve of the three-index combined diagnosis was 0.748, C-statistic analysis also showed the same result.
Conclusion: No history of hypertension, lobar cerebral hemorrhage, and coagulation dysfunction may be predictors of the risk of occult malignancies in patients with cerebral hemorrhage.
Keywords: cerebral hemorrhage, clinical features, malignant tumor, risk factors
Introduction
Cerebrovascular disease is a major cause of acquired disability,1 and intracranial hemorrhage (ICH) is a fatal stroke with high morbidity and mortality.2–4 Apart from vascular diseases, cancer is the leading cause of death worldwide.5 Several risk factors are associated with cerebral hemorrhage and cancer, and both diseases may affect patients simultaneously. Cerebrovascular disease is a common complication of malignant tumors, and it is the second most common complication of malignancy in the central nervous system, including ischemic and hemorrhagic strokes, of which intracranial hemorrhage accounts for nearly 50% of the cases.6,7 Some studies have shown that systemic tumors increase the risk of cerebral hemorrhage. A retrospective study conducted by Zöller et al in Sweden with 820,491 cases showed that the incidence of intracerebral hemorrhage was 2.2%, 1.4%, and 1.3% after 6, 6–12, and 15 years, respectively, of a diagnosis of malignant tumor, which was significantly higher than that in the normal population.8 Moreover, a study found that among the patients registered in the Danish Malignancy Centre, the incidence of stroke 1 year before the diagnosis of malignancy was two times higher than that of the control group,9 suggesting that malignant tumors are directly or indirectly involved in the occurrence of cerebral hemorrhage, this is defined as malignant tumor-associated cerebral hemorrhage.8,10 The pathogenesis of malignant tumor-associated cerebral hemorrhage may be related to intratumoral hemorrhage, coagulation dysfunction, venous sinus thrombosis, ischemic stroke hemorrhagic transformation, hypertension, aneurysm, trauma, and reversible posterior cerebral syndrome.7,11–15 Tumors associated with intracerebral hemorrhage are not uncommon in clinical practice; however, intracerebral hemorrhage rarely occurs as the first manifestation of a malignancy, and there are only a few relevant studies.16 Huang et al17 described the clinical manifestations and possible pathogenesis of 17 cases of patients with malignant tumor with intracerebral hemorrhage as the first manifestation; however, they did not compare them with those in common intracerebral hemorrhage. In other existing reports, the incidence varied from 1% to 10%,12,15 which may be due to the differences in study groups. In different studies, 4%18 or 6%19 of patients who underwent surgical treatment for intracerebral hemorrhage were found to have occult malignancies, while 1%20 and 4%21 of occult malignancies were found during autopsy and imaging examinations, respectively.
In addition, cancer is an independent predictor of poor prognosis in patients with intracerebral hemorrhage. Compared with non-cancer patients, patients with systemic cancer have higher mortality and poorer prognosis after intracerebral hemorrhage.22,23 The clinical characteristics, treatment plan, and prognosis of malignant tumor-associated cerebral hemorrhage may differ from those of common acute intracerebral hemorrhage. Therefore, early identification of occult systemic tumors with intracerebral hemorrhage as the first manifestation is required for better and timely development of appropriate clinical protocols for patients. This study aimed to investigate the clinical characteristics and potential risk factors of the first onset of cerebral hemorrhage in patients with occult malignant tumors.
Materials and Methods
Study Population
This study included patients with acute or subacute cerebral hemorrhage (within 14 days of onset) who were hospitalized in the First Affiliated Hospital of Zhengzhou University between January 2017 and December 2020. Patients with occult malignant tumors that had a first onset of cerebral hemorrhage were enrolled into the tumor group, and patients without occult tumors in the same period were randomly enrolled in the control group. There were no statistically significant differences in age and sex between the two groups of patients using propensity score matching. The inclusion criteria for the tumor group were as follows: (1) Patients with neurological impairment and new cerebral hemorrhage confirmed by imaging examination. The diagnostic criteria are based on the 2015 AHA/ASA (American Heart Association/American Stroke Association) Guideline on Spontaneous Intracerebral Hemorrhage.24 (2) Patients who were pathologically confirmed to have systemic malignant tumor during hospitalization. (3) Patients with tumors that did not undergo pathological evaluation during hospitalization, but were diagnosed with malignant tumors after computed tomography (CT), magnetic resonance imaging (MRI), magnetic resonance spectroscopy (MRS), and other imaging examinations, and after consultation with two or more imaging experts and oncologists (during the final follow-up, patients with malignant tumors were confirmed by pathology). The exclusion criteria for the tumor group were as follows: (1) Age <18 years; (2) past cerebral infarction or other neurological diseases; (3) a clear history of the existence of a solid tumor prior to admission; and (4) newly discovered tumor was a hematological tumor or a primary intracranial tumor. The above criteria were jointly determined by an oncologist, a neurologist, and a neuroimaging expert. This study is a cross-sectional study and strictly complies with the Declaration of Helsinki. It also was approved by the Research and Clinical Trial Ethics Committee of the First Affiliated Hospital of Zhengzhou University (2019-KY-123). Almost all of the patients had gave the informed consent for the study, as two patients were deprived of the ability to give informed consent due to health condition, their written informed consent to the participation of this study was obtained from their legal guardian/next of kin.
Data Collection
Individual clinical data were collected, including age; gender; history of smoking; drinking; hypertension; diabetes; routine blood tests; neutrophil-to-lymphocyte ratio (NLR); liver and kidney function; blood glucose concentration; coagulation function; tumor markers; brain CT, MRI imaging, MRA, angiography, lung CT, and other imaging examinations; pathological types; metastasis; and the method used to treat the tumor. NIHSS score on admission and mRS score 90 days after intracerebral hemorrhage were also recorded. The NIHSS score was used to assess the degree of neurological impairment at admission, and the mRS score was used to assess the prognosis after cerebral hemorrhage. The follow-up data were obtained by clinical or telephonic follow-up every 6 months, clinical disability in patients at the last follow-up were recorded as final mRS score. Based on previous studies, coagulation dysfunction16 is defined as (satisfying any of the following conditions): platelet count <100 × 109/L; international standard ratio>1.5; activated partial thrombin time >45 S; prothrombin time(PT)>15 S; diffuse intravascular coagulation (DIC): fibrinogen < 2 g/L and D-dimer >290 µg/L. According to the SMASH-U classification,25 the causes of cerebral hemorrhage are divided into vascular structural damage, drugs, cerebral amyloid angiopathy(CAA), systemic diseases, hypertension, and unknown causes.
Statistical Analysis
Data were analyzed using SPSS software (version 26.0; International Business Machines Corporation, Chicago, IL, USA) and R software (version 3.5.2). We compared the demographic and clinical characteristics between tumor group and control group. If the Shapiro–Wilk test showed that continuous variables were normally distributed, they were expressed as means and standard deviations (SD) and were compared using t-tests. Otherwise, data were expressed as medians (interquartile range) and compared using the Mann–Whitney U-test. Classification variables were expressed as frequency (percentage, %) and were compared using the chi-square test or continuity correction of chi-square. Variables with P <0.1, were included in a multivariate model. Multivariate logistic regression was performed to analyze the independent risk predictors of patients with occult malignant tumors with the first onset of cerebral hemorrhage. Receiver operating characteristic (ROC) curve analysis and C-statistic analysis were used to test the predictive ability of absence of hypertension history, cerebral hemorrhage, and coagulation dysfunction for occult malignant tumors. A two-tailed P-value of <0.05 was considered statistically significant.
Results
Demographics and General Characteristics
Among the 4183 patients with acute cerebral hemorrhage, 23 were included in the tumor group, accounting for 0.55% of the patients. They included 18 males (78.3%) and 5 females (21.7%), with an average age of 59.52±12.17 (range, 30–79) years, and the median age was 59 years. A total of 92 patients with an average age of 59.57±11.93 years were enrolled in the control group. The tumor group had less cases of hypertension than the control group (52.2% vs 81.5%, P<0.05), while diabetes (4.3% vs 12%), smoking history (39.1% vs 30.4%), drinking history (43.3% vs 25%), and coronary heart disease (4.3% vs 8.7%) were not significantly different between the two groups (Table 1).
 |
Table 1 Comparison of Clinical Data Between Patients with Occult Malignant Tumor and Common Cerebral Hemorrhage |
Tumor-Related Clinical Data
Among the 23 cases, there were nine cases of lung cancer, three cases of renal cancer, two cases of thyroid cancer, one case each of thoracic malignancy, esophageal cancer, gastric cancer, liver cancer, cholangiocellular cancer, colon cancer, prostate cancer, ovarian cancer, and chorioepithelial cancer; of these, 13 patients were pathologically diagnosed with malignant tumors within 7 days, and the other 10 patients did not undergo pathological examination, but all of them underwent imaging examinations and were highly suggestive of malignant tumors, and were ultimately pathologically confirmed as malignant tumors during follow-up. Eight of the 23 patients (34.8%) were tested for tumor markers, and had elevated levels of one or more tumor markers, including CA125, CA211, CA724, CA153, carcinoembryonic protein, TPSA, FPSA, and HCG. Six of the 23 patients developed metastasis, and 12 patients received treatment (one surgery, six chemotherapy, one chemoembolization of the tumor supplying artery, one radiotherapy, one radio ion implantation, one steroid, and two fresh frozen plasma for anticoagulant dysfunction.
Clinical Data of Cerebral Hemorrhage in Tumor Group
In the tumor group, symptoms of intracerebral hemorrhage, such as limb weakness (60.9%), loss of consciousness (60.9%), speech disorders (47.8%), headache (43.5%), dizziness (39.1%), and nausea and vomiting (34.8%) were more common. Symptoms of limb convulsions (17.4%), sensory disturbance (14.3%), visual impairment (8.7%), and coma (4.3%) were less common. According to the SMASH-U classification, the incidence of hypertensive intracerebral hemorrhage in the tumor group was less than that in the control group (30.4% vs 55.4%, P<0.05), while the incidence of arteriovenous malformation (4.3% vs 9.8%) and CAA (30.4% vs 18.5%) was not statistically significant. There was also no statistically significant difference in the NIHSS score between the tumor and control groups (Table 1). In the tumor group, 90 days after cerebral hemorrhage, mRS score: 0–1 score: 3 cases (13.1%), 2–3 score: 6 cases (26.1%), 4–5 score: 9 cases (39.1%), 6 score: 5 cases (21.7%).There were no significant differences between the two groups in the treatment of intracerebral hemorrhage (surgery or drug therapy). There was also no difference in mRS score between the two groups at 90 days after discharge, but there was a significant difference in the final mRS score between the two groups at the end of follow-up. Compared with the control group, the final mRS score was higher (3 vs 2, P<0.05) and the follow-up time (21 vs 30.5 months, P<0.05) was shorter in the tumor group.
Laboratory Examination Data
There were no significant differences in WBC, RBC, HB, PLT, ALC, and AMC between the two groups (P>0.05), but the ANC absolute value of neutrophil) (4.17 vs 6.36, P<0.05) and NLR (2.74 vs 5.46, P<0.05) levels of patients in the tumor group were lower than those of patients in the control group. There were no statistically significant differences in liver function (liver enzyme), renal function (BUN, creatinine), and blood lipid (cholesterol, triglyceride, high-density lipoprotein, and low-density lipoprotein) and blood glucose levels between the two groups (P>0.05). However, the tumor group had more patients with coagulation dysfunction than the control group (52.2% vs 29.3%, P<0.05), which were characterized by prolonged PT (52.2% vs 29.3%, P<0.05) and increased D-dimer levels (60.9% vs 34.8%, P<0.05); additionally, one patient in the tumor group had DIC (Table 1).
Imaging Examination Data
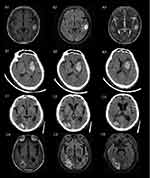
All patients underwent CT or MRI. In the tumor group, intracerebral hemorrhage occurred mainly in the supratentorial area, distributed in both hemispheres (left, 12; right, 7; bilateral, 3) or occurred as subtentorial hemorrhage (1). The most common site of bleeding was located in the cerebral lobar (43.48%), followed by the basal ganglia (21.7%), thalamus (13.04%), cerebellum (4.35%), and multi-site bleeding (13.04%) (Figure 1). Compared with the control group, there was no significant difference in the distribution of bleeding sites in the left and right hemispheres of the brain in the tumor group (left: 52.2% vs 38.8%, right: 30.4% vs 52.2%, P>0.05). Compared with the control group, the site of hemorrhage in the tumor group was more commonly located in the cerebral lobar regions (43.5% vs 19.6%, P<0.05) (Table 1). The average amount of cerebral hemorrhage in the tumor group was 17.05±11.60mL, and the distribution of bleeding volume was as follows: 0–10 mL, seven cases; 10–20 mL, four cases; 20–30 mL, six cases; >30 mL, two cases (because the amount of bleeding could not be calculated, four patients were not included in the analysis due to bleeding into the ventricle or bleeding in multiple locations). Thus, the amount of bleeding between the two groups was not compared.
The Predictors of Occult Tumors are Evaluated by Multivariate Logistic Regression
Variables with P-values of <0.1, which were clinically considered to be closely related to the dependent variables, were included in the multivariate model. Multivariate logistic regression was performed to analyze the independent risk predictors of occult malignant tumors in patients with the first onset of cerebral hemorrhage, and this revealed that no history of hypertension (OR: 3.141, 95% CI: 1.107–8.916), coagulation dysfunction (OR: 3.176, 95% CI: 1.131–8.913), and lobar cerebral hemorrhage (OR: 3.465, 95% CI: 1.172–10.243) were independent predictors of occult tumors, while NLR (OR: 0.917, 95% CI: 0.829–1.015) was not included in the regression equation (Table 2).
 |
Table 2 Multivariate Logistic Regression Analysis of Occult Tumor Predictors |
ROC Curve Analysis of Predictors and C-Statistic Analysis
ROC curve analysis was used to test the predictive ability of the absence of hypertension history, cerebral hemorrhage, and coagulation dysfunction for occult malignant tumors. ROC curve analysis was carried out for these three factors alone, combined with pair, and combined with three factors, which showed that the combined diagnosis of the three factors had the best predictive ability; the area under the curve of three-index combined diagnosis was 0.748 (Table 3). C-statistic showed the same results as the ROC curve analysis (Table 4).
 |
Table 3 ROC Curve Analysis of Predictors |
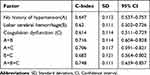 |
Table 4 C-Statistic Analysis of Predictors |
Discussion
We analyzed the clinical characteristics of 23 cases of occult systemic tumors with cerebral hemorrhage as the first manifestation, and found that there were fewer patients with hypertension, diabetes, smoking history, drinking history, and coronary heart disease history, and this shows that traditional vascular risk factors may not be the main cause of cerebral hemorrhage in patients with occult malignancies, which is consistent with the reports of previous studies.17 We further compared the etiology classifications of the two groups with reference to the SMASH-U classification and found that the tumor group had less cases of hypertensive cerebral hemorrhage than the control group, which has a certain prompting effect on the diagnosis of occult malignant tumors, and for further exclusion, malignant tumor-related examinations provide reference significance. Previous reports have shown that tumor-related intracerebral hemorrhage is located in the lobar of the brain, accounting for 60% of cases.14 Similar results were obtained in this study, and the difference was statistically significant when compared with the control group. In addition, according to the definition of coagulation dysfunction,16 in this study, 12 of the 23 patients had coagulation dysfunction manifesting mainly as prolonged PT and elevated D-dimer levels, which is consistent with the findings of previous reports and suggests that thrombin dysfunction is a possible mechanism of tumor-related intracerebral hemorrhage.14,26 Moreover, this indicates that abnormal coagulation may be the main cause of intracerebral hemorrhage in patients who do not have conventional vascular risk factors; however, coagulation dysfunction only accounts for a small proportion of common cerebral hemorrhage.27 Some studies have shown that the most common causes of malignant tumor-related ICH may be intratumoral hemorrhage and abnormal coagulation,10,16 and abnormal coagulation is usually caused by a variety of mechanisms, including abnormal platelet and abnormal coagulation factors. Platelet abnormalities occur in patients with bone marrow suppression and tumor infiltration after chemotherapy or radiotherapy, and are mostly seen in those with hematological malignancies. Abnormal coagulation factors are usually caused by vitamin K deficiency caused by liver failure, malnutrition, or DIC.10 In a study of 1117 patients with solid tumors, the incidence of chronic low-grade DIC in solid tumors was reported to be 6.8%.28 It has also been reported that recurrent hemorrhagic stroke may be the initial manifestation of occult malignancy and may be related to DIC.29,30
The mechanism of paratumor DIC is not fully understood, but it is undoubtedly different from that of sepsis-associated DIC, where extensive endothelial damage is pathogenic.31 In the early stages of cancer, coagulants, such as plasma cancer marker, inflammatory factors, and tissue factors, produced by the tumor31,32 may cause an increase in platelets in the blood, lead to a hypercoagulable state, activate the coagulation system, produce excessive thrombin, and then increase the consumption of platelets, coagulation factors, and blood coagulation inhibitors, which puts the body in a state of DIC and increases the risk of intracranial hemorrhage.33,34
However, in patients with solid tumors, this process is usually characterized by a chronic or subacute, well-compensated state. In this state, it may be difficult to find abnormalities in routine laboratory coagulation tests, and in some circumstances, such as surgery, the balance may be tilted, leading to thrombosis or bleeding, which can lead to cerebral hemorrhage and the discovery of occult malignancies.35 Interestingly, ischemic stroke due to hypercoagulability during chronic DIC is also possible, but is less common.28 In addition, coagulation dysfunction is an independent risk factor for mortality after surgical treatment in patients with cancer complicated with intracerebral hemorrhage, suggesting that coagulation dysfunction is closely related to the prognosis of cancer-related intracerebral hemorrhage.36 We also found that the NLR in the tumor group was relatively lower than that in the control group. Immune-associated inflammation also plays an important role in ICH-induced brain injury and malignant tumors.37,38 NLR is an indicator of poor prognosis of some malignant tumors and cerebral hemorrhage, and is an easily measured inflammatory marker;39–45 however, it may not be an independent predictor of occult neoplasms due to the limited sample size in our study. In addition, there was no significant difference in mRS score 90 days after discharge between the two groups. However, final mRS score of the tumor group was higher than that of the control group during the follow-up period, which may be caused by the effect of tumor with death as the observation end point.
Furthermore, multivariate logistic regression analysis suggested that only no history of hypertension, lobar cerebral hemorrhage, and coagulation dysfunction were independent predictors of occult malignancies in patients with cerebral hemorrhage, and the combined diagnosis could increase the efficiency of the prediction. However, the specific mechanism of this occurrence remains to be prospectively studied in a large sample in future, so as to explore an available forecasting model. 12–15 In this study, lung CT was performed on all 10 patients who had lung masses, and lung cancer was subsequently diagnosed. One patient also underwent lung CT due to pulmonary infection and was diagnosed with liver cancer after an incidental discovery of the liver mass. Tumor markers were detected in eight of the 23 patients, all of whom had one or more elevated tumor marker levels, which indicated that lung CT and tumor markers were necessary to improve the detection rate of combined lung cancer when the cause of intracerebral hemorrhage is unknown; previous studies have also shown that elevated plasma CEA and CA199 levels are associated with the pathogenesis of lung cancer related cerebral hemorrhage (LCRCH), and that the index derived from independent risks should serve as a specific biomarker of LCRCH.26
Limitations
This study is a single-center retrospective study, although there were no statistical differences in age and sex between both groups by propensity score matching, we did not balance other differences that may have caused tumors, and the unbalanced sample size of each group may have led to bias in the results. Therefore, a prospective multi-center study with more cases is needed to further verify the conclusions.
Conclusion
In summary, having no history of hypertension, lobar cerebral hemorrhage, and coagulation dysfunction may be predictors of the risk of occult malignancies in patients with cerebral hemorrhage, and this may help in the early detection of occult tumors.
Acknowledgments
This work was supported by the National Science and Technology Basic Resources Survey of China (2018FY100900). We also thank Editage (www.editage.cn) for English language editing.
Disclosure
The authors declare that they have no conflicts of interest.
References
1. GBD 2015 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1603–1658.
2. González-Pérez A, Gaist D, Wallander MA, McFeat G, García-Rodríguez LA. Mortality after hemorrhagic stroke: data from general practice (The health improvement network). Neurology. 2013;81:559–565.
3. Poon MT, Fonville AF, Al-Shahi Salman R. Long-term prognosis after intracerebral haemorrhage: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2014;85:660–667.
4. Katsuki M, Kakizawa Y, Nishikawa A, et al. Endoscopic hematoma removal of supratentorial intracerebral hemorrhage under local anesthesia reduces operative time compared to craniotomy. Sci Rep. 2020;10(1):10389.
5. GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1459–1544.
6. Adams HP
7. Rogers LR. Cerebrovascular complications in patients with cancer. Semin Neurol. 2004;24:453–460.
8. Zöller B, Ji J, Sundquist J, Sundquist K. Risk of haemorrhagic and ischaemic stroke in patients with cancer: a nationwide follow-up study from Sweden. Eur J Cancer. 2012;48:1875–1883.
9. Andersen KK, Olsen TS. Risk of ischemic and hemorrhagic strokes in occult and manifest cancers. Stroke. 2018;49:1585–1592.
10. Dardiotis E, Aloizou AM, Markoula S, et al. Cancer-associated stroke: pathophysiology, detection and management (Review). Int J Oncol. 2019;54:779–796.
11. Graus F, Rogers LR, Posner JB. Cerebrovascular complications in patients with cancer. Medicine. 1985;64:16–35.
12. Velander AJ, DeAngelis LM, Navi BB. Intracranial hemorrhage in patients with cancer. Curr Atheroscler Rep. 2012;14:373–381.
13. Minette SE, Kimmel DW. Subdural hematoma in patients with systemic cancer. Mayo Clin Proc. 1989;64:637–642.
14. Zhang YY, Chan DK, Cordato D, Shen Q, Sheng AZ. Stroke risk factor, pattern and outcome in patients with cancer. Acta Neurol Scand. 2006;114:378–383.
15. Kuga Y, Waga S, Itoh H. Intracranial hemorrhage due to brain metastasis from hepatocellular carcinoma–case report. Neurol Med Chir (Tokyo). 1990;30:768–771.
16. Navi BB, Reichman JS, Berlin D, et al. Intracerebral and subarachnoid hemorrhage in patients with cancer. Neurology. 2010;74:494–501.
17. Huang G, Chen L, Qin C, et al. Cerebral hemorrhage as the initial manifestation in patients with systemic cancer. Int J Neurosci. 2018;128:48–54.
18. Scott M. Spontaneous intracerebral hematoma caused by cerebral neoplasms. Report of eight verified cases. J Neurosurg. 1975;42:338–342.
19. Abrahams NA, Prayson RA. The role of histopathologic examination of intracranial blood clots removed for hemorrhage of unknown etiology: a clinical pathologic analysis of 31 cases. Ann Diagn Pathol. 2000;4:361–366.
20. Kothbauer P, Jellinger K, Falment H. Primary brain tumour presenting as spontaneous intracerebral haemorrhage. Acta Neurochir. 1979;49:35–45.
21. Schrader B, Barth H, Lang EW, et al. Spontaneous intracranial haematomas caused by neoplasms. Acta Neurochir. 2000;142:979–985.
22. Gon Y, Todo K, Mochizuki H, Sakaguchi M. Cancer is an independent predictor of poor outcomes in patients following intracerebral hemorrhage. Eur J Neurol. 2018;25:128–134.
23. Murthy SB, Shastri A, Merkler AE, et al. Intracerebral hemorrhage outcomes in patients with systemic cancer. J Stroke Cerebrovasc Dis. 2016;25:2918–2924.
24. Hemphill JC
25. Meretoja A, Strbian D, Putaala J, et al. SMASH-U: a proposal for etiologic classification of intracerebral hemorrhage. Stroke. 2012;43:2592–2597.
26. Qin K, Chen Y, Long H, et al. The biomarkers and potential pathogenesis of lung cancer related cerebral hemorrhage. Medicine (Baltimore). 2019;98:e15693.
27. Quinones-Hinojosa A, Gulati M, Singh V, Lawton MT. Spontaneous intracerebral hemorrhage due to coagulation disorders. Neurosurg Focus. 2003;15:E3.
28. Sallah S, Wan JY, Nguyen NP, Hanrahan LR, Sigounas G. Disseminated intravascular coagulation in solid tumors: clinical and pathologic study. Thromb Haemost. 2001;86:828–833.
29. Koenig MA, Maleszewski J, Winters B. Multiple hemorrhagic strokes from DIC associated with occult large cell carcinoma. Neurocrit Care. 2006;5:210–212.
30. Prado MB
31. Bick RL, Strauss JF, Frenkel EP. Thrombosis and hemorrhage in oncology patients. Hematol Oncol Clin North Am. 1996;10:875–907.
32. Hisada Y, Mackman N. Tissue factor and cancer: regulation, tumor growth, and metastasis. Semin Thromb Hemost. 2019;45:385–395.
33. Levi M, Sivapalaratnam S. Disseminated intravascular coagulation: an update on pathogenesis and diagnosis. Expert Rev Hematol. 2018;11:663–672.
34. Levi M. Disseminated intravascular coagulation in cancer: an update. Semin Thromb Hemost. 2019;45:342–347.
35. Levi M. Management of cancer-associated disseminated intravascular coagulation. Thromb Res. 2016;140(Suppl 1):S66–S70.
36. Yamaki VN, Telles JPM, Paiva WS, Teixeira MJ, Neville IS. Surgical treatment of spontaneous intracranial hemorrhage in patients with cancer: analysis of prognostic factors. J Clin Neurosci. 2021;83:140–145.
37. Singh R, Mishra MK, Aggarwal H. Inflammation, immunity, and cancer. Mediators Inflamm. 2017;2017:6027305.
38. Saand AR, Yu F, Chen J, Chou SH. Systemic inflammation in hemorrhagic strokes - a novel neurological sign and therapeutic target? J Cereb Blood Flow Metab. 2019;39:959–988.
39. Lattanzi S, Brigo F, Trinka E, Cagnetti C, Di Napoli M, Silvestrini M. Neutrophil-to-lymphocyte ratio in acute cerebral hemorrhage: a system review. Transl Stroke Res. 2019;10:137–145.
40. Lattanzi S, Cagnetti C, Provinciali L, Silvestrini M. Neutrophil-to-lymphocyte ratio predicts the outcome of acute intracerebral hemorrhage. Stroke. 2016;47:1654–1657.
41. Templeton AJ, McNamara MG, Šeruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106:dju124.
42. Simonaggio A, Elaidi R, Fournier L, et al. Variation in neutrophil to lymphocyte ratio (NLR) as predictor of outcomes in metastatic renal cell carcinoma (mRCC) and non-small cell lung cancer (mNSCLC) patients treated with nivolumab. Cancer Immunol Immunother. 2020;69:2513–2522.
43. Ethier JL, Desautels D, Templeton A, Shah PS, Amir E. Prognostic role of neutrophil-to-lymphocyte ratio in breast cancer: a systematic review and meta-analysis. Breast Cancer Res. 2017;19:2.
44. Miyamoto R, Inagawa S, Sano N, Tadano S, Adachi S, Yamamoto M. The neutrophil-to-lymphocyte ratio (NLR) predicts short-term and long-term outcomes in gastric cancer patients. Eur J Surg Oncol. 2018;44:607–612.
45. Çalışkan S, Sungur M, Kaba S, Özsoy E, Koca O, Öztürk Mİ. Neutrophil-to-lymphocyte ratio in renal cell carcinoma patients. Folia Med (Plovdiv). 2018;60:553–557.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.