Back to Journals » Infection and Drug Resistance » Volume 15
Clinical Characteristics and Prognosis Analysis of Acinetobacter baumannii Bloodstream Infection Based on Propensity Matching
Authors Wang J, Zhang J, Wu ZH , Liu L, Ma Z , Lai CC , Luo YG
Received 30 August 2022
Accepted for publication 14 November 2022
Published 30 November 2022 Volume 2022:15 Pages 6963—6974
DOI https://doi.org/10.2147/IDR.S387898
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Jinghui Wang,1 Jun Zhang,2 Zhuang-hao Wu,1 Lei Liu,3 Zijun Ma,1 Cheng-cheng Lai,1 Yong-gang Luo1
1Department of Intensive Care Unit, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, People’s Republic of China; 2Department of Pharmacy, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, People’s Republic of China; 3Department of Cardiovascular Surgery, The Second Affiliated Hospital of Zhengzhou University, Zhengzhou, People’s Republic of China
Correspondence: Yong-gang Luo, Department of Intensive Care Unit, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, People’s Republic of China, Tel +86-371-66916536, Email [email protected]
Purpose: In view of the fact that Acinetobacter baumannii bloodstream infection(BSI) is a great threat to human survival, early identification of the risk factors affecting prognosis will be of great benefit to the clinic.
Patients and Methods: A propensity score matching method was used to collect patients identified with Acinetobacter baumannii BSI from 2016 to 2020 from a reputable hospital in China.
Results: A total of 398 patients were considered. According to the 28-day prognosis, they were divided into the survival group 150 (37.7%) and the death group 248 (62.3%), and the prognosis was analyzed. Subsequently, Propensity score matching was adjusted for variables with p-values
Conclusion: The existence of drug resistance with Acinetobacter baumannii only leads to Inappropriate empirical antibiotic therapy, ultimately, Inappropriate empirical antibiotic therapy was the direct predictor of mortality.
Keywords: Acinetobacter baumannii, bloodstream infection, propensity score matching, prognosis, inappropriate empirical antibiotic therapy
Introduction
Acinetobacter baumannii is notorious for its ability to colonize hospital settings and the increase of multidrug-resistant bacteria.1–3 It is also constantly attracting widespread attention worldwide.4 An international cohort study by EUROBACT5 showed that Acinetobacter baumannii was the most common pathogen of nosocomial bloodstream infection (BSI) in intensive care unit (ICU). In China, where non-fermenting Gram-negative bacilli causing BSI, Acinetobacter baumannii became the second most pathogenic bacterium after Pseudomonas aeruginosa.6 According to reports, the mortality rate of Acinetobacter baumannii BSI ranges between 29% and 73%.7–9 Therefore, exploring the clinical characteristics and risk factors for death in Acinetobacter baumannii BSI patients will be of great benefit to clinical work.
The risk factors of adverse outcomes of Acinetobacter baumannii BSI are constantly being explored, some studies have shown that excessive use of steroids before the occurrence of bacteremia and septic shock during hospitalization were significantly associated with the poor prognosis.10 A multicenter study from five hospitals in South Korea showed that the severity of the patient’s disease was essential factor affecting the prognosis of Acinetobacter baumannii BSI,11 Son, et al12 reported that the 30-day mortality of Acinetobacter baumannii BSI is inextricably linked to the nature of the infection source and source control, the severity of bacteremia, and inappropriate definitive treatment. Unfortunately, the risk factors of death in patients with Acinetobacter baumannii BSI in other researchs remained inconsistent; the reasons may be as follows, the existence of the study population differences, failed to differentiate between Acinetobacter baumannii colonization or infection, could not to adjust for confounding factors such as disease severity and comorbidities, nonetheless, in order to achieve more accurate and precise results, it is necessary to recognize prognostic factors influencing Acinetobacter baumannii BSI by taking an improved method, it can effectively improve the survival rate and lighten the burden of disease with Acinetobacter baumannii BSI patients. Propensity score matching can effectively balance the baseline differences and reduce the influence of bias and confounding factors in the study. As studied by Joe Amoah,13 propensity score analysis is generally more favorable than traditional regression analysis when estimating causal effects using observed data.
This study further explored the clinical characteristics of BSI, adjusted the confounding factors by using propensity score matching, and explored the factors influencing the 28-day mortality of Acinetobacter baumannii BSI patients. The study design is shown in Figure 1.
 |
Figure 1 Study design of Acinetobacter baumannii BSI patients. |
Materials and Methods
Study Subjects and Inclusion Criteria
The study was carried out in the first affiliated hospital of Zhengzhou University, Henan Province. This is a famous tertiary hospital in the province. The hospital was retrospectively analyzed for at least one blood culture (BC) positive for Acinetobacter baumannii BSI from 2016 to 2020, and simultaneously, met the diagnostic criteria for BSI as proposed by the CDC.14 Only the first positive culture specimen were retained for patients with multiple bacteremia episodes. Patients were aged <18 years, and those discharged within 24 hours of admission were excluded. Patients with peripheral BC which were multiple- bacterial infections would not also not be noticed.
This study was approved by the Scientific Research and Clinical trial Ethics Committee of the first affiliated Hospital of Zhengzhou University (Code 2019-KY-330). Patient consent to participate was waived according to local Ethics Committee due to the retrospective and anonymous characteristics of the study. The study was conducted in accordance with the Declaration of Helsinki.
Variables and Definitions
Through the hospital database, we got the patient’s demographics(gender, age), admitted to the department during BC, clinical parameters(hospital before, ICU before, hospital before infection onset ICU before infection onset). Any comorbidity(diabetes, hypertension, hypertension, cardiovascular disease, cardiopulmonary, resuscitation, malignancy, renal failure, pulmonary infection, respiratory failure). Invasive procedures before bacteremia(mechanical ventilation, surgery), results of drug sensitivity test, laboratory data(White Blood Cell Counts. Procalcitonin (PCT), C-Reactive Protein (CRP), and Lactic Acid (lac)), Severity of disease(APACHE II, SOFA, GCS, septic shock), Antimicrobial regimens before positive BC. Key data such as adverse events and outcomes, If necessary, they would be followed up by telephone to obtain out-of-hospital survival information. Made the clinical data of patients as complete as possible. What interested us was the prognosis after 28 days. The death group is defined as patients who died within 28 days (≤28) from the start of the BC collection day, and the survival group refers to patients who survived more than 28 days.
Inappropriate empirical antibiotic therapy is defined as no antibiotic of any sensitive class being given before a positive BC result according to the drug sensitivity test results. In this study, the duration of antibiotics included in the efficacy analysis ≥ 24 hours.
Multidrug resistance (MDR) is characterized by insensitivity to three or more antibiotics within the antibacterial spectrum. Extensive drug-resistance (XDR) is defined as insensitivity to almost all types of antibiotics except one or two types of antibiotics. Pandrug resistance (PDR) refers to that is insensitivity to all types of antimicrobials currently being used in clinical settings.15
Strain Identification and Drug Sensitivity Testing
Use of the VITEK 2 System (BioMérieux, CRaponne, France) for identification of strains in blood samples, Susceptibility Testing by Disk Diffusion, Minimum inhibitory concentration (MIC) was determined by agar dilution; the interpretation of drug sensitivity test results is based on the recommendations issued by the European Committee for antibiotic susceptibility testing (EUCAST).16 It is worth mentioning that the results of drug sensitivity suggest that Acinetobacter baumannii, which is intermediately sensitive to antibiotics, is identified as drug-resistant.
Statistical Analysis
SPSS25.0(IBM Corp., Armonk, NY, USA) software was used for analysis. For continuous variables with normal distributions, the mean ± standard deviation (mean ± SD) was used, and for non-normal distributions, the median[interquartile range] (Median[IQR]). was used. Categorical variables were expressed as frequencies and percentages. The prognostic factors of Acinetobacter baumannii BSI 28-day mortality were screened by binomial Logistic regression, and the odds ratio and 95% confidence interval were calculated at the same time. Variables with P values < 0.05 in the univariate logistic analysis were included in the multivariable regression model, and we considered the results with P values <0.05 to be statistically significant.
Subsequently, the survival group was matched with the death group by using 1:1 matching for propensity score to reduce possible bias due to unbalanced baseline characteristics between the two groups, the variables of baseline data p value < 0.2 in univariate logistic regression were used as independent predictors to calculate propensity score, and the caliper value was set at 0.2. The 1: 1 nearest neighbor matching method was used to match each survival patient with the death patient separately. After matching propensity score, the matched samples were analyzed by univariate and multivariate paired logistics regression analysis. P < 0.05 was statistically significant. Furthermore, we evaluated the differences between the survival and the death group regarding inappropriate empiric therapy.
Results
Characteristics of the Study Population
This single-center retrospective study included 422 patients and excluded 24, of whom 17 were <18 years old and seven were discharged within 24 hours of admission. 398 eligible patients were considered. The majority were male patients 264 (66.3%), and the average age of 398 patients was 53.0 ±15.9 years old, the hospital stay ranged from 2 to 209 days, and 371 (93.2%) patients had at least one comorbidity. The most common were pulmonary infection 290 (72.9%) and respiratory failure 195 (49.0%). Up to 328 (82.4%) patients underwent invasive procedures before the onset of bacteremia. We found that Acinetobacter baumannii was detected in 316 patients (79.4%) during ICU hospitalization, and the median length of stay in ICU prior to infection onset was 6.0 days [3.0 12.0] days. In our population, 345 patients (86.7) were classified as Carbapenem-resistant Acinetobacter baumannii (CRAB). The SOFA score was 10.0 ± 5.4 on average for all patients. The demographic, clinical, and laboratory characteristics of the 398 participants are summarized in Table 1.
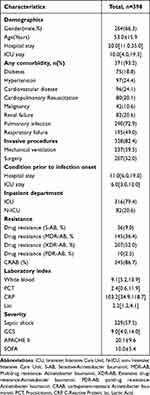 |
Table 1 Demographic and Clinical Characteristics of 398 Patients with Acinetobacter baumannii BSI |
Risk Factors for 28-Day Mortality in Patients with Acinetobacter baumannii BSI
According to the 28-day prognosis, the patients were divided into the survival group (150 cases, 37.7%) and the death group (248 cases, 62.3%). During the 5-year study, the overall mortality rate was as high as 62.3%. Univariate analysis showed that the distribution of gender (P=0.913) and age (P=0.148) was similar between the survival group and the death group. Compared with the death group, the length of stay in the survival group was significantly longer (31.0 vs 15.0, P < 0.001), as shown in Table 2. Patients in the death group were more likely to have renal failure, pulmonary infection, respiratory failure, and so on; the differences were statistical significance. The most common comorbidity was pulmonary infection 209 (84.3%), and patients who received cardiopulmonary resuscitation (P < 0.001) or invasive procedures (P < 0.001) during hospitalization were at significantly increased risk of death. Comparing the drug resistance between the survival group and the death group, it was observed that the drug resistance rate of the death group to carbapenem was significantly higher than that of the survival group (P < 0.001). Most of the dead patients were distributed in ICU (91.5% vs 8.5%, P < 0.001). Univariate analysis identified laboratory indicators associated with 28-day mortality in Acinetobacter baumannii BSI, including PCT, CRP, and lac. in terms of disease severity, a higher APACHE score or SOFA score was a potential risk factor for 28-day mortality.
 |
Table 2 Univariate Logistic Regression Analysis for Risk Factors of 28-Day Mortality for Patients with Acinetobacter baumannii BSI |
The factors with statistical significance in the univariate Logistic analysis were included in the multivariate Logistic regression analysis. The results were shown in Table 3. The prolongation of hospital stay is a protective factor for the 28-day mortality of Acinetobacter baumannii BSI, and the risk of mortality in patients undergone cardiopulmonary resuscitation in the hospital was 6.389 times higher than that in patients without cardiopulmonary resuscitation. Patients with renal failure were also an independent risk factor for death within 28 days of Acinetobacter baumannii BSI, consistent with univariate analysis, the Higher APACHE score was an independent risk factor for death.
 |
Table 3 Multivariate Logistic Regression Analysis for Risk Factors of 28-Day Mortality for Patients with Acinetobacter baumannii BSI |
Risk Factors for 28-Day Mortality in Patients with Acinetobacter baumannii BSI After a Propensity Scoring Match
Propensity score matching was adjusted for variables with P<0.2 in univariate logistic regression, with a total of 63 data sets after matching, and details of the matched patients are shown in Table 4. Based on the paired univariate logistics analysis of the matched population, we found that the potential risk factors between the survival group and the death group were substantially reduced, and the death group was more likely to receive mechanical ventilation. Compared with the survival group, the dead patients’ SOFA scores were higher, which lead to higher 28-day mortality. A higher APACHE score is no longer an independent risk factor for death. Furthermore, we incorporated the above factors into the paired multivariate logistics regression analysis. Table 5 depicts that mechanical ventilation in hospital, the high SOFA score at the time of infection, or septic shock are independent risk factors for 28-day mortality of Acinetobacter baumannii BSI.
 |
Table 4 Univariate Logistic Regression Analysis for Risk Factors of 28-Day Mortality for Patients with Acinetobacter baumannii BSI After Propensity Score Matching |
 |
Table 5 Multivariate Logistic Regression Analysis for Risk Factors of 28-Day Mortality for Patients with Acinetobacter baumannii BSI After Propensity Scoring Match |
Empirical Antibiotic Therapy for Patients with Acinetobacter baumannii BSI
Table 6 shows the association between inadequate empirical antibiotic therapy and mortality before and after propensity matching, with 175 (44%) patients receiving inappropriate empirical therapy before propensity score matching. Patients in the death group were more likely to receive inappropriate empirical treatment than those in the survival group. However, there was no significant correlation between the two groups. After propensity score matching, patients in the death group were still more likely to receive inappropriate empirical treatment than those in the survival group. Thankfully, we found that receiving inappropriate empirical treatment will significantly increase the risk of 28-day death of Acinetobacter baumannii BSI.
Discussion
The incidence of BSI with high morbidity and mortality is increasing worldwide.17 Although effective antibiotic use is constantly updated, BSI remains one of the seven leading causes of death in middle-and high-income countries.18 Acinetobacter baumannii was on the Priority List of Antibiotic-Resistant Bacteria in the Global essential Study Category by the World Health Organization (WHO).19 A multicenter cohort study conducted in 24 countries found that It was the most common pathogen that caused hospital-acquired BSI.5 An analysis of an observational study conducted in 12 large tertiary hospitals in 7 Italian regions reported a 30-day mortality of 73.6% for BSI caused by Acinetobacter baumannii.20 A systematic review of matched cohort and case-control studies21 concluded that the infection with Acinetobacter baumannii was associated with increased mortality. The BSI caused by Acinetobacter baumannii has been paid more and more attention in the clinic because of its high fatality rate.22 Therefore, studying the prognostic factors of Acinetobacter baumannii BSI is necessary.
The increasing use of carbapenem-resistant drugs and the tenacious adaptability of Acinetobacter baumannii make its Drug-resistant strains increasingly tricky.23,24 For a long time, the question of whether drug resistance is a high risk factor for death of Acinetobacter baumannii BSI has remained ambiguous, and This study found that The resistance of Acinetobacter baumannii had nothing to do with a worse clinical prognosis before and after Propensity score matching, this was in line with previous reports in the relevant literature;9,25 however, some studies still supported that drug resistance could increase the mortality of Acinetobacter baumannii BSI patients.26,27 Interestingly, Santoro et al28 discovered that BSI caused by MDR bacteria did not significantly increase mortality after precise stratification of bacterial resistance patterns. On the other hand, BSI caused by XDR gram-negative bacteria was a powerful predictor of death. In our opinion, the presence of drug resistance in Acinetobacter baumannii will only give rise to more and more inappropriate empirical antibiotic therapy, and ultimately, it is the inappropriate empirical antibiotic therapy that is a direct predictor of mortality. A matched control study based on comorbidity, age, and septic shock may towards a better understanding of the role of antibiotic resistance in the clinical outcome of patients with Acinetobacter baumannii.29
In this study, compared with the survival group, people in the death group received more Inappropriate empirical antibiotic therapy before the propensity scoring match, then, the results in the matching queue based on the propensity score, showed that inappropriate empirical antibiotic therapy remained frequently in the death group and significantly affected the prognosis of patients with Acinetobacter baumannii BSI. Some researchs demonstrated that controlling the patient’s baseline status and risk factors of death, especially the patient’s comorbidities and severity of the disease, which were key confounding factors in the relationship between appropriate empirical antibiotic treatment and patient mortality, can more effectively derive the efficacy of empiric antibiotics.30,31 Subsequently based on that evidence, we found out, As soon as possible to carry out the appropriate empiric therapy seemed to be a more attractive strategy compared with the positive experience therapy of broad-spectrum antibiotics which are widely used.22,32 Even every day matters.33 Of course, not only the empirical antibiotic therapy but also the definitive therapy was related to the prognosis of patients with Acinetobacter baumannii BSI. Some studies showed that colistin-containing therapies were associated with survival rates. Accepted loading doses of colistin in patients with CRAB improved their 30-day survival.34,35 Cancer patients with CRAB infection, who received a long course (≥14 days) of colistin had a lower 30-day mortality but the same nephrotoxicity compared with the short course (<14 days).36 For further, the available studies have not reached a consistent conclusion on whether colistin-based combination therapies can reduce the mortality of Acinetobacter baumannii BSI patients.37–40 Therefore, more clinical data are needed for observational analysis in order to improve the prognosis of Acinetobacter baumannii BSI. This is also the direction that our study will focus on in the future.
Acinetobacter baumannii BSI prefers patients with severe diseases. In this study, APACHE, SOFA, and septic shock were used to evaluate the patient’s condition, which is unique and comprehensive. The prognostic analysis only showed that a higher APACHE score was related to a worse prognosis before propensity matching; then, SOFA score and septic shock demonstrated good predictive power for 28-day mortality of Acinetobacter baumannii BSI in the matched population. We confirmed that a higher SOFA score and septic shock were reliable predictors of mortality in conditions that control for comorbidities and disease severity,41 which significantly affect prognosis, and the APACHE score was no longer an independent predictor of death; our study may indicate. Compared with the APACHE score on admission, the factor that can really judge the prognosis of Acinetobacter baumannii BSI patients lies in the severity of bacteremia, or perhaps the APACHE score may be a vital factor in judging the prognosis of some other diseases. However, for patients with Acinetobacter baumannii BSI, there is still a lack of ability to predict prognosis. Worthy of further research. And the matched population was more likely to receive inappropriate empirical therapy, which coincided with the research of Su-Jung Chen et al.42 The difference was that we added the indicator of septic shock to assess the severity of disease, because neither APACHE nor SOFA score included information such as septic shock; however, septic shock often means severe infection, which usually occurs in critically ill patients, They are more likely to die after a lethal hit. We agreed that it was a good indicator for evaluating the severity of the disease and predicting the mortality of bacteremia. Our finding was in accordance with recent literature reports.43,44
In our study, more than half of the patients came from ICU, meaning more invasive procedures. The pulmonary infection has become the primary comorbidity in this cohort. Ultimately, mechanical ventilation during hospitalization was considered to increase the mortality of Acinetobacter baumannii BSI Significantly, it was suggested that pulmonary colonization might be the primary source of Acinetobacter baumannii BSI; further study is needed. In addition, most of the patients who received mechanical ventilation were in serious condition, which may support that the severity of the disease can significantly affect the 28-day prognosis of Acinetobacter baumannii BSI.
It is rare for our research to use propensity score matching in analyzing the factors related to the 28-day prognosis of the Acinetobacter baumannii BSI. Compared with simple logistics regression analysis, propensity score matching adjusted for more confounding factors and equalized differences in baseline to make the results more convincing. In addition, we conducted multivariate analyses to ensure that statistically significant confounders of clinical credibility were retained in our final multivariate model to reflect the actual relationship between research indicators and mortality. Thus, that is, patients with Acinetobacter baumannii BSI who have higher SOFA score at the time of infection, with septic shock, or mechanical ventilation, and Inappropriate empirical antibiotic therapy were more likely to be fatally hit within 28 days and had a poor prognosis.
The limits of research are as follows. Firstly, there is an inevitable selection bias in the design of the retrospective study and we could not exclude the effect of unmeasured variables or unknown confounders, for example, we only collected information on mechanical ventilation and surgery for invasive procedures but failed to include the more common invasive procedures such as the central venous catheter; secondly, our research may lack the ability to distinguish the death caused by Acinetobacter baumannii from that caused by other factors, which is also a matter of concern in the future. In the end, single-center research may lack some universality, multicenter, prospective research is needed to identify further prognostic factors affecting patients with Acinetobacter baumannii BSI.
Conclusion
In summary, we showed that patients with Acinetobacter baumannii BSI had a high 28-day mortality rate. For such patients, clinicians should pay close attention to the patient’s conditions, especially those with high SOFA scores at the time of infection, with septic shock during hospitalization, or mechanical ventilators, by reason of such patients tend to predict poor clinical outcomes; moreover, providing patients with appropriate empirical antibiotic therapy can best improve survival.
Data Sharing Statement
Data are available upon reasonable request to the corresponding author.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was funded by Young And Middle-aged Scientific And Technological Innovation Jie-qing Talent Project, grant number YXKC2021042 and the Henan Province’s Medical Science And Technology Research Provincial And Ministerial Joint Construction Of Key Projects, grant number SBGJ202102081, Henan Province Science and Technology Research Project grant number 212102310321.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Dijkshoorn L, Nemec A, Seifert H. An increasing threat in hospitals: multidrug-resistant Acinetobacter baumannii. Nat Rev Microbiol. 2007;5(12):939–951. doi:10.1038/nrmicro1789
2. Ashuthosh KC, Hegde A, Rao P, Manipura R. Multidrug-resistant Acinetobacter baumannii - the modern menace: a retrospective study in a tertiary hospital in Mangalore. Infect Drug Resist. 2020;13:2181–2187. doi:10.2147/IDR.S249123
3. Ibrahim S, Al-Saryi N, Al-Kadmy IMS, Aziz SN. Multidrug-resistant Acinetobacter baumannii as an emerging concern in hospitals. Mol Biol Rep. 2021;48(10):6987–6998. doi:10.1007/s11033-021-06690-6
4. Asif M, Alvi IA, Rehman SU. Insight into Acinetobacter baumannii: pathogenesis, global resistance, mechanisms of resistance, treatment options, and alternative modalities. Infect Drug Resist. 2018;11:1249–1260. doi:10.2147/IDR.S166750
5. Tabah A, Koulenti D, Laupland K, et al. Characteristics and determinants of outcome of hospital-acquired bloodstream infections in intensive care units: the EUROBACT international cohort study. Intensive Care Med. 2012;38(12):1930–1945. doi:10.1007/s00134-012-2695-9
6. Change in antimicrobial resistance of pathogens from blood specimens: surveillance report from China Antimicrobial resistance surveillance system in 2014–2019. Chin J Infect Control. 2021;20(02):124–133.
7. Townsend J, Park AN, Gander R, et al. Acinetobacter infections and outcomes at an academic medical center: a disease of long-term care. Open Forum Infect Dis. 2015;2(1):ofv023. doi:10.1093/ofid/ofv023
8. Leao AC, Menezes PR, Oliveira MS, Levin AS. Acinetobacter spp. are associated with a higher mortality in intensive care patients with bacteremia: a survival analysis. BMC Infect Dis. 2016;16:386. doi:10.1186/s12879-016-1695-8
9. Gu Z, Han Y, Meng T, et al. Risk factors and clinical outcomes for patients with Acinetobacter baumannii bacteremia. Medicine. 2016;95(9):e2943. doi:10.1097/MD.0000000000002943
10. Ballouz T, Aridi J, Afif C, et al. Risk factors, clinical presentation, and outcome of Acinetobacter baumannii bacteremia. Front Cell Infect Microbiol. 2017;7:156. doi:10.3389/fcimb.2017.00156
11. Kim T, Lee EJ, Park SY, et al. Natural prognosis of carbapenem-resistant Acinetobacter baumannii bacteremia in patients who did not receive appropriate antibiotic treatment: a retrospective multicenter study in Korea. Medicine. 2018;97(43):e12984. doi:10.1097/MD.0000000000012984
12. Son HJ, Cho EB, Bae M, et al. Clinical and microbiological analysis of risk factors for mortality in patients with carbapenem-resistant Acinetobacter baumannii bacteremia. Open Forum Infect Dis. 2020;7(10):ofaa378. doi:10.1093/ofid/ofaa378
13. Amoah J, Stuart EA, Cosgrove SE, et al. Comparing propensity score methods versus traditional regression analysis for the evaluation of observational data: a case study evaluating the treatment of gram-negative bloodstream infections. Clin Infect Dis. 2020;71(9):e497–e505. doi:10.1093/cid/ciaa169
14. Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36(5):309–332. doi:10.1016/j.ajic.2008.03.002
15. Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281. doi:10.1111/j.1469-0691.2011.03570.x
16. The European Committee on Antimicrobial Susceptibility Testing. Zone diameter breakpoints for rapid antimicrobial susceptibility testing (RAST) directly from blood culture bottles. Version 4.0; 2022. Available from: http://www.eucast.org.
17. Goto M, Al-Hasan MN. Overall burden of bloodstream infection and nosocomial bloodstream infection in North America and Europe. Clin Microbiol Infect. 2013;19(6):501–509. doi:10.1111/1469-0691.12195
18. Valles J, Calbo E, Anoro E, et al. Bloodstream infections in adults: importance of healthcare-associated infections. J Infect. 2008;56(1):27–34. doi:10.1016/j.jinf.2007.10.001
19. Tacconelli E, Carrara E, Savoldi A, et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018;18(3):318–327. doi:10.1016/S1473-3099(17)30753-3
20. Russo A, Bassetti M, Ceccarelli G, et al. Bloodstream infections caused by carbapenem-resistant Acinetobacter baumannii: clinical features, therapy and outcome from a multicenter study. J Infect. 2019;79(2):130–138. doi:10.1016/j.jinf.2019.05.017
21. Falagas ME, Bliziotis IA, Siempos II. Attributable mortality of Acinetobacter baumannii infections in critically ill patients: a systematic review of matched cohort and case-control studies. Crit Care. 2006;10(2):R48. doi:10.1186/cc4869
22. Du X, Xu X, Yao J, et al. Predictors of mortality in patients infected with carbapenem-resistant Acinetobacter baumannii: a systematic review and meta-analysis. Am J Infect Control. 2019;47(9):1140–1145. doi:10.1016/j.ajic.2019.03.003
23. Ayobami O, Willrich N, Suwono B, Eckmanns T, Markwart R. The epidemiology of carbapenem-non-susceptible Acinetobacter species in Europe: analysis of EARS-net data from 2013 to 2017. Antimicrob Resist Infect Control. 2020;9(1):89. doi:10.1186/s13756-020-00750-5
24. Giammanco A, Cala C, Fasciana T, Dowzicky MJ, Bradford PA. Global assessment of the activity of tigecycline against multidrug-resistant gram-negative pathogens between 2004 and 2014 as part of the tigecycline evaluation and surveillance trial. mSphere. 2017;2(1). doi:10.1128/mSphere.00310-16
25. Blot S, Vandewoude K, De Bacquer D, Colardyn F. Nosocomial bacteremia caused by antibiotic-resistant gram-negative bacteria in critically ill patients: clinical outcome and length of hospitalization. Clin Infect Dis. 2002;34(12):1600–1606. doi:10.1086/340616
26. Liu Q, Li W, Du X, et al. Risk and prognostic factors for multidrug-resistant Acinetobacter Baumannii complex bacteremia: a retrospective study in a tertiary hospital of West China. PLoS One. 2015;10(6):e0130701. doi:10.1371/journal.pone.0130701
27. Pfaller MA, Carvalhaes CG, Smith CJ, Diekema DJ, Castanheira M. Bacterial and fungal pathogens isolated from patients with bloodstream infection: frequency of occurrence and antimicrobial susceptibility patterns from the SENTRY Antimicrobial surveillance program (2012–2017). Diagn Microbiol Infect Dis. 2020;97(2):115016. doi:10.1016/j.diagmicrobio.2020.115016
28. Santoro A, Franceschini E, Meschiari M, et al. Epidemiology and risk factors associated with mortality in consecutive patients with bacterial bloodstream infection: impact of MDR and XDR bacteria. Open Forum Infect Dis. 2020;7(11):ofaa461. doi:10.1093/ofid/ofaa461
29. Metan G, Sariguzel F, Sumerkan B. Factors influencing survival in patients with multi-drug-resistant Acinetobacter bacteraemia. Eur J Intern Med. 2009;20(5):540–544. doi:10.1016/j.ejim.2009.05.005
30. McGregor JC, Rich SE, Harris AD, et al. A systematic review of the methods used to assess the association between appropriate antibiotic therapy and mortality in bacteremic patients. Clin Infect Dis. 2007;45(3):329–337. doi:10.1086/519283
31. Lemos EV, de la Hoz FP, Einarson TR, et al. Carbapenem resistance and mortality in patients with Acinetobacter baumannii infection: systematic review and meta-analysis. Clin Microbiol Infect. 2014;20(5):416–423. doi:10.1111/1469-0691.12363
32. Kadri SS, Lai YL, Warner S, et al. Inappropriate empirical antibiotic therapy for bloodstream infections based on discordant in-vitro susceptibilities: a retrospective cohort analysis of prevalence, predictors, and mortality risk in US hospitals. Lancet Infect Dis. 2021;21(2):241–251. doi:10.1016/S1473-3099(20)30477-1
33. Lodise TP, Kanakamedala H, Hsu WC, Cai B. Impact of incremental delays in appropriate therapy on the outcomes of hospitalized adult patients with gram-negative bloodstream infections: “every day matters”. Pharmacotherapy. 2020;40(9):889–901. doi:10.1002/phar.2446
34. Katip W, Uitrakul S, Oberdorfer P. Clinical efficacy and nephrotoxicity of the loading dose colistin for the treatment of carbapenem-resistant Acinetobacter baumannii in critically ill patients. Pharmaceutics. 2021;14(1):31. doi:10.3390/pharmaceutics14010031
35. Katip W, Oberdorfer P, Kasatpibal N. Effectiveness and nephrotoxicity of loading dose colistin-meropenem versus loading dose colistin-imipenem in the treatment of carbapenem-resistant Acinetobacter baumannii infection. Pharmaceutics. 2022;14(6):1266.
36. Katip W, Uitrakul S, Oberdorfer P. Short-course versus long-course colistin for treatment of carbapenem-resistant A.baumannii in cancer patient. Antibiotics. 2021;10(5):484. doi:10.3390/antibiotics10050484
37. Tamma PD, Aitken SL, Bonomo RA, Mathers AJ, van Duin D, Clancy CJ. Infectious diseases society of America guidance on the treatment of ampC beta-lactamase-producing enterobacterales, carbapenem-resistant Acinetobacter baumannii, and Stenotrophomonas maltophilia infections. Clin Infect Dis. 2022;74(12):2089–2114. doi:10.1093/cid/ciab1013
38. Paul M, Daikos GL, Durante-Mangoni E, et al. Colistin alone versus colistin plus meropenem for treatment of severe infections caused by carbapenem-resistant gram-negative bacteria: an open-label, randomised controlled trial. Lancet Infect Dis. 2018;18(4):391–400. doi:10.1016/S1473-3099(18)30099-9
39. Katip W, Oberdorfer P. Clinical efficacy and nephrotoxicity of colistin alone versus colistin plus vancomycin in critically ill patients infected with carbapenem-resistant Acinetobacter baumannii: a propensity score-matched analysis. Pharmaceutics. 2021;13(2):162.
40. Katip W, Uitrakul S, Oberdorfer P. A comparison of colistin versus colistin plus meropenem for the treatment of carbapenem-resistant Acinetobacter baumannii in critically ill patients: a propensity score-matched analysis. Antibiotics. 2020;9(10):647. doi:10.3390/antibiotics9100647
41. Digiovine BCC, Watts C, Higgins M. The attributable mortality and costs of primary nosocomial bloodstream infections in the intensive care unit. Am J Respir Crit Care Med. 1999;160:976–981. doi:10.1164/ajrccm.160.3.9808145
42. Chen SJ, Chao TF, Chiang MC, et al. Prediction of patient outcome from Acinetobacter baumannii bacteremia with Sequential Organ Failure Assessment (SOFA) and Acute Physiology and Chronic Health Evaluation (APACHE) II scores. Intern Med. 2011;50(8):871–877. doi:10.2169/internalmedicine.50.4312
43. Papadimitriou-Olivgeris M, Fligou F, Spiliopoulou A, et al. Risk factors and predictors of carbapenem-resistant Pseudomonas aeruginosa and Acinetobacter baumannii mortality in critically ill bacteraemic patients over a 6-year period (2010-15): antibiotics do matter. J Med Microbiol. 2017;66(8):1092–1101. doi:10.1099/jmm.0.000538
44. Niu T, Xiao T, Guo L, et al. Retrospective comparative analysis of risk factors and outcomes in patients with carbapenem-resistant Acinetobacter baumannii bloodstream infections: cefoperazone-sulbactam associated with resistance and tigecycline increased the mortality. Infect Drug Resist. 2018;11:2021–2030. doi:10.2147/IDR.S169432
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

