Back to Journals » Clinical Interventions in Aging » Volume 18
Clinical Assessment Tools for the Detection of Cognitive Impairment and Hearing Loss in the Ageing Population: A Scoping Review
Authors Ferguson MA, Nakano K, Jayakody DM
Received 2 August 2023
Accepted for publication 7 November 2023
Published 7 December 2023 Volume 2023:18 Pages 2041—2051
DOI https://doi.org/10.2147/CIA.S409114
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Maddalena Illario
Melanie A Ferguson,1– 3 Kento Nakano,4 Dona MP Jayakody1,3,4
1School of Allied Health, Faculty of Health Sciences, Curtin University, Perth, Australia; 2Curtin enAble Institute, Faculty of Health Sciences, Curtin University, Perth, Australia; 3Centre for Ear Sciences, Medical School, University of Western Australia, Perth, Australia; 4Ear Science Institute Australia, Perth, Australia
Correspondence: Melanie A Ferguson, Curtin School of Allied Health, Kent Street, Bentley, Perth, 6102, Western Australia, Australia, Tel +61 8 6457 0572, Email [email protected]
Objective: There is a strong association between cognitive impairment and hearing loss, both highly prevalent in the ageing population. Early detection of both hearing loss and cognitive impairment is essential in the management of these conditions to ensure effective and informed decisions on healthcare. The main objective was to identify existing and emerging cognitive and auditory assessment tools used in clinical settings (eg, memory clinics, audiology clinics), which manage the ageing population.
Methods: A scoping review of peer-reviewed publications and results were reported according to the PRISMA-ScR guidelines.
Results: A total of 289 articles were selected for data extraction. The majority of studies (76.1%) were conducted in 2017 or later. Tests of global cognitive function (ie, Mini-Mental State Exam, Montreal Cognitive Assessment) were the most commonly used method to detect cognitive impairment in hearing healthcare settings. Behavioral hearing testing (ie, pure-tone audiometry) was the most commonly used method to detect hearing loss in cognitive healthcare settings. Objective, physiological measures were seldom used across disciplines.
Conclusion: Preferences among clinicians for short, accessible tests likely explain the use of tests of global cognitive function and behavioral hearing tests. Rapidly evolving literature has identified inherent limitations of administering global cognitive function tests and pure-tone testing in an ageing population. Using electrophysiological measures as an adjunct to standard methods of assessment may provide more reliable information for clinical recommendations in those with cognitive and hearing impairment, and subsequently achieve better healthcare outcomes.
Plain Language Summary: 1. Hearing loss in mid-life (45– 65 years) is the number one modifiable risk factor for dementia, therefore early detection of hearing loss and cognitive impairment are essential to manage both conditions effectively.2. Tests of global cognitive function (eg, MMSE, MOCA) were the most commonly used tests to detect cognitive impairment in hearing healthcare clinics.3. Pure-tone audiometry was the most commonly used test to detect hearing loss in cognitive healthcare settings.4. Objective electrophysiological measures can overcome difficulties in assessing cognition and hearing loss in people with dementia, and can be a useful adjunct to existing assessment tests.
Keywords: hearing loss, cognition, assessment, ageing, early detection
Introduction
With an increasing global population and better healthcare outcomes, the proportion of individuals entering the ageing population is increasing.1 Subsequently, the proportion of age-related morbidities is on the rise. Hearing loss, present in approximately half of those aged 75 years and over, is ranked 3rd out of 328 conditions in years lived with disability.2 Hearing loss most commonly occurs as a result of ageing (ie, presbycusis), with changes in the peripheral and central auditory system. Since the seminal paper by Lin et al, attention has been directed towards the association between hearing loss and cognitive impairment.3,4 Untreated hearing loss in midlife (45–65 years) has since been reported as the number one modifiable risk factor for dementia.5,6 Dementia is characterized by impaired memory, thinking and behavior, commonly resulting from pathologies affecting the brain such as Alzheimer’s disease.7 In the early stages of dementia, deficits in peripheral and central auditory processing have been observed.8–10 Therefore, there is growing interest in developing methods of early detection for hearing loss and cognitive impairment in the adult population, as early detection allows for earlier intervention and better health outcomes.11 An important consideration of this “early detection, early intervention” agenda is for healthcare clinicians to be equipped with the necessary tools to detect those at risk of hearing loss and cognitive impairment in midlife, as well as in the ageing population.
Detecting Cognitive Impairment in the Ageing Population
Effective cognitive assessment tools test multiple domains of cognition to identify different patterns of impairment.11 Examples include tests of global cognitive function, such as the Mini-Mental State Exam (MMSE) and the Montreal Cognitive Assessment (MoCA). These tests are commonly used by hearing healthcare clinicians screening for cognitive impairment.12 However, sensory deficits (eg, hearing loss) have the potential to impact adversely on cognitive test performance, leading to a subsequent overestimation of cognitive decline.13,14 Adapted versions of cognitive assessment tools have therefore been developed to address this impact (eg, written-MMSE;15,16 MoCA-Hearing-Impaired,17 and more recently, a validated version (MOCA-H) in English18 and in German;19 Cambridge Neuropsychological Test Automated Battery (CANTAB),20 Repeated Battery for the Assessment of Neuropsychological Status for Hearing Impaired Individuals (RBANS-H)).21 Other computerised test batteries that can be used for assessment of cognitive impairment are the RBANS, Cogstate,22 ALACog,23 the CDR computerized assessment system (CDR system),24 and Factors of Longitudinal Attention, Memory and Executive Function (FLAME) test batteries.25 While these adapted cognitive tests are more suitable for those with hearing loss, it is unclear whether exclusively visual tests are equivalent to those that cross multiple modalities. Indeed, previous reviews have suggested that cognitive screeners, such as the MOCA, are not optimally sensitive to detecting mild cognitive impairment (MCI);26 further, said tests tend to have poorer specificity for MCI,27 potentially contributing to over-referral. The final diagnosis of cognitive decline, however, includes a comprehensive clinical evaluation by a clinical expert.
More recently, the use of objective measures in the assessment of cognitive impairment has gained interest. Two systematic reviews have identified the use of auditory event-related potentials as biomarkers for cognitive impairment and Alzheimer’s disease.28,29 Auditory-event-related potentials (eg, P200; P300) were shown to be valuable biomarkers of Alzheimer’s disease, and have the potential to be integrated as a complementary assessment as part of a clinical neuropsychological test battery.28 An investigation into routine clinical practice is needed to determine the effectiveness of current measures and assess the practicality of using biomarkers in clinic.
Detecting Hearing Loss in the Ageing Population
Management of hearing loss plays a vital role in improving health-related outcomes more generally, but specifically for people with MCI and people with dementia.30,31 Individuals with untreated mild, moderate and severe hearing loss in midlife are two, three and five times, respectively, more likely to develop dementia.3 Therefore, early and timely detection of hearing loss and uptake of appropriate interventions are essential. There is increasing evidence for the positive impact of hearing aids, the most common management for hearing loss, on cognitive function.32 More recently, the large, well-conducted, randomized controlled ACHIEVE trial conducted across a three-year period showed that hearing intervention (including hearing aids and audiological counselling) might improve cognition in older adults at risk for cognitive decline.33 However, there was no evidence of improved cognition in those with decreased risk of cognitive decline. Pure-tone audiometry (PTA) administered by a trained audiologist is the “gold-standard” clinical method of assessing hearing sensitivity,34 although limitations of administering PTA are evident. Firstly, PTA requires access to specialized equipment and trained personnel that are limited for many non-audiological healthcare clinics.35 Secondly, there is evidence to suggest that PTA may be unreliable in individuals with greater severities of dementia.36 In addition to using auditory evoked potentials (ie, P300, MMN) as potential biomarkers for cognitive impairment, some of the auditory evoked potentials (ie, Auditory Brainstem responses, Auditory steady-state responses) can also be used to establish this population’s hearing thresholds objectively.37 However, recent practice recommendations for the clinical management of people with dementia and comorbid auditory impairment have not discussed the role of electrophysiological assessment in different clinical contexts.38,39 There is a growing need for easily accessible and reliable auditory assessment tools for those with MCI and people with dementia that can be administered by healthcare clinicians without specialized audiological training (eg, clinicians in memory clinics).
Overall Aim and Research Questions
Due to the wide-ranging, heterogeneous nature of the research questions across two conditions (ie, hearing loss and cognitive impairment) and settings (eg, audiology and memory clinics), a scoping review was conducted. Previous reviews have identified cognitive assessment tools used in populations with hearing loss, focusing specifically on screening.11,12,40 Whereas, one review explored assessments of hearing function in individuals living with cognitive impairment specifically in long-term care homes.39
The overall aim of this review was to provide a comprehensive overview of existing and emerging tools to detect cognitive impairment and hearing loss across different clinical settings (primarily cognitive assessment in audiology clinics and hearing assessment in memory clinics). The review is intended to identify gaps in the evidence, and further determine the strengths and limitations of using assessments across healthcare disciplines and settings, to help inform future evidence-based recommendations and developments of care for older adults. The specific research questions were:
- What screening and/or diagnostic tools, and their frequency, are used in the assessment of dementia and/or cognitive impairment in individuals with a hearing loss?
- What screening and/or diagnostic tools, and their frequency, are used in the assessment of hearing loss in individuals with cognitive impairment or dementia?
- In what settings (eg, audiology clinics, memory clinics) are these screening and/or diagnostic tools being used?
Methods
Design
The scoping review was conducted according to the methodological framework proposed by Arksey & O’Malley,41 later adapted by Levac et al,42 and which adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews (PRISMA-ScR) guidelines.43 In contrast to a systematic review, a scoping review does not evaluate the quality of selected studies, thus any biases or methodological issues in the studies are not addressed.
Identifying Relevant Studies
A structured electronic literature search was conducted using the following databases: Embase, Cochrane Library, Web of Science, PubMed, and Scopus. The search strategy was developed by the authors and library staff from the University of Western Australia (see Supplementary Document A). A hand-search of the bibliographies of included papers was performed to identify any additional papers. A final search was run prior to submission of the article (28.11.22).
Study Selection
Two independent reviewers screened the titles and abstracts of the articles prior to full-text review according to the eligibility criteria. Inclusion criterion were (i) human adults, aged ≥18 years, assessed for hearing loss and/or cognitive impairment. Exclusion criteria were (i) full-text articles not available, or not available in English, (ii) no information on methods of clinical assessment, (iii) population with physical conditions or disorders that may potentially confound assessments, (iv) neuroimaging studies, (v) population that was prelingually deafened. A third reviewer was blinded to the review of six initial full-text articles to ensure inter-rater reliability prior to the screening. Any disagreements between the two reviewers were arbitrated by the third reviewer.
Data Extraction
Data from included articles were extracted and charted in a collection form against the following parameters (Supplementary Table 1): First-named author, year of publication, sample size, assessment setting, cognitive assessment(s), hearing assessment(s). The prevalence of cognitive and hearing assessments across articles was separated into two time periods, pre-2017 and 2017 onwards because the influential Lancet Commission report on dementia prevention, intervention and care, first reported hearing loss in mid-life as the largest modifiable risk factor for dementia.6 Data from studies performing a secondary analysis, or being reported in multiple articles, are displayed in Supplementary Table 1 but not included in the manuscript in order to exclude duplicate results.
Results
Literature Search
The search initially identified 3992 articles, and after duplicates were removed, 3381 article titles and abstracts were screened (Figure 1). Of the 3381 records screened, 2995 articles were excluded as they did not meet the inclusion criteria. Ten articles were irretrievable, and 416 articles were retrieved for full-text review that either met the inclusion criteria or for which there was uncertainty about inclusion. Following exclusion of 173 articles (for reasons see Figure 1), 243 articles were included from the original search. A re-run of the searches performed prior to submission of the manuscript yielded an additional 46 articles, leading to a total of 289 articles for inclusion. Sixty-nine articles (23.9%) were published between 1989 and 2016 (pre-2017), with the remaining 220 articles (76.1%) published from 2017 onwards (post-2017). Ten articles presented secondary analysis of data. Therefore, data were extracted from a total of 279 studies. Assessment tools of cognitive and hearing function according to their categorized domains and percentage used across the studies are presented in Supplementary Table 2. Some studies used more than one assessment tool.
 |
Figure 1 PRISMA-Scr flowchart of the literature search.43 |
Assessment Tools of Cognitive Function
Tests of global cognitive function administered by interview were the most frequently used method for detecting cognitive impairment in patient populations with hearing loss (68.3% of articles). The MMSE in its original format was the most commonly administered test (n = 127 studies), followed by the MoCA (n = 48 studies), both showing a greater number post-2017 compared to pre-2017. Adapted versions of the MMSE and MoCA to account for hearing loss were administered in 14 and seven studies, respectively. Furthermore, in two studies published post-search, there are now validated English18 and German19 versions of the MOCA-H for adults with hearing impairment. Due to the wide-range of global cognitive function tests used, tests that were used in only a single study pre- or post-2017 were categorized as “other” with corresponding references provided (eg, Blessed Information-Memory Concentration Test). Self-report measures and questionnaires were used to assess cognitive function in 25.6% of articles, with patient-reported memory complaints (n = 14 studies) and the Clinical Dementia Rating Scale (n = 12 studies) being the most used, especially post-2017. Objective biomarkers of cognitive function were seldom used, with electrophysiological measures (eg, cortical auditory evoked potentials; auditory steady state responses) used in four studies pre-2017 and three studies post-2017.
Assessment of cognitive subdomains was performed by either cognitive test batteries assessing multiple domains, or shorter domain-specific tests (eg, verbal fluency; executive function; visuospatial ability). Full neurocognitive test batteries were administered in 11.8% of articles, mostly post-2017, with the Repeatable Battery for the Assessment of Neuropsychological Status being the most commonly used in six studies post-2017. The most-used cognitive subdomain tests assessed learning and memory, verbal fluency, executive function, and processing speed, most post-2017. Tests of free, immediate and delayed recall (n = 42 studies), trail-making (n = 42 studies), digit-symbol coding/substitution (n = 40 studies), and controlled oral word association (n = 37 studies) were most commonly used for detecting cognitive impairment.
Assessment Tools of Hearing Function
Behavioral measures of hearing function were most frequently used for detecting hearing loss in patient populations with cognitive impairment, administered in 73.4% of total articles. Pure-tone audiometry (PTA) was the most commonly used test both pre-2017 and post-2017 (n = 169 studies), followed by speech audiometry (n = 74 studies). Screening audiometry was used in 29 studies, and the whisper test in four studies. Self-report measures and clinical interviews were used to assess hearing function in 39.1% of total articles, the majority post-2017. Patient-reported hearing problems obtained using questionnaires that were not validated measures were the most frequently used self-report method (n = 54 studies). This was followed by the Hearing Handicap Inventory for the Elderly questionnaire (n = 22 studies), which is the most commonly used self-reported outcome measure in audiology.44 Other self-report measures, such as the SSQ and IOI-HA were documented in 28 unique studies. Objective assessment of hearing function was performed in 11 studies, most post-2017, through electrophysiological measures (eg, auditory evoked potentials). Of these electrophysiological measures, distortion product otoacoustic emissions were the most common measure to determine hearing thresholds (n = 7 studies), followed by auditory brainstem responses (n = 6 studies).
Hybrid Assessment Tools
Certain studies obtained measures of word recognition and recall through the use of hybrid assessment tools (eg, Word Auditory Recognition and Recall Measure; Sentence-final Word Identification and Recall test). As these are not used to identify individuals with hearing loss or cognitive impairment, they were not included in Supplementary Table 2.
Assessment of Cognitive Function in Hearing Healthcare Research
We were particularly interested in the number of cognitive assessment tools administered in hearing healthcare research (ie, audiology, ENT) (Supplementary Table 3). As high-quality aged care is an important research priority,45 studies performed in aged care facilities were also included. The results were broadly similar to those in Supplementary Table 2. Tests of global cognitive function (MMSE; MoCA) were the most commonly used measure for the clinical assessment of cognitive function (n = 27; n = 20 studies respectively). Cognitive test batteries were rarely used in hearing healthcare research studies, with the RBANS-H (n = 5 studies) and the ALAcog test battery (n = 4 studies) being the most common. Cognitive subdomains were occasionally assessed individually (n = 18 studies). Self-report measures were used in 10 studies performed in hearing healthcare clinics. No objective electrophysiological measures (ie, auditory evoked potentials) were used by hearing healthcare clinicians and aged-care staff to measure brain function.
Assessment of Hearing Function in Cognitive Healthcare Research
Similarly, we were interested in the number of hearing assessment tools administered in memory clinics, geriatric clinics, Alzheimer’s centers, and aged-care facilities (Supplementary Table 4), with results broadly similar to Supplementary Table 2. Behavioral measures of hearing function were reported in fifteen studies, with PTA used as a diagnostic measure of hearing loss in seven studies, and pure-tone threshold screening used in seven studies. Two studies administered speech audiometry testing to the patient population, and one study used the whisper test. A range of self-reported measures were used, equally pre-2017 and post-2017. One study used distortion product otoacoustic emissions as an objective measure of hearing thresholds, and another used cortical automated threshold estimation.
Discussion
The aim of this review was to identify existing and emerging tools commonly used to assess cognitive impairment and hearing loss in the ageing population. We were particularly interested in cognitive assessment tools used in hearing healthcare settings, and assessments of hearing function used in cognitive healthcare settings to identify which tests might be recommended for clinical and research use in a screening capacity to identify those at risk of hearing loss or cognitive decline/dementia. Although general population screening for dementia is not recommended, there is an opportunity to target populations at risk.46
Cognitive Assessment in Individuals with Hearing Loss
The tests of global cognitive function (ie, MoCA and MMSE) were the most commonly used tests to screen for cognitive impairment in adults with hearing loss, particularly post-2017. Characteristics of these tests include quick administration time, minimal training requirements, and ease of accessibility, explaining the high favorability amongst clinicians and researchers. Although such measures allow for cognitive screening, the confounding effect of hearing loss should be accounted for. Few studies opted for versions of cognitive screening tools modified for use in those with hearing loss, developed as early on as 1989.15–17 Although used for research and clinical purposes, most current adapted cognitive screening tools are not standardized and validated for use in adults with hearing loss.12 Clinicians should also be made aware of the other limitations of cognitive screening tools. Such tests have shown poor predictability of impairment when compared to results of extensive assessment of cognitive subdomains.47 These tests also require comparison to factor-corrected normative data, are likely to be influenced by ceiling effects and restricted score range, and are limited in their assessment of cognitive subdomains, all resulting in a greater likelihood of misclassification for cognitive impairment.48
Cognitive test batteries assist in diagnosis and enable the comprehensive assessment of multiple domains of cognitive function (eg, attention, executive function, verbal fluency, working memory, learning, visuospatial ability, and global cognitive function).48 Although these test batteries have greater power in determining functional capacity, routine application in clinic is limited by long administration time, need for specialized training, and expertise in interpretation, making it unsuitable for use by inexperienced clinicians. This is echoed in our findings that show cognitive test batteries were seldom used, with few studies integrating assessments of certain cognitive subdomains that have been associated with poorer performance in individuals with hearing loss (eg, executive functioning; learning and memory).49,50
The use of electrophysiological measures as a biomarker of cognitive impairment is evidently rare in the assessment of individuals with hearing loss. Yet, there is potential for use as an adjunct to routine clinical assessment of cognitive impairment. Auditory event-related potentials have shown significant abnormalities in both amplitude and latency in individuals living with Alzheimer’s disease and mild cognitive impairment.28,29,51 Specifically, a meta-analysis conducted by Tarawneh et al showed the promising role of these potentials (eg, P300) in discriminating between those at higher risk of cognitive decline from normal-ageing individuals.29 Biomarkers of cognitive impairment may also be reflected in clinical measures of listening effort (eg, pupillometry).52 These electrophysiological measures are not without drawbacks (see Figure 2). Implementing these electrophysiological measures in clinical settings require additional resources including trained professionals and access to equipment.
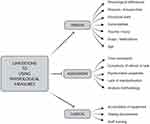 |
Figure 2 Limitations of physiological measures of functional assessment in clinic. For detailed discussion on the factors limiting physiological testing, see references.53,54 |
Hearing Assessment in Individuals with Cognitive Impairment
Pure-tone audiometry was the most commonly used method of hearing assessment in populations with cognitive impairment. Although a reliable indicator of hearing thresholds (ie, hearing sensitivity), PTA may not provide reliable estimates of an individual’s functional hearing ability, particularly in background noise.55 In addition, the ability to complete PTA may be compromised by the severity of cognitive impairment in the subject.56 In their systematic review, Bott et al found that testing for hearing thresholds could not be completed in up to 44% of people with dementia.36 Adaptations to standard audiological testing procedure that can accommodate those individuals unable to complete behavioral tests are important, and include the use of objective measures (eg, auditory evoked potentials).57 Particularly, as recent practice guidelines promote the sole use of behavioral screening tools in clinics managing people with dementia.58
When contemplating other modalities of auditory testing in this population, it is important to consider the applicability for researchers and clinicians that lack experience and advanced audiological training. Such is the case for aged-care facilities and memory clinics, where the prevalence of hearing loss in the cognitively impaired population has been markedly under-recognized.59,60 In their review, Hobler et al identified alternative measures of hearing loss to PTA that are favored amongst aged-care and allied healthcare staff, such as questionnaires and the whisper test.40,61,62 The whisper test does not require additional specialized staff, prolonged administration time, nor access to restricted equipment; however, its clinical utility has long been contentious.63–65 Concerns surrounding the reliability in the general population have resulted from a lack of standardization, reproducibility, and low sensitivity.66,67 Self-report questionnaires are valuable in the general population and also in individuals diagnosed with MCI. However, those with more severe dementia are unlikely to provide reliable responses,62,68 although proxy measures by carers are an option. In line with people with dementia’s needs and perspectives on audiology testing, incorporating a robust and fit-for-purpose hearing test in healthcare practices is an urgent priority.59,69,70
This scoping review identified 11 studies where electrophysiological measures of hearing thresholds were obtained. Examples of measures included were distortion product otoacoustic emissions, auditory steady state responses and auditory brainstem responses, either tone-evoked (single-ear, or parallel) or broadband click-evoked. These measures enable objective assessment of hearing thresholds in individuals unable to provide reliable responses to standard audiological testing, and can serve as a means to identify a hearing disorder.71 Such is the case in neonatal and pediatric populations,72 as well as in populations with autism and dementia where significant correlations between behavioral thresholds and auditory evoked potentials have been found.73,74 However, challenges faced by healthcare practices include, but are not limited to: long administration time; electromagnetic interference; arousal state of the subject (ie, muscle movement, stress, anxiety); experience of clinician; and equipment restrictions. Further research is required to overcome the limitations associated with the use of reliable, objective functional assessment of hearing in healthcare practices.
Limitations and Biases
Although the scope of this review was broad, tests of central auditory processing were not reported in this review, as we were more focused on assessments of peripheral hearing loss in the ageing population due to its high prevalence. Furthermore, a number of studies did not report the setting at which testing was administered. However, as the data are relatively proportionate when comparing Supplementary Tables 3 and 4 to Supplementary Table 2, it is likely that the results are broadly representative of each clinical setting. There is also evidence that other conditions comorbid with cognitive impairment and hearing loss have been associated with the progression of Alzheimer’s disease and dementia (eg, cardiovascular disease, depression, traumatic brain injury).6 This review did not include assessments of comorbid conditions, although it is noted that these conditions are likely confounders in the assessment of cognitive and hearing function.
Conclusions
In order to address the concerns and symptoms for cognitive impairment and hearing loss, a range of assessment tools have been used across different healthcare disciplines that manage the ageing population. This review has found that tests of global cognitive function (eg, MMSE, MOCA) and behavioral hearing tests (ie, pure-tone audiometry) are the most common existing tools used across healthcare disciplines, despite their inherent limitations. These limitations may be overcome by using emerging and objective measures that are less influenced by the effect of comorbid conditions and subjectivity on assessment. Combined integration of testing modalities (ie, subjective, behavioral, and objective) may provide more reliable results, although barriers to widespread application are apparent. Further research is needed to develop methods for overcoming such barriers that inhibit the implementation of reliable, standardized testing in clinic in order for recommendations to be made to achieve better healthcare outcomes for the ageing population.
Funding
This work was partially supported by funding from the William Demant Foundation.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Christensen K, Doblhammer G, Rau R, Vaupel JW. Ageing populations: the challenges ahead. Lancet. 2009;374(9696):1196–1208. doi:10.1016/S0140-6736(09)61460-4
2. Collaborators GBDCo D. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1151–1210. doi:10.1016/S0140-6736(17)32152-9
3. Lin FR, Metter EJ, O’Brien RJ, Resnick SM, Zonderman AB, Ferrucci L. Hearing loss and incident dementia. Arch Neurol. 2011;68(2):214–220. doi:10.1001/archneurol.2010.362
4. Kiessling J, Pichora-Fuller MK, Gatehouse S, et al. Candidature for and delivery of audiological services: special needs of older people. Int J Audiol. 2003;42(Suppl 2):2S92–101. doi:10.3109/14992020309074650
5. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413–446. doi:10.1016/S0140-6736(20)30367-6
6. Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017;390(10113):2673–2734. doi:10.1016/S0140-6736(17)31363-6
7. Alzheimer’s Association. 2022 Alzheimer’s disease facts and figures. Alzheimers Dement. 2022. doi:10.1002/alz.12638
8. Quaranta N, Coppola F, Casulli M, et al. The prevalence of peripheral and central hearing impairment and its relation to cognition in older adults. Audiol Neurootol. 2014;19(Suppl 1):10–14. doi:10.1159/000371597
9. Tarawneh HY, Menegola HK, Peou A, Tarawneh H, Jayakody DMP. Central auditory functions of Alzheimer’s disease and its preclinical stages: a systematic review and meta-analysis. Cells. 2022;11(6):1007. doi:10.3390/cells11061007
10. Lau K, Dimitriadis PA, Mitchell C, Martyn-St-James M, Hind D, Ray J. Age-related hearing loss and mild cognitive impairment: a meta-analysis and systematic review of population-based studies. J Laryngol Otol. 2022;136(2):103–118. doi:10.1017/S0022215121004114
11. Pye A, Charalambous AP, Leroi I, Thodi C, Dawes P. Screening tools for the identification of dementia for adults with age-related acquired hearing or vision impairment: a scoping review. Int Psychogeriatr. 2017;29(11):1771–1784. doi:10.1017/S104161021700120X
12. Raymond M, Barrett D, Lee DJ, Peterson S, Raol N, Vivas EX. Cognitive screening of adults with postlingual hearing loss: a systematic review. Otolaryngol Head Neck Surg. 2021;164(1):49–56. doi:10.1177/0194599820933255
13. Fullgrabe C. On the possible overestimation of cognitive decline: the impact of age-related hearing loss on cognitive-test performance. Front Neurosci. 2020;14:454. doi:10.3389/fnins.2020.00454
14. Volter C, Gotze L, Dazert S, Wirth R, Thomas JP. Impact of hearing loss on geriatric assessment. Clin Interv Aging. 2020;15:2453–2467. doi:10.2147/CIA.S281627
15. Uhlmann RF, Teri L, Rees TS, Mozlowski KJ, Larson EB. Impact of mild to moderate hearing loss on mental status testing. Comparability of standard and written Mini-Mental State Examinations. J Am Geriatr Soc. 1989;37(3):223–228. doi:10.1111/j.1532-5415.1989.tb06811.x
16. De Silva ML, McLaughlin MT, Rodrigues EJ, Broadbent JC, Gray AR, Hammond-Tooke GD. A mini-mental status examination for the hearing impaired. Age Ageing. 2008;37(5):593–595. doi:10.1093/ageing/afn146
17. Lin VY, Chung J, Callahan BL, et al. Development of cognitive screening test for the severely hearing impaired: hearing-impaired MoCA. Laryngoscope. 2017;127(Suppl 1):S4–S11. doi:10.1002/lary.26590
18. Dawes P, Reeves D, Yeung WK, et al. Development and validation of the Montreal cognitive assessment for people with hearing impairment (MoCA-H). J Am Geriatr Soc. 2023;71(5):1485–1494. doi:10.1111/jgs.18241
19. Völter C, Fricke H, Faour S, et al. Validation of the German Montreal-Cognitive-Assessment-H for hearing-impaired. Front Aging Neurosci. 2023;15:1209385. doi:10.3389/fnagi.2023.1209385
20. Jayakody DMP, Friedland PL, Eikelboom RH, Martins RN, Sohrabi HR. A novel study on association between untreated hearing loss and cognitive functions of older adults: baseline non-verbal cognitive assessment results. Clin Otolaryngol. 2018;43(1):182–191. doi:10.1111/coa.12937
21. Claes AJ, Mertens G, Gilles A, et al. The repeatable battery for the assessment of neuropsychological status for hearing impaired individuals (RBANS-H) before and after cochlear implantation: a protocol for a prospective, longitudinal cohort study. Front Neurosci. 2016;10:512. doi:10.3389/fnins.2016.00512
22. Hammers D, Spurgeon E, Ryan K, et al. Validity of a brief computerized cognitive screening test in dementia. J Geriatr Psychiatry Neurol. 2012;25(2):89–99. doi:10.1177/0891988712447894
23. Völter C, Götze L, Falkenstein M, Dazert S, Thomas JP. Application of a computer-based neurocognitive assessment battery in the elderly with and without hearing loss. Clin Interventions Aging. 2017;12:1681–1690. doi:10.2147/CIA.S142541
24. Dwolatzky T, Dimant L, Simon ES, Doniger GM. Validity of a short computerized assessment battery for moderate cognitive impairment and dementia. Int Psychogeriatr. 2010;22(5):795–803. doi:10.1017/S1041610210000621
25. Brooker H, Williams G, Hampshire A, et al. FLAME: a computerized neuropsychological composite for trials in early dementia. Alzheimer’s Dementia. 2020;12(1):e12098–n/a. doi:10.1002/dad2.12098
26. Weissberger GH, Strong JV, Stefanidis KB, Summers MJ, Bondi MW, Stricker NH. Diagnostic accuracy of memory measures in Alzheimer’s dementia and mild cognitive impairment: a systematic review and meta-analysis. Neuropsychol Rev. 2017;27(4):354–388. doi:10.1007/s11065-017-9360-6
27. Breton A, Casey D, Arnaoutoglou NA. Cognitive tests for the detection of mild cognitive impairment (MCI), the prodromal stage of dementia: meta‐analysis of diagnostic accuracy studies. Int J Geriatr Psychiatry. 2019;34(2):233–242. doi:10.1002/gps.5016
28. Yue T, Chen Y, Zheng Q, Xu Z, Wang W, Ni G. Screening tools and assessment methods of cognitive decline associated with age-related hearing loss: a review. Front Aging Neurosci. 2021;13:677090. doi:10.3389/fnagi.2021.677090
29. Tarawneh HY, Mulders W, Sohrabi HR, Martins RN, Jayakody DMP. Investigating auditory electrophysiological measures of participants with mild cognitive impairment and Alzheimer’s disease: a systematic review and meta-analysis of event-related potential studies. J Alzheimers Dis. 2021;84(1):419–448. doi:10.3233/JAD-210556
30. Crosbie B, Ferguson M, Wong G, Walker DM, Vanhegan S, Dening T. Giving permission to care for people with dementia in residential homes: learning from a realist synthesis of hearing-related communication. BMC Med. 2019;17(1):54. doi:10.1186/s12916-019-1286-9
31. World Health Organization. International Statistical Classification of Diseases and Related Health Problems.
32. Sanders ME, Kant E, Smit AL, Stegeman I, Bayer A. The effect of hearing aids on cognitive function: a systematic review. PLoS One. 2021;16(12):e0261207. doi:10.1371/journal.pone.0261207
33. Lin FR, Pike JR, Albert MS, et al. Hearing intervention versus health education control to reduce cognitive decline in older adults with hearing loss in the USA (ACHIEVE): a multicentre, randomised controlled trial. Lancet. 2023;402(10404):786–797. doi:10.1016/S0140-6736(23)01406-X
34. Baiduc RR, Poling GL, Hong O, Dhar S. Clinical measures of auditory function: the cochlea and beyond. Dis Mon. 2013;59(4):147–156. doi:10.1016/j.disamonth.2013.01.005
35. Frank A, Goldlist S, Mark fraser AE, Bromwich M. Validation of SHOEBOX QuickTest hearing loss screening tool in individuals with cognitive impairment. Front Digit Health. 2021;3:724997. doi:10.3389/fdgth.2021.724997
36. Bott A, Meyer C, Hickson L, Pachana NA. Can adults living with dementia complete pure-tone audiometry? A systematic review. Int J Audiol. 2019;58(4):185–192. doi:10.1080/14992027.2018.1550687
37. Paulraj MP, Subramaniam K, Yaccob SB, Adom AH, Hema CR. Auditory evoked potential response and hearing loss: a review. Open Biomed Eng J. 2015;9(1):17–24. doi:10.2174/1874120701509010017
38. Littlejohn J, Bowen M, Constantinidou F, et al. International practice recommendations for the recognition and management of hearing and vision impairment in people with dementia. Gerontology. 2022;68(2):121–135. doi:10.1159/000515892
39. Leroi I, Constantinidou F, Langenbahn D, Heyn P, Yeung WK, Dawes P. Hearing and vision impairment in people with dementia: a guide for clinicians. Arch Phys Med Rehabil. 2020;101(9):1667–1670. doi:10.1016/j.apmr.2020.04.012
40. Hobler F, McGilton KS, Wittich W, et al. Hearing screening for residents in long-term care homes who live with dementia: a scoping review. J Alzheimers Dis. 2021;84(3):1115–1138. doi:10.3233/JAD-215087
41. Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19–32. doi:10.1080/1364557032000119616
42. Levac D, Colquhoun H, O’Brien KK. Scoping studies: advancing the methodology. Implement Sci. 2010;5(1):69. doi:10.1186/1748-5908-5-69
43. Tricco AC, Lillie E, Zarin W, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–473. doi:10.7326/M18-0850
44. Granberg S, Dahlström J, Möller C, Kähäri K, Danermark B. The ICF Core Sets for hearing loss--researcher perspective. Part I: systematic review of outcome measures identified in audiological research. Int J Audiol. 2014;53(2):65–76. doi:10.3109/14992027.2013.851799
45. Royal Commission. Royal Commission into aged care quality and safety. Final report; 2021.
46. Dyer SM, Laver K, Pond CD, Cumming RG, Whitehead C, Crotty M. Clinical practice guidelines and principles of care for people with dementia in Australia. Aust Fam Physician. 2016;45(12):884–889.
47. Moafmashhadi P, Koski L. Limitations for interpreting failure on individual subtests of the Montreal Cognitive Assessment. J Geriatr Psychiatry Neurol. 2013;26(1):19–28. doi:10.1177/0891988712473802
48. Roebuck-Spencer TM, Glen T, Puente AE, et al. Cognitive screening tests versus comprehensive neuropsychological test batteries: a national academy of neuropsychology education paper†. Arch Clin Neuropsychol. 2017;32(4):491–498. doi:10.1093/arclin/acx021
49. Schubert CR, Cruickshanks KJ, Fischer ME, et al. Sensory impairments and cognitive function in middle-aged adults. J Gerontol a Biol Sci Med Sci. 2017;72(8):1087–1090. doi:10.1093/gerona/glx067
50. Huber M, Roesch S, Pletzer B, Lukaschyk J, Lesinski-Schiedat A, Illg A. Cognition in older adults with severe to profound sensorineural hearing loss compared to peers with normal hearing for age. Int J Audiol. 2020;59(4):254–262. doi:10.1080/14992027.2019.1687947
51. Shahmiri E, Jafari Z, Noroozian M, Zendehbad A, Haddadzadeh Niri H, Yoonessi A. Effect of mild cognitive impairment and Alzheimer disease on auditory steady-state responses. Basic Clin Neurosci. 2017;8(4):299–306. doi:10.18869/nirp.bcn.8.4.299
52. Peelle JE. Listening effort: how the cognitive consequences of acoustic challenge are reflected in brain and behavior. Ear Hear. 2018;39(2):204–214. doi:10.1097/AUD.0000000000000494
53. Morrison C, Rabipour S, Knoefel F, Sheppard C, Taler V. Auditory event-related potentials in mild cognitive impairment and Alzheimer’s disease. Curr Alzheimer Res. 2018;15(8):702–715. doi:10.2174/1567205015666180123123209
54. Winn MB, Wendt D, Koelewijn T, Kuchinsky SE. Best practices and advice for using pupillometry to measure listening effort: an introduction for those who want to get started. Trends Hear. 2018;22:2331216518800869. doi:10.1177/2331216518800869
55. Littlejohn J, Blackburn D, Venneri A. Understanding the links between hearing impairment and dementia: development and validation of the Social and Emotional Impact of Hearing Impairment (SEI-HI) questionnaire. Neurol Sci. 2020;41(12):3711–3717. doi:10.1007/s10072-020-04492-5
56. McClannahan KS, Chiu YF, Sommers MS, Peelle JE. Test-retest reliability of audiometric assessment in individuals with mild dementia. JAMA Otolaryngol Head Neck Surg. 2021;147(5):442–449. doi:10.1001/jamaoto.2021.0012
57. Association. AS-L-H. Guidelines for audiological screening. Available from: www.asha.org/policy.
58. Meyer C, Hickson L. Nursing management of hearing impairment in nursing facility residents. J Gerontol Nurs. 2020;46(7):15–25. doi:10.3928/00989134-20200605-04
59. Wolski L, Leroi I, Regan J, et al. The need for improved cognitive, hearing and vision assessments for older people with cognitive impairment: a qualitative study. BMC Geriatr. 2019;19(1):328. doi:10.1186/s12877-019-1336-3
60. Allen NH, Burns A, Newton V, et al. The effects of improving hearing in dementia. Age Ageing. 2003;32(2):189–193. doi:10.1093/ageing/32.2.189
61. Wittich W, Hobler F, Jarry J, McGilton KS. Recommendations for successful sensory screening in older adults with dementia in long-term care: a qualitative environmental scan of Canadian specialists. BMJ Open. 2018;8(1):e019451. doi:10.1136/bmjopen-2017-019451
62. Wittich W, Jarry J, Hobler F, McGilton KS. Agreement on the use of sensory screening techniques by nurses for older adults with cognitive impairment in long-term care: a mixed-methods consensus approach. BMJ Open. 2019;9(9):e027803. doi:10.1136/bmjopen-2018-027803
63. Reiss M, Reiss G. Zur Bedeutung orientierender Horprufungen [Value of preliminary hearing tests]. Wien Med Wochenschr. 2003;153(3–4):73–75. German. doi:10.1046/j.1563-258x.2003.02005.x
64. Kauffman MA, Moron DG, Bruno V, Boatman DF, Reich SG. Re: how accurate are bedside hearing tests? Neurology. 2007;69(13):
65. Uhlmann RF, Rees TS, Psaty BM, Duckert LG. Validity and reliability of auditory screening tests in demented and non-demented older adults. J Gen Intern Med. 1989;4(2):90–96. doi:10.1007/BF02602346
66. Pirozzo S, Papinczak T, Glasziou P. Whispered voice test for screening for hearing impairment in adults and children: systematic review. BMJ. 2003;327(7421):967. doi:10.1136/bmj.327.7421.967
67. Labanca L, Guimaraes FS, Costa-Guarisco LP, Couto EAB, Goncalves DU. Triagem auditiva em idosos: avaliacao da acuracia e reprodutibilidade do teste do sussurro [Screening of hearing in elderly people: assessment of accuracy and reproducibility of the whispered voice test]. Cien Saude Colet. 2017;22(11):3589–3598. Portuguese. doi:10.1590/1413-812320172211.31222016
68. Trigg R, Jones RW, Skevington SM. Can people with mild to moderate dementia provide reliable answers about their quality of life? Age Ageing. 2007;36(6):663–669. doi:10.1093/ageing/afm077
69. Hobler F, Argueta-Warden X, Rodriguez-Monforte M, Escrig-Pinol A, Wittich W, McGilton KS. Exploring the sensory screening experiences of nurses working in long-term care homes with residents who have dementia: a qualitative study. BMC Geriatr. 2018;18(1):235. doi:10.1186/s12877-018-0917-x
70. McDonough A, Dookhy J, McHale C, Sharkey J, Fox S, Kennelly SP. Embedding audiological screening within memory clinic care pathway for individuals at risk of cognitive decline-patient perspectives. BMC Geriatr. 2021;21(1):691. doi:10.1186/s12877-021-02701-0
71. Tarawneh HY, Sohrabi HR, Mulders WH, Martins RN, Jayakody DMP. Comparison of auditory steady-state responses with conventional audiometry in older adults. Front Neurol. 2022;13. doi:10.3389/fneur.2022.924096
72. Bakhos D, Marx M, Villeneuve A, Lescanne E, Kim S, Robier A. Electrophysiological exploration of hearing. Eur Ann Otorhinolaryngol Head Neck Dis. 2017;134(5):325–331. doi:10.1016/j.anorl.2017.02.011
73. Tas A, Yagiz R, Tas M, Esme M, Uzun C, Karasalihoglu AR. Evaluation of hearing in children with autism by using TEOAE and ABR. Autism. 2007;11(1):73–79. doi:10.1177/1362361307070908
74. Villeneuve A, Hommet C, Aussedat C, Lescanne E, Reffet K, Bakhos D. Audiometric evaluation in patients with Alzheimer’s disease. Eur Arch Otorhinolaryngol. 2017;274(1):151–157. doi:10.1007/s00405-016-4257-1
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
