Back to Journals » International Journal of General Medicine » Volume 17
Clinical Application of Serum Interleukin-6 Combined with Inflammatory Cytokines in the Dynamic Monitoring of Patients with Acute Cholecystitis
Authors Shan D, Wang Q, Heng X, Wu X
Received 18 October 2023
Accepted for publication 14 January 2024
Published 8 February 2024 Volume 2024:17 Pages 503—508
DOI https://doi.org/10.2147/IJGM.S444839
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Hyam Leffert
Danping Shan, Qiyao Wang, Xiang Heng, Xiaoyan Wu
Department of Clinical Laboratory, The Second Hospital of Jiaxing, Jiaxing, Zhejiang, People’s Republic of China
Correspondence: Xiaoyan Wu; Xiang Heng, Department of Clinical Laboratory, The Second Hospital of Jiaxing, 1518 Huanchen North Road, Jiaxing, Zhejiang, 314000, People’s Republic of China, Email [email protected]; [email protected]
Objective: To investigate the dynamic fluctuations of serum interleukin-6 (IL-6), procalcitonin (PCT), and neutrophil counts in individuals diagnosed with acute cholecystitis. Additionally, the research seeks to investigate the potential clinical significance of these biomarkers in the early stages of acute cholecystitis.
Methods: This retrospective cohort study included one hundred patients with acute cholecystitis (60 with mild acute cholecystitis and 40 with severe cholecystitis) admitted to our hospital between January 2022 and December 2022 were included. The levels of various cytokines, PCT and neutrophils in serum on days 1, 3, 5, and 7 were dynamically detected. The difference in each indicator between the two groups was analysed, and the diagnostic value of each indicator for acute cholecystitis was evaluated using a receiver operating characteristic (ROC) curve.
Results: IL-6 and PCT levels and neutrophil counts were significantly higher in patients with moderate and severe cholecystitis than those in those with mild cholecystitis (P < 0.01). The AUC values for the three indicators were all greater than 60%, and the AUC value for the joint diagnosis of the three indicators reached 90%.
Conclusion: Serum interleukin-6 combined with PCT and neutrophil count is helpful to determine the degree of disease development in patients with acute cholecystitis. The advantage of dynamic monitoring of the three indicators is that the detection is simple and worthy of clinical promotion.
Keywords: serum interleukin-6, procalcitonin, PCT, neutrophil count, acute cholecystitis
Acute cholecystitis is an acute disease of the digestive system and is common in clinical practice. After disease onset, patients often feel severe colic or distention pain in the upper right abdomen, occasionally accompanied by symptoms such as fever, nausea, and vomiting.1 Acute cholecystitis can be divided into mild acute cholecystitis and severe acute cholecystitis based on condition. Severe acute cholecystitis involves not only the deterioration of symptoms but also various complications, which can cause death.2 The development of timely and reasonable intervention is very important for treatment and can effectively reduce the incidence of complications. Therefore, it is important to accurately determine the degree of development of acute cholecystitis and to look for serological indicators that are significantly correlated with acute cholecystitis severity to guide the early diagnosis and treatment of acute cholecystitis.
Studies in China and abroad report that inflammation is common in patients with acute cholecystitis and that inflammation-related indicators such as C-reactive protein (CRP) and procalcitonin (PCT) are often correlated with disease severity.3,4 CRP can increase in bacterial infection, viral infection, acute rejection, cardiovascular diseases and surgery.5 However, CRP is meaningful only 2–3 days after admission; therefore, it cannot be used as an early predictor of acute cholecystitis severity.6 PCT is a precursor of calcitonin, an inflammatory transmitter produced by the stimulation of macrophages and monocytes in organs after infection. An increase in this indicator suggests bacterial or fungal infection. Therefore, the detection of PCT indicators can help assess whether a patient needs infection prevention or corresponding antibiotic treatment.7,8 However, the increase in PCT in cholecystitis has been shown to not be significant.9 Due to limitations in the sensitivity or specificity of a single indicator, it is important to combine multiple inflammatory indicators in clinical practice to improve sensitivity and specificity in the assessment of the severity of acute cholecystitis. This study planned to dynamically monitor serum IL-6, IL-10, PCT and neutrophil markers for 100 patients with acute cholecystitis and analysed the clinical application value of the joint detection of related inflammatory indicators in patients with acute cholecystitis.
Objects and Methods
One hundred patients with acute cholecystitis admitted to our hospital between January 2022 and December 2022 were included in this study. The diagnostic criteria of acute cholecystitis were based on the “Tokyo Guidelines 2018”:5 pain, tenderness, and a mass present in the upper right abdomen; systemic inflammatory response; and imaging showing manifestations of acute cholecystitis. The grading criteria for moderate-to-severe acute cholecystitis were as follows: white blood cells >18 × 109/L; a palpable mass in the upper right abdomen; duration of the onset of >72 h; and severe local inflammation: gangrenous cholecystitis, peri-gallbladder abscess, gallbladder peritonitis, and liver abscess. Acute cholecystitis was diagnosed when one of the above four criteria was met. All patients were admitted to the hospital within 24 h after symptom onset. Obesity, metabolic diseases, diabetes, hypertension, hepatobiliary and pancreatic diseases, renal failure and chronic inflammatory diseases caused by other diseases were excluded.
Detection Methods
Five millilitres of fasting cubital venous blood was collected from all patients on days 1, 3, 5, and 7 after admission to the hospital. All blood samples were centrifuged, and the serum obtained was cryopreserved in a −80 °C freezer. IL-2, IL-4, IL-6, IL-10, TNF-α and IFN-γ were detected by flow cytometry. The flow cytometry instrument was a BD canto flow cytometer (United States). Cytokine detection kits were purchased from Cellgene Biotech (Jiangxi). The electrochemiluminescence method was used for PCT detection. The assay kits were purchased from Roche, USA, and the procedures were performed in strict accordance with the instruction manuals. For complete blood count, neutrophil count was measured using a Sysmex XN9000 automatic haematology analyser.
The normal reference ranges of each detection indicator were as follows: IL-2, ≤11.4 pg/mL; IL-4, ≤12.9 pg/mL;IL-6, ≤20.0 pg/mL; IL-10, ≤5.9 pg/mL; TNFα, ≤5.5 pg/mL; IFNγ, ≤17.3 pg/mL; PCT, (0.000–0.050) ng/mL; and absolute neutrophil count: (1.8–6.3) *10^9/L.
For statistical analysis, GraphPad Prism 6.0 software was used for data processing. Measurement data (x±s) were compared using the t-test. Receiver operating characteristic (ROC) curves were used to evaluate the predictive value of IL-6, PCT and neutrophil levels for acute cholecystitis. P<0.05 was considered statistically significant.
Results
Patient Characteristics
One hundred patients with acute cholecystitis from hepatobiliary surgery department were included in this study. Among them, 56 males were 52.3±4.61 years of age, and 44 females were 51.4±5.84 years of age. They were divided into mild acute cholecystitis (M-C) and moderate-to-severe acute cholecystitis (MS-C) groups. The 100 patients with acute cholecystitis were divided into a mild group (60 cases) and a moderate-to-severe group (40 cases).
Dynamic Changes in Serum Cytokine Levels in Patients with Acute Cholecystitis
First, magnetic bead flow cytometry was used to analyse the expression levels of IL-2, IL-4, IL-6, IL-10, TNFα and IFNγ on days 1, day 3, day 5, and day 7 after hospitalization (Table 1).
 |
Table 1 Expression Levels of Serum Cytokines in Patients with Acute Cholecystitis at Different Time Points |
The IL-6 level in MS-C group was significantly higher than that in M-C group at different time points (P<0.001). Among them, the IL-6 level was the highest on the first day of hospitalization, an effect that may be related to active treatment after hospitalization.
The IL-2 level in MS-C group was significantly higher than that in M-C group on days 1 (P<0.05), but there was no significant difference on day 3, day 5 and day 7 (P>0.05).
In addition, the IL-10 level in MS-C group was significantly higher than that in M-C group on days 1, 3, and 5 (P<0.05), but there was no significant difference on day 7 (P>0.05).
However, the serum levels of IL-4, TNFα, and IFNγ were not significantly increased at different time points after hospitalization, and there were no significant differences (P>0.05).
Dynamic Changes in Serum PCT Levels in Patients with Acute Cholecystitis
PCT concentrations at admission were significantly increased in the acute cholecystitis group, with concentrations of 2.72±0.95 ng/mL in M-C group and 9.26±2.36 ng/mL in MS-C group. The serum PCT concentration in patients in MS-C group was significantly higher than that in patients in M-C group at day 1 (P=0.003), day 3 (P=0.001), day 5 (P=0.001) and day 7 (P=0.001). The PCT concentrations in different groups of patients with cholecystitis peaked on the 1st day after admission and then gradually decreased (Figure 1).
Dynamic Changes in Peripheral Neutrophil Counts in Patients with Acute Cholecystitis
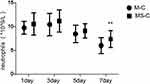
The neutrophil count in the peripheral blood of patients with acute cholecystitis was significantly increased on day 1 of hospitalization, 9.80 ± 1.34 * 10^9/L in M-C group and 10.60 ± 2.35 * 10^9/L in MS-C group. Neutrophil counts in patients in MS-C group were significantly higher than those in patients in M-C group on day 7 of hospitalization (P=0.01). The neutrophil counts in different groups of patients with cholecystitis gradually decreased after treatment (Figure 2).
ROC Curve Analysis of Serum IL-6 and PCT Levels and Neutrophil Counts in Patients with Acute Cholecystitis
Using ROC curves, we evaluated the clinical value of IL-6, PCT and neutrophil levels in differentiating patients with acute cholecystitis and moderate-to-severe acute cholecystitis. The results showed that on day 1, the AUC for IL-6 was 0.7511 (95% CI was 0.66–0.82, the sensitivity was 45.67%, and the specificity was 88.33%); the AUC for PCT was 0.7085 (95% CI was 0.62–0.78, the sensitivity was 50.50%, and the specificity was 82.25%); the AUC for neutrophil count was 0.6255 (95% CI was 0.52–0.72, the sensitivity was 60.67%, and the specificity was 55.33%); the AUC for combination of the three index was 0.8622 (95% CI was 0.65–0.85, the sensitivity was 65.50%, and the specificity was 92.00%), as shown in Figure 3.
 |
Figure 3 Receiver operating characteristic (ROC) curves analysis of serum IL-6 and PCT levels and neutrophil counts in patients with acute cholecystitis. |
Discussion
Acute cholecystitis is a common disease in hepatobiliary surgery departments. Different degrees of severity of cholecystitis require different management strategies, which have different effects of prognosis. Therefore, correctly assessing the severity, classification and staging of acute cholecystitis is important for the timely treatment and prognosis of cholecystitis in clinical practice.
Interleukin-6 (IL-6) is a pleiotropic cytokine with a wide range of functions. IL-6 can regulate the growth and differentiation of a variety of cells and has been shown to regulate the immune response and acute phase inflammation.10–12 Literature reports in China and abroad13 indicate that IL-6 increases to different degrees during the inflammatory response in the liver, gallbladder, pancreas, and intestinal tract and during the systemic inflammatory response, and in tumours. In this study, the level of serum IL-6 in patients with acute cholecystitis was significantly positively correlated with disease severity, and the expression level was highest on the first day of admission and declined with timely treatment. This finding is also consistent with the results of relevant studies conducted in China and abroad. This indicates that IL-6 levels at different time points can predict the severity and treatment effect of acute cholecystitis to some extent. In addition, PCT may be produced earlier than IL-6 and recently has served as the main monitoring indicator for severe infection. PCT production can be induced by the body in response to bacterial endotoxin.14 As reported in the literature,15 PCT has high specificity and sensitivity in systemic infection, and PCT concentration is often correlated with the severity of inflammation. This study has shown that the serum PCT concentration of patients with acute cholecystitis is significantly higher than the reference range and that the serum PCT concentration of patients with moderate-to-severe cholecystitis is significantly higher than that in patients with mild cholecystitis. After reasonable clinical treatment, the PCT level will gradually decrease and return to a level close to normal. We analysed the absolute count of peripheral blood neutrophils in patients with acute cholecystitis and found that counts were increased in patients with different degrees of acute cholecystitis but that there was no significant correlation with disease severity. With timely treatment, the absolute neutrophil count will gradually decrease. According to literature reports, for patients with severe inflammation, the neutrophil count increases rapidly under the function and mediation of inflammatory factors such as IL-6.16 This is also consistent with our data. In summary, the dynamic observation of serum IL-6 and PCT and neutrophil count is important for evaluating the condition and prognosis of patients with acute cholecystitis. ROC curves were used to analyse the diagnostic value of IL-6 and PCT levels and neutrophil count for patients with acute cholecystitis. The results showed that the three had good predictive value for different degrees of acute cholecystitis and that the combined detection of the three had higher sensitivity and specificity. Dynamic monitoring of IL-6 and PCT levels and neutrophil count is convenient and has high accuracy. Doctors can refer to these indicators when developing intervention programs and considering the choice of antibiotics for infection prevention.
The aim of this study was to analyse the dynamic changes in IL-6 and PCT levels and neutrophil count in patients with acute cholecystitis and to explore their potential application value in early diagnosis, staging, severity staging, diagnosis of complications, and clinical guidance for acute cholecystitis treatment. Large-sample, extensive and in-depth studies are needed to standardized these methods to better guide clinical diagnosis and treatment.
Patient Consent for Publication
All patients provided verbal and written consent for publication.
Ethics Approval and Consent to Participate
This study complied with the tenets of the Declaration of Helsinki and was approved by the Medical Ethics Committee of the Second Affiliated Hospital of Jiaxing University with the ethics number of JXEY-2022ZFYJ276. All patients offering clinical materials provided written informed consent and signed informed consent forms.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Gallaher JR, Charles A. Acute cholecystitis: a review. JAMA. 2022;327(10):965–975. doi:10.1001/jama.2022.2350
2. Yokoe M, Hata J, Takada T, et al. Tokyo Guidelines 2018: diagnostic criteria and severity grading of acute cholecystitis (with videos). J Hepatobiliary Pancreat Sci. 2018;25:41–54. doi:10.1002/jhbp.515
3. Lee SO, Yim SK. [management of acute Cholecystitis]. Korean J Gastroenterol. 2018;71:264–268. Korean. doi:10.4166/kjg.2018.71.5.264
4. Sato N, Kinoshita A, Imai N, et al. Inflammation-based prognostic scores predict disease severity in patients with acute cholecystitis. Eur J Gastroenterol Hepatol. 2018;30:484–489. doi:10.1097/MEG.0000000000001063
5. Sethwala AM, Goh I, Amerena JV. Combating inflammation in cardiovascular disease. Heart Lung Circ. 2021;30:197–206. doi:10.1016/j.hlc.2020.09.003
6. Steffens S, Mach F. Inflammation and atherosclerosis. HERZ. 2004;29:741–748. doi:10.1007/s00059-004-2634-9
7. Pierrakos C, Velissaris D, Bisdorff M, Marshall JC, Vincent JL. Biomarkers of sepsis: time for a reappraisal. Crit Care. 2020;24:287. doi:10.1186/s13054-020-02993-5
8. Mierzchala-Pasierb M, Lipinska-Gediga M. Sepsis diagnosis and monitoring - procalcitonin as standard, but what next? Anaesthesiol Intensive Ther. 2019;51:299–305. doi:10.5114/ait.2019.88104
9. Yaow C, Chong R, Chan KS, Chia C, Shelat VG. Should procalcitonin be included in acute cholecystitis guidelines? A systematic review. Medicina (Kaunas). 2023;60:59. doi:10.3390/medicina60010059
10. Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6:a16295. doi:10.1101/cshperspect.a016295
11. Dawson RE, Jenkins BJ, Saad MI. IL-6 family cytokines in respiratory health and disease. Cytokine. 2021;143:155520. doi:10.1016/j.cyto.2021.155520
12. Kang S, Tanaka T, Narazaki M, Kishimoto T. Targeting interleukin-6 signaling in clinic. Immunity. 2019;50:1007–1023. doi:10.1016/j.immuni.2019.03.026
13. Johnson C, Han Y, Hughart N, McCarra J, Alpini G, Meng F. Interleukin-6 and its receptor, key players in hepatobiliary inflammation and cancer. Transl Gastrointest Cancer. 2012;1:58–70. doi:10.3978/j.issn.2224-4778.2011.11.02
14. Maruna P, Nedelnikova K, Gurlich R. Physiology and genetics of procalcitonin. Physiol Res. 2000;49(1):S57–S61.
15. Matwiyoff GN, Prahl JD, Miller RJ, et al. Immune regulation of procalcitonin: a biomarker and mediator of infection. Inflamm Res. 2012;61:401–409. doi:10.1007/s00011-012-0439-5
16. Liew PX, Kubes P. The neutrophil’s role during health and disease. Physiol Rev. 2019;99:1223–1248. doi:10.1152/physrev.00012.2018
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.