Back to Journals » International Journal of General Medicine » Volume 17
Clinical Application of a New Cesarean Scar Pregnancy Classification and Evaluation System and a Risk Scoring System
Authors Fu P, Zhang L , Zhou T, Wang S, Liu R
Received 18 October 2023
Accepted for publication 9 January 2024
Published 16 January 2024 Volume 2024:17 Pages 115—126
DOI https://doi.org/10.2147/IJGM.S445327
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Woon-Man Kung
Peiying Fu,* Ling Zhang,* Ting Zhou, Shixuan Wang, Ronghua Liu
Department of Obstetrics and Gynecology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Ronghua Liu, Department of Obstetrics and Gynecology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, 1095 Jiefang Anv., Wuhan, Hubei, 430030, People’s Republic of China, Tel/Fax +86-27-83663078, Email [email protected]
Objective: Cesarean scar pregnancy (CSP) is an uncommon form of ectopic pregnancy that carries the risk of severe bleeding. To date, there has not been a universally accepted classification and treatment strategy. We performed this study to establish a risk scoring system and new CSP classification system for CSP and evaluate its efficacy.
Methods: A total of five groups were generated based on different methods of treatment, and the factors that increase the risk of intraoperative bleeding were examined in our center from 2013 to 2018. The construction of a risk scoring system in this study was based on the use of the chi-square test and multivariate logistic regression analysis. To determine the appropriate cutoff scores, receiver operating characteristic (ROC) curves and the area under the curve (AUC) were generated.
Results: We identified the main high-risk factors for excessive intraoperative hemorrhage during CSP surgery through univariate and multivariate analyses. Within this investigation, the risk factors included gestational sac location and gestational sac diameter. Through analysis, an optimal cutoff score of 3 was determined, and the area under the ROC curve was calculated to be 0.8113 (95% CI=0.7696– 0.8531). A score ranging from 0– 3 was classified as low risk, while a score ranging from 5– 7 was classified as high risk. Additionally, a new classification system for CSP has been established based on sonographic parameters. We also established a diagnostic and treatment process for CSP patients according to the risk scoring method and new CSP classification.
Conclusion: We identified the high-risk factors associated with bleeding during CSP surgery and developed a scoring system incorporating these factors. The utilization of this novel CSP typing method, in conjunction with the risk scoring system, can effectively inform doctors in their decision-making process concerning treatment strategies for patients with CSP.
Keywords: cesarean scar pregnancy, scoring system, risk, classification, treatment
Introduction
Cesarean scar pregnancy (CSP) presents mainly as the implantation of a blastocyst within myometrial scar tissue in the anterior lower uterine segment. When a patient suffers from hemorrhagic shock, emergency surgery may be needed, and the uterus may be removed. If surgery is not performed in time, the life of patients may be endangered. CSP is currently categorized into two or three subtypes according to the site of the cesarean section scar and its relationship with the cesarean section scar.1,2 Several scholars have proposed different classification methods. Specifically, Du et al proposed one type of CSP based on the size of the cesarean scar defect (CSD) and demonstrated its value in evaluating the risk of CSP and guiding the choice of treatment strategies.3 However, both of these methods have many drawbacks. For example, these classification methods are based primarily on whether the gestational sac is located within the uterine cavity or on the thickness of the scar, which may lead to poor practicability. Therefore, choosing an appropriate treatment approach is not conducive to doctors, and there is no agreement on the best management method for CSP.
When making treatment decisions, doctors are influenced by various factors, such as the patient’s overall condition, CSP classification, blood flow status, and level of experience. Presently, a growing number of doctors opt for treatment methods that align with the CSP classification. Irrespective of the classification approach, the most suitable treatment modality for type I CSP is dilatation and curettage (D&C) or hysteroscopy. From an economic perspective, some scholars suggest D&C as the more suitable option. Even so, some doctors may consider pretreatment with methotrexate (MTX) or uterine artery embolization (UAE) before surgery.4–6 Whether these approaches are appropriate is worth discussing. Hysteroscopy in conjunction with laparoscopy for type I CSP is the most successful option, but for type I CSP, D&C could be the most cost-effective option. Some doctors also use scar thickness as the main basis for choosing a treatment plan. A recent study reported that a CSP with a large gestational sac (over 10 mm) at presentation should be referred for surgical treatment.7 There are similar reports in our research center suggesting that ultrasound-guided D&C may be a reliable treatment.8,9 Therefore, there is no significant controversy regarding the choice of treatment plan for type I CSP.
However, there are significant differences in the treatment options for type II and III CSP and in the variety of treatments chosen for these patients. In one randomized clinical trial (RCT), CSP patients with any of the three subtypes (types I, II and III) underwent ultrasound-guided curettage with or without uterine artery embolization (UAE). Among the patients in this clinical trial were also patients with type II and III CSP, and it is worth discussing whether this clinical trial is feasible.10 However, more type II or type III CSP patients than type I CSP patients underwent operative resection and uterine repair combined with UAE, temporary occlusion of the artery, high-intensity focused ultrasound (HIFU) or hysteroscopy.11,12 The objectives of integrating treatment modalities include the mitigation of intraoperative hemorrhage and the reduction of surgical hazards. Nevertheless, the utilization of UAE in the management of stable CSP patients may substantially escalate hospitalization expenses. In another investigation, laparoscopy, hysteroscopy, or curettage was employed to treat type II and III CSP patients.13,14 There is uncertainty in the method of choice for these types of CSP among doctors, especially regarding D&C; the thin lower segment of the uterus cannot be corrected by D&C, and catastrophic hemorrhage, which is detrimental to patient fertility, can occur during the operation.
Based on these factors, it is necessary to explore new CSP typing methods to provide better guidance for doctors in selecting appropriate treatment strategies. The aim of this retrospective study was to establish a novel CSP classification system according to sonographic characteristics and the risk score. Based on the amount of intraoperative bleeding, we intended to identify high-risk factors for bleeding using the chi-square test and single-factor logistic regression analysis. Then, scores were assigned to these factors to distinguish whether the patient was at low or high risk. Finally, we validated the effectiveness of our proposed classification and scoring system based on different treatment plans.
Methods
Patients and Inclusion Criteria
The present study received approval from the Ethics Committee of Tongji Hospital. Prior to each procedure, all patients provided informed consent. The standard adopted by many doctors was proposed by Vial (type Ia and type IIa)1 and a Chinese expert opinion on the diagnosis and treatment of CSP (type Ib, type IIb and type IIIb).2 In accordance with the two classification methods, we conducted appropriate categorizations for all patients. The inclusion criteria were established based on the findings of our previous study.15 Briefly, the inclusion criteria for this study were as follows: pregnancy with a history of cesarean section in the lower uterine segment; CSP diagnosed by transvaginal ultrasound imaging; lower uterine incision scar with gestational sac or mixed mass; a thinning or absent myometrial layer; or a gestational sac implanted or infiltrated into the muscle layer of the lower uterine segment.
Surgical Approach
We chose a certain treatment strategy (ultrasound-guided D&E, hysteroscopy-guided D&E, laparoscopy-guided D&E, laparoscopy or laparotomy) based on the ultrasound examination results of each patient, the type of CSP, and the patient’s symptoms and vital signs. The operations were performed by experienced gynecologists.
Assessment of Intraoperative Blood Loss
Estimated intraoperative blood loss was recorded during surgery. The intraoperative bleeding volume was assessed by measuring the weight change in the gauzes and through operator estimation. Excessive intraoperative hemorrhage was defined as bleeding equal to or greater than 200 mL.
Analysis of Clinical and Ultrasound Characteristics
The study included various clinical and ultrasound characteristics, such as age, urban residency, vaginal bleeding, abdominal pain, gestational age, number of artificial abortions, number of cesarean sections, interval since the last cesarean section, fetal heartbeat, perioperative human chorionic gonadotropin (HCG) level, perioperative hemoglobin, intraoperative bleeding volume, and treatment approach. All patients underwent thorough examinations conducted by proficient ultrasound doctors who meticulously recorded data pertaining to the gestational sac diameter, gestational sac width, gestational sac area, and myometrial thickness. The examination involved assessing the relationship between the gestational sac and the scar, including any outward protrusion or vascular status. Subsequently, the doctor conducted CSP typing based on these findings.
Follow-Up
The initial follow-up schedule was once per week from the first day after discharge until the serum HCG levels returned to normal. The first phase of follow-up included assessing for vaginal bleeding and serum HCG levels and performing a routine blood test and ultrasonography. During the second phase, follow-up was conducted once every three months for one year. The follow-up data included menstrual status, pregnancy status, ovarian function and endocrine levels in addition to the above items.
Statistical Analysis
Statistical analysis software (SAS) version 9.4 was used for the data analysis. The data are expressed as the mean ± standard deviation. Between-group comparisons were conducted using two-sample t tests, while analysis of variance was utilized for multiple-group comparisons. Count data are presented as the frequency and composition ratio. Between-group comparisons were assessed using the chi-square test, and Fisher’s exact test was used for data that did not meet the requirements of the chi-square test. A P value less than 0.05 indicated a statistically significant difference. To screen for independent risk factors associated with endpoint events, a single-factor logistic regression analysis was initially employed to identify potential risk factors exhibiting a significant correlation (P<0.2) with the endpoint event; these risk factors were subsequently considered candidate variables. Subsequently, multiple factor logistic regression analysis was conducted to determine the independent risk factors influencing the endpoint event using the inclusion criterion of P<0.05. The ROC curve area evaluation model was used to ascertain the optimal cutoff point through analysis of the area under the ROC curve, thus indicating its predictive capability. For internal validation and assessment of stability, the tenfold cross-validation method was utilized. The accuracy of the scoring system was verified using the Hosmer‒Lemeshow goodness of fit test (HL test).
Results
Study Population
From 2013–2018, 935 women suspected of having CSP were treated at our hospital. Of these patients, 29 failed to meet the diagnostic criteria for CSP. As a result, 906 patients were eligible for the study after they met the inclusion criteria. However, 28 of these patients chose expectant treatment; therefore, 878 patients were ultimately included in this study. All the included patients were divided into five groups according to the therapy type: group 1, ultrasound-guided D&E, 251 patients; group 2, hysteroscopy-guided D&E, 198 patients; group 3, laparoscopy-guided D&E, 45 patients; group 4, laparoscopy, 91 patients; and group 5, laparotomy, 145 patients. The baseline characteristics and clinical data of the patients are presented in Table 1.
 |
Table 1 Univariate Analysis of Clinical and Sonographic Characteristics of Women with Cesarean Scar Pregnancy by Intraoperative Bleeding Volume |
Screening of High-Risk Factors Affecting Blood Loss and Establishment of the ROC Curve of the Risk Scoring System
Based on the intraoperative estimated bleeding volume of 200 mL, all patients were divided into two groups (534 patients with a bleeding volume < 200 mL and 121 patients with a bleeding volume ≥ 200 mL). Many of the clinical factors, such as age, residence, number of artificial abortions, number of gestational sacs (GSs), interval from the last cesarean section (CS), gestational sac width, gestational sac area (cm2), fetal heartbeat, main symptoms (vaginal bleeding and abdominal pain), preoperative HCG and first HCG after the operation, were similar between the two groups. However, there were significant differences in other factors, including gestational sac location, gestational age, gestational sac diameter, remnant myometrial thickness, color Doppler signal, operation methods, preoperative hemoglobin, and median and first hemoglobin after operation, between the two groups. Subsequently, multivariate logistic regression analysis was conducted to examine the associations between factors and compute the odds ratios (ORs) of the identified risk factors, resulting in the identification of two factors (gestational sac location and gestational sac diameter). The variables used in the logistic regression and their odds ratios are shown in Table 2.
 |
Table 2 Multivariate Risk Assessment and Model Establishment |
Next, a scoring system was devised to assess the risk of CSP. Each predictor was assigned a weight, with the weight of its predictive factor (represented by the smallest β value) set at 1 point. For the remaining predictors, their β values were divided by the minimum β value and rounded to the nearest whole number to determine their respective weights. Subsequently, the Hosmer–Lemeshow test was employed to evaluate the model’s goodness of fit, and distinct scores were allocated to the subgroups of the two factors based on their odds ratios (ORs).
Among the variables examined, gestational sac location (infiltration) had the highest odds ratio (OR) (21.28; 95% confidence interval: 7.45 to 60.74), followed by gestational sac diameter (≥4 cm), with an OR of 1.23. In this particular scoring system, a score ranging from 0 to 3 was classified as low risk, while a score ranging from 5 to 7 was classified as high risk. Patients in the high-risk group were 17 times more likely to experience intraoperative bleeding than were those in the low-risk group. Our analysis utilizing the HL test demonstrated that the assigned scores were predominantly aligned with the prediction line, indicating a satisfactory level of accuracy for the scoring systems (Figure 1). Furthermore, the discrimination performance of the CSP risk prediction model was evaluated by utilizing the area under the receiver operating characteristic (ROC) curve (AUC). The identification of a score of 3 as the optimal cutoff yielded a prediction model sensitivity of 71% and specificity of 84%. The calculated area under the ROC curve was determined to be 0.8113, with a 95% confidence interval of 0.7696–0.8531 (Figure 2).
New CSP Classification Based on Ultrasound Characteristics
We propose a new classification system for CSP based on the three-dimensional ultrasound results of patients. In our system, CSP is classified into one of three types: type I, type II or type III. Each type of CSP can be divided into two subtypes: a and b. According to the proximity of the pregnancy sac to the scar, type I CSP is divided into Ia (the gestational sac located in the lower segment of the uterine cavity) and Ib (the gestational sac located in the cervical canal). Type II CSP combines with CSD. The scars were divided into IIa (T ≥ 0.2 cm) and IIb (0.1 cm ≤ T<0.2 cm) according to their thickness. The scar thickness of type III CSP is less than 0.1 cm, and there is no normal muscle layer; however, the gestational sac does not protrude outward (IIIa). The gestational sac of the type IIIb CSP obviously protruded outward without a normal muscular layer (Figure 3A–C and Table 3). Each CSP classification and subclassification corresponded to a unique set of Doppler ultrasound imaging characteristics (Figure 3D–F).
 |
Table 3 The Type of Cesarean Scar Pregnancy on Ultrasound |
Verification of CSP Type on Ultrasound and the Risk Scoring System in Clinical Cases
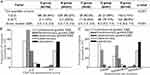
Our prior research revealed challenges in selecting an appropriate surgical strategy for patients with type IIa or type IIb. In this investigation, novel ultrasound-based typing techniques and risk scoring systems were employed to validate clinical data, aiming to address the aforementioned concerns. A scoring system was used for validation. The outcomes revealed notable differences in the proportions of patients in the high-risk and low-risk groups, as well as differences in scores across five distinct groups (Figure 4A). Strikingly, disparities were observed in the treatment regimens utilized by patients who fell within two distinct scoring ranges. Within the 0–3 score group, hysteroscopy constituted the largest proportion, followed by curettage and laparoscopy. Conversely, in the groups with scores ranging from 5–7, the majority of patients underwent laparoscopy or laparotomy (Figure 4B). This study confirmed the efficacy of our risk prediction scoring system and its applicability in the clinical management of CSP.
Next, employing our novel ultrasound-guided CSP typing method, we assessed the distribution of surgical approaches among all patients. Notably, for patients classified as having the closer type, the predominant approach employed was dilation and evacuation (D&E), with the highest proportion of patients undergoing ultrasound-guided D&E, followed by hysteroscopy-guided D&E. The use of laparoscopy or laparotomy was minimal. The percentage of patients classified as implantation patients who underwent hysteroscopy-guided D&E significantly increased, followed by patients who underwent ultrasound-guided D&E and laparoscopy-guided D&E. In the case of patients with an infiltration type, laparoscopy or laparotomy was the preferred surgical approach for the majority of patients (Figure 4C).
Diagnosis and Treatment Mode of CSP Patients
When a patient’s diagnosis of CSP was confirmed through three-dimensional ultrasound, the risk score was determined based on the size and location of the gestational sac, and the patient was subsequently classified based on the ultrasound findings. Patients with a score of 0–3 underwent either D&C or hysteroscopy. Conversely, patients with a score of 5–7 underwent operative resection (laparoscopy or laparotomy), as illustrated in Figure 5. Additionally, treatment should be tailored to the specific subtype of CSP. In cases where the pregnancy is determined to be of the closer type, direct dilation and curettage (D&C) is recommended. Conversely, if the pregnancy is identified as an implantation type, it is necessary to incorporate risk scoring in the decision-making process. The specific methods outlined above should be consulted for guidance. In instances where the pregnancy is classified as an infiltration type, operative resection (either laparoscopy or laparotomy) is the preferred treatment approach.
Discussion
The incidence of CSP is on the rise due to the increasing number of women with a previous cesarean scar. However, there is currently no universally accepted clinical guidance for classifying CSP, and the definitions of sonographic characteristics associated with CSP also lack consistency. The objective of this study was to develop a novel classification and risk scoring system utilizing ultrasound manifestations of CSP. Our findings demonstrated that the newly devised CSP classification and scoring system can be used to assess the risk associated with this condition and aid in the determination of appropriate clinical treatment strategies.
Notably, there is currently no universally accepted treatment approach for CSP. However, a study reported the use of HIFU ablation followed by ultrasound-guided D&C as a potential treatment option for patients diagnosed with either type I or type II CSP.16 The utilization of HIFU pretreatment for all type Ia CSP patients appears to be unsuitable, while the application of D&C treatment for all type IIb CSP patients remains controversial. Conversely, Yang et al observed that individuals with type IIb and type IIIb CSP typically opt for immediate termination of their pregnancies during early gestation rather than pursuing expectant management.17 Nonetheless, some doctors have indicated hysteroscopic treatment as the optimal choice for treating CSP,18 while others hold divergent perspectives on this matter. Excision of the CSP and subsequent repair of the scar may constitute a more favorable management approach, offering potential benefits for future fertility.19 These challenges were also encountered within our institution, where the multitude of available treatment options posed difficulty for physicians in selecting the most appropriate course of action. Previous research has indicated that certain high-risk factors, such as a gestational sac exceeding 4.5 cm or a myometrial thickness less than 2 mm, can contribute to the persistence of CSP following initial surgical intervention.20 In our study, we observed that the highest score was achieved when the gestational sac diameter surpassed 4 cm, and we additionally employed a scar thickness of 2 mm as the threshold for CSP classification. These results were consistent with the results disclosed in the aforementioned literature.
Therefore, early diagnosis of CSP and good sonographic characteristics are particularly important for guiding the selection of treatment strategies. An increasing number of doctors are paying attention to the impact of CSD on pregnancy after another cesarean section. One study suggested that during scarred uterine pregnancy, the lower uterine segments deform from a V shape to a U shape and eventually develop uterine rupture.21 Using the modified Delphi method, Jordans et al generated a sonographic evaluation system for CSP in early pregnancy.22 However, this system is relatively complex, lacks classification, lacks clinical guidance and is not suitable for advanced gestation. Another study used the intensity ratio of cesarean scar tissue to myometrium on contrast-enhanced ultrasound (CEUS) as a quantitative index to diagnose CSP.23 Neither of these studies provided a specific treatment plan. Therefore, we developed a new CSP classification system based on the location of the gestational sac, the relationship between the gestational sac and the scar, whether the mass protruded outside the serosa of the lower segment of the uterine scar and the thickness of the scar. Our purpose was to evaluate the risk of CSP and guide the selection of treatment procedures. The surgical techniques employed in our study, along with the subsequent postoperative results, further substantiate the aforementioned objectives.
Additionally, misdiagnosis or improper management of CSP can potentially lead to the development of placenta accreta spectrum (PAS) disorders or result in life-threatening hemorrhage. Consequently, the investigation of high-risk factors associated with CSP is important for its diagnosis and treatment. A residual myometrial thickness less than 2 mm is considered a significant risk factor for major hemorrhage during curettage.24 Furthermore, Du et al discovered that the area of CSD could serve as a predictive measure for the safety of hysteroscopic procedures in patients with CSP. However, importantly, all the patients included in this study exhibited high risk factors, such as a mass protruding into the abdominal cavity or a discontinuous muscle layer.25 Given these circumstances, it is worth considering whether hysteroscopic surgery truly benefits patients of this nature, as niche resection may be a more suitable alternative.
The correlation between the gestational age at CSP and estimated blood loss was found to be significant in certain studies;26,27 however, our present study did not yield similar results. Notably, the two typing methods for CSP mentioned in our study were also based on high-risk factors, such as scar thickness and protrusion into the abdominal cavity. Another retrospective study utilized the HCG index (<17,757.0 mIU/mL) and gestational sac size (<10.4 mm) as indicators of successful systemic MTX treatment for CSP.28 Nevertheless, it is widely accepted that MTX alone is no longer considered an effective treatment strategy for CSP. Furthermore, our study substantiated the significance of gestational sac size as a prominent risk factor, thereby emphasizing its crucial role in informing the choice of treatment intervention. Additionally, we identified the location of the gestational sac as another influential risk factor that, when combined with gestational sac size, can be used to assess the likelihood of bleeding in patients with CSP effectively and aid in determining appropriate treatment strategies. More interestingly, the risk scoring system, developed using two factors, was applied to our patients to effectively evaluate the risk associated with CSP. Notably, the treatment approaches chosen by doctors remained consistent.
Although we did not directly compare the economic disparities between the D&C and hysteroscopy groups, the literature indicates that opting for D&C can result in reduced surgical expenses without compromising surgical outcomes.29 Consequently, we advocate for the selection of D&C, as D&C offers cost savings while ensuring comparable surgical outcomes. Hence, D&C is preferred for low-risk CSP patients, as our previous research has substantiated the superiority of laparoscopic surgery over open surgery.15 Consequently, our primary focus will be on considering laparoscopy for high-risk CSP patients.
Importantly, the retrospective nature of our analysis of previous cases in this study introduces inevitable selection bias, accompanied by some gaps in the medical records. Furthermore, the absence of follow-up data renders the postoperative fertility status of each patient unknown. A notable strength of this retrospective study is the substantial number of patients involved.
In this study, we presented a risk scoring system, a novel CSP classification method, and a diagnostic and treatment flowchart. These tools enable physicians to select appropriate treatment strategies. Additionally, we demonstrated the feasibility of this process. However, further validation using data from multiple centers is warranted. Therefore, conducting RCTs to validate these systems is imperative, as they hold the potential to establish a more robust clinical foundation for the standardized diagnosis and treatment of CSP.
Data Sharing Statement
The datasets generated during the current study are not publicly available.
Ethical Disclosure
The Ethics Committee of Tongji Hospital, Huazhong University of Science and Technology, approved this study (No: TJ-IRB20191214). The study was conducted in accordance with the ethics of the 2013 Helsinki World Medical. Association Declaration.
Disclosure
The authors declare no competing interests.
References
1. Vial Y, Petignat P, Hohlfeld P. Pregnancy in a cesarean scar. Ultrasound Obstet Gynecol. 2000;16(6):592–593. doi:10.1046/j.1469-0705.2000.00300-2.x
2. Family Planning Group, Obstetrics and Gynecology Branch, Chinese Medical A ssociation. Expert consensus on diagnosis and treatment of uterine scar pregnancy after cesarean section (2016). Chin J Obstet Gynecol. 2016;51:568–572. Chinese.
3. Du Q, Liu G, Zhao W. A novel method for typing of cesarean scar pregnancy based on size of cesarean scar diverticulum and its significance in clinical decision-making. J Obstet Gynaecol Res. 2020;46(5):707–714. doi:10.1111/jog.14226
4. Wang W, Chen Y, Yang Y, et al. High-intensity focused ultrasound compared with uterine artery chemoembolization with methotrexate for the management of cesarean scar pregnancy. Int J Gynaecol Obstet. 2022;158(3):572–578. doi:10.1002/ijgo.14036
5. Diakosavvas M, Kathopoulis N, Angelou K, et al. Hysteroscopic treatment of cesarean scar pregnancy: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2022;270:42–49. doi:10.1016/j.ejogrb.2021.12.038
6. Pickett CM, Minalt N, Higgins OM, et al. A laparoscopic approach to cesarean scar ectopic pregnancy. Am J Obstet Gynecol. 2022;226(3):417–419. doi:10.1016/j.ajog.2021.11.021
7. Dior UP, Palma-Dias R, Reidy KL, et al. Cesarean scar pregnancies: incidence and factors associated with conversion to surgery from medical management. J Minim Invasive Gynecol. 2018;26:919–927. doi:10.1016/j.jmig.2018.09.771
8. Zeng Z, Ding SP, Zeng X, et al. The value of transvaginal ultrasound in clinical surgical treatment of cesarean scar pregnancy. J Huazhong Univ Sci Technolog Med Sci. 2017;37(4):536–540. doi:10.1007/s11596-017-1769-x
9. Wang S, Li Y, Ma X. Lower uterine segment thickness in assessing whether cesarean scar pregnancy patients could be treated with suction curettage. J Matern Fetal Neonatal Med. 2020;33(19):3332–3337. doi:10.1080/14767058.2018.1531118
10. Tang Y, Zhang Y, Tang H, et al. A comparison of ultrasound guided curettage with and without uterine artery embolization on controlling intraoperative blood loss for a cesarean scar pregnancy treatment: study protocol for a randomized clinical trial. Front Endocrinol. 2021;12:651273. doi:10.3389/fendo.2021.651273
11. Su X, Yang M, Na Z, et al. Application of laparoscopic internal iliac artery temporary occlusion and uterine repair combined with hysteroscopic aspiration in type III cesarean scar pregnancy. Am J Transl Res. 2022;14(3):1737–1741.
12. Yang J, Li B, Liu J, et al. A new modified hysteroscopic-laparoscopic surgery for cesarean scar pregnancy of stable type III. Int J Gen Med. 2021;14:2289–2295. doi:10.2147/IJGM.S308768
13. Huang L, Zhao L, Shi H. Clinical efficacy of combined hysteroscopic and laparoscopic surgery and reversible ligation of the uterine artery for excision and repair of uterine scar in patients with type II and III cesarean scar pregnancy. Med Sci Monit. 2020;26:e924076. doi:10.12659/MSM.924076
14. Fu P, Zhou T, Cui P, et al. Selection of laparoscopy or laparotomy for treating cesarean scar pregnancy: a retrospective study. Int J Gen Med. 2022;15:7229–7240. doi:10.2147/IJGM.S369884
15. Kathopoulis N, Chatzipapas I, Samartzis K, et al. Laparoscopic management of cesarean scar pregnancy: report of two cases with video-presentation of different operative techniques and literature review. J Gynecol Obstet Hum Reprod. 2021;50(8):102066. doi:10.1016/j.jogoh.2021.102066
16. Liu Y, Yin Q, Xu F, et al. Clinical efficacy and safety of high-intensity focused ultrasound (HIFU) ablation in treatment of cesarean scar pregnancy (CSP) I and II. BMC Pregnancy Childbirth. 2022;22(1):607. doi:10.1186/s12884-022-04848-z
17. Yang X, Zheng W, Zhang H, et al. Expectant management of cesarean scar pregnancy in 13 patients. J Matern Fetal Neonatal Med. 2022;35(25):8066–8071. doi:10.1080/14767058.2021.1940942
18. Tang Q, Qin Y, Zhou Q, et al. Hysteroscopic treatment and reproductive outcomes in cesarean scar pregnancy: experience at a single institution. Fertil Steril. 2021;116(6):1559–1566. doi:10.1016/j.fertnstert.2021.06.015
19. Miller CE, McKenna MM. Is hysteroscopic treatment of cesarean scar pregnancy the best option? Fertil Steril. 2021;116(6):1567. doi:10.1016/j.fertnstert.2021.09.034
20. Zhang Y, Chen L, Zhou M, et al. Risk factors of persistent cesarean scar pregnancy after dilation and curettage: a matched case–control study. Taiwan J Obstet Gynecol. 2020;59(2):237–242. doi:10.1016/j.tjog.2020.01.011
21. Kawakami K, Yoshizato T, Kurokawa Y, et al. New ultrasonographic risk assessment of uterine scar dehiscence in pregnancy after cesarean section. J Med Ultrason. 2023;50(1):89–96. doi:10.1007/s10396-022-01265-9
22. Jordans IPM, Verberkt C, De Leeuw RA, et al. Definition and sonographic reporting system for cesarean scar pregnancy in early gestation: modified Delphi method. Ultrasound Obstet Gynecol. 2022;59(4):437–449. doi:10.1002/uog.24815
23. Li H, Liu X, Xie L, et al. Diagnostic accuracy and cut-off of contrast-enhanced ultrasound in caesarean scar pregnancy. Eur J Obstet Gynecol Reprod Biol. 2020;246:117–122. doi:10.1016/j.ejogrb.2020.01.036
24. Wu D-F, Zhang H-X, He W, et al. Experience in management of cesarean scar pregnancy and outcomes in a single center. J Int Med Res. 2022;50(10):3000605221123875. doi:10.1177/03000605221123875
25. Du Q, Zhao W. Exploring the value of cesarean section diverticulum area to predict the safety of hysteroscopic management for cesarean scar pregnancy patients. Int J Gynaecol Obstet. 2022;156(3):488–493. doi:10.1002/ijgo.13682
26. Yang X, Zheng W, Wei X, et al. Management of cesarean scar pregnancy: importance of gestational age at diagnosis and disease type-A single center’s 5 years of experience involving 223 cases. Front Surg. 2023;15:1055245. doi:10.3389/fsurg.2023.1055245
27. Zhang Y, Zhang Z, Liu X, et al. Risk factors for massive hemorrhage during the treatment of cesarean scar pregnancy: a systematic review and meta-analysis. Arch Gynecol Obstet. 2021;303(2):321–328. doi:10.1007/s00404-020-05877-9
28. Mitsui T, Mishima S, Ohira A, et al. hCG values and gestational sac size as indicators of successful systemic methotrexate treatment in cesarean scar pregnancy. Taiwan J Obstet Gynecol. 2021;60(3):454–457. doi:10.1016/j.tjog.2021.03.011
29. Qian ZD, Huang LL, Zhu XM. Curettage or operative hysteroscopy in the treatment of cesarean scar pregnancy. Arch Gynecol Obstet. 2015;292(5):1055–1061. doi:10.1007/s00404-015-3730-1
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.