Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 17
Clinical and Psychological Impact of Chronic Pain in People with Chronic Obstructive Pulmonary Disease
Authors Tanaka T , Okita M, Jenkins S, Kozu R
Received 23 January 2022
Accepted for publication 3 April 2022
Published 22 April 2022 Volume 2022:17 Pages 893—903
DOI https://doi.org/10.2147/COPD.S359223
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Takako Tanaka,1,2 Minoru Okita,1 Sue Jenkins,3 Ryo Kozu1
1Department of Physical Therapy Science, Nagasaki University Graduate School of Biomedical Sciences, Nagasaki, Japan; 2Department of Rehabilitation, Tagami Hospital, Nagasaki, Japan; 3Institute for Respiratory Health and Physiotherapy Department, Sir Charles Gairdner Hospital, Perth, Western Australia
Correspondence: Takako Tanaka, Department of Physical Therapy Science, Nagasaki University Graduate School of Biomedical Sciences, 1-7-1 Sakamoto, Nagasaki, 852-8520, Japan, Tel +81 95 819 7919, Fax +81 95 819 7919, Email [email protected]
Purpose: The presence of pain can be associated with an exaggerated negative cognitive and emotional response, leading to worsening of existing symptoms. This study aimed to describe the multifaceted impact of chronic pain on cognition, emotional and physical health in people with chronic obstructive pulmonary diseases (COPD) and to explore the clinical impact of pain.
Patients and Methods: A prospective, cross-sectional multicenter study was carried out in 68 people with COPD (COPD group) and 65 community-dwelling age-matched participants (control group). Participants were assessed for the presence of chronic pain, pain location, intensity and catastrophizing, pain-related fear (kinesiophobia), anxiety and depression, physical activity, and sleep duration. The COPD group also completed assessments of dyspnea, exercise tolerance (6-minute walk distance [6MWD]), and activities of daily living (ADL).
Results: The prevalence of pain was higher in the COPD group (85% vs 51%, p< 0.001). The COPD group reported pain located in neck/shoulder, upper back, thorax and upper limbs, while the control group had more pain in the lower back. Pain catastrophizing and kinesiophobia were reported by 28% and 67% vs 9% and 42%, in the COPD and control groups respectively (both p< 0.05). People with COPD and pain (n=58) reported greater dyspnea (p< 0.001), and impairment in ADL (p< 0.05), and lower 6MWD and physical activity (both p< 0.01) compared to COPD participants without pain (n=10).
Conclusion: This study demonstrated that, compared to community-dwelling participants, there is a higher prevalence of chronic pain in people with COPD. Pain combined with dyspnea may impact adversely on cognitive function and lead to anxiety and depression, as well as greater impairment in exercise tolerance, physical activity, and ADL. These results suggested that it is necessary to assess the symptoms of chronic pain and inflect in chronic pain coping strategies.
Keywords: chronic obstructive pulmonary disease, chronic pain, cognitive function, physical function, dyspnea
Introduction
The characteristic symptoms of chronic obstructive pulmonary diseases (COPD) are dyspnea, reduced exercise tolerance and impaired health-related quality of life.1 Anxiety and depression are also frequently observed in people with COPD.1 In addition to these symptoms, more recently, there has been an increased awareness that pain is a common symptom in people with COPD.2 An earlier systematic review reported that pain affects between 45% and 96% of the people with COPD.3 According to an interview-based study of pain experiences, people with COPD reported that pain had adverse effects on their breathing, walking ability, daily activities and sleep, and contributed to the development of anxiety and depression.4 Both pain and dyspnea are unpleasant sensory and emotional experiences with physiological and psychological consequences.5 Therefore, dyspnea and pain in people with COPD are likely to interact and increase the total symptom burden, leading to greater challenges in clinical management.
Pain is defined as “An unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage”.6 In people with low back pain and arthritis, the presence of pain can be associated with an exaggerated negative cognitive and emotional response to actual as well as anticipated pain, referred to as pain catastrophizing.7 People with pain catastrophizing can be worried about how increased pain leads to progressive disability. Therefore, it leads to anxiety and depression. Previous studies have reported that COPD people with chronic pain have psychological disorder such as anxiety or depression.8,9 If individual people perceive by pain be anxiety, they fall into the loop described as a fear-avoidance mode.6 Since people with chronic pain often have a range of signs and symptoms in addition to pain, it is recommended to perform a multifaceted evaluation such as such as physical function, cognition, and emotion in order to comprehensively understand their pain.6 Further, an association between pain and reduced physical activity has been reported in those with COPD10 which is important given that low levels of physical activity are associated with greater mortality in this population.11 Moreover, a previous study reported that pulmonary rehabilitation did not relieve the sensation of pain or the patients’ response such as general health, mood, enjoyment of life, anxiety or fear avoidance behavior to COPD with pain.9 Although there are reports of the cognitive, emotional, and physical aspects associated with chronic pain in people with COPD, few have included a systematically multifaceted assessment.12–14 Specifically, few studies have included assessment of cognitive aspects such as pain catastrophizing and fear of movement.15,16
The primary aim of this study was to describe the characteristic multifaceted impact of chronic pain on cognition, emotional and physical health in people with stable COPD and to compare with an age-matched sample of community-dwelling older participants. The secondary aim was to explore the pain impact upon physical function and psychological aspect in people with COPD.
Materials and Methods
Study Design and Participants
This was a prospective, cross-sectional multicenter study carried out in people with COPD and a group of community-dwelling age-matched participants. The COPD participants (COPD group) comprised those with clinically stable disease17,18 who attended pulmonary rehabilitation at Hozenkai Tagami Hospital (Nagasaki, Japan), Utsunomiya Medical Clinic or Kirigaoka Tsuda Hospital (Fukuoka, Japan). Stable COPD was defined as having symptoms are well managed, pulmonary decline is minimized and no exacerbation in the previous 4 weeks.17,18 The community-dwelling participants (Control group) were recruited from a community-based exercise class. Recruitment took place between November 2017 and May 2018. For the COPD group, inclusion criteria comprised confirmed diagnosis of COPD on spirometry and receiving optimal medical management. Control participants were required to have no history of lung disease such as bronchiectasis, asthma and interstitial lung diseases in addition to COPD, and no smoking history as inclusion criteria. People with COPD were excluded from participation if they had experienced an exacerbation in the previous 4 weeks. Exclusion criteria common to both groups were the presence of concurrent malignancy (including lung cancer) that can cause chronic pain, a diagnosis of cognitive impairment or suspected cognitive dysfunction, and soft tissue or musculoskeletal injury within the last 4 weeks. All participants provided written informed consent prior to data collection. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the Human Ethics Review Committee of Nagasaki University Graduate School of Biomedical Sciences on October 10, 2017 (approval number 17082117).
Procedures
All participants attended a single visit. Firstly, participants were asked a screening question relating to chronic pain, “In the last past month have you experienced any pain-related symptoms? If yes, were these symptoms present for at least the last 6 months?”19 Those who answered yes to the second question were considered to have chronic pain and underwent the following assessments and completed them by themselves in a quiet environment of each room. The remaining participants (ie, those without chronic pain) underwent all assessments other than those related to pain (ie, location, intensity, catastrophizing and fear).
Location of Pain
The participant was asked to circle the location(s) of their pain on a body chart comprising standardized body regions based on 45 anatomical areas.20
Pain Intensity
This was assessed by documenting intensity at the site of maximum pain experienced over the past month using a numerical rating scale from 0 (“no pain”) to 10 (“worst imaginable pain”).21
Pain Catastrophizing
This was assessed using the Japanese version of the Pain Catastrophizing Scale (PCS-J).22 The scale comprises 13 items and can be scored to provide an overall composite measure of catastrophizing (total score) or as three subscales that represent rumination, helplessness and magnification. The PCS-J instructions ask the responder to reflect on past painful experiences and to indicate the degree to which they experienced thoughts or feelings when experiencing pain on a 5-point Likert scale (0–4), with a total score ranging from 0 to 52; higher scores indicate greater catastrophizing. A score above 30 (cut-off) indicates a high level of catastrophizing. The PCS-J has been shown to be reliable and valid in Japanese people with chronic pain.23
Pain-Related Fear
Pain-related fear was assessed using the Japanese version of the Tampa Scale for Kinesiophobia (TSK-J), which is a self-report measure developed to assess fear of movement-related pain. The scale comprises 17 questions which identify fear-related to activities with responses to each question scored on a 4-point Likert scale (0–4) with a maximum total score of 68.24 A score greater than 37 indicates a high degree of kinesiophobia.25 The TSK-J is valid and reliable for detecting kinesiophobia in the Japanese population.26
Anxiety and Depression
This was assessed using the Hospital Anxiety and Depression Scale (HADS) which consists of 14 items, 7 of which assess anxiety and seven assess depression. Response options to each question are assigned a score from 0 to 3 with higher scores representing greater feelings of anxiety and/or depression. Scores of 7 or lower were considered “normal”, scores of 8–10 taken to represent “borderline abnormal” and a score of 11 or higher considered to be suggestive of a “clinical diagnosis of anxiety or depression”.27
Physical Activity
Physical activity was measured with a uniaxial accelerometer (Lifecorder GS; Suzuken Corporation; Nagoya, Japan) over a 7-day period. The accelerometer records vertical acceleration as counts and activity times per day. The accelerometer was worn on a waistband at all times except when showering or sleeping. The step count of adults when walking around an outdoor track with this accelerometer has an intramodel reliability of 0.998.28
Sleep Duration
Participants were asked to report their average daily sleep time over the past month by responding to a question about sleep duration taken from the Japanese version of the Pittsburgh Sleep Quality Index.29
In addition, the following evaluations were also undertaken in the COPD group.
Spirometry
Lung function was measured using a portable automatic calibrated spirometer (Autospiro AS-507®; MINATO MEDICAL SCIENCE, Osaka, Japan). We measured forced expiratory volume in the first second (FEV1) and forced vital capacity (FVC) in accordance with published guidelines.30 Each participant repeated the manoeuvres until three reliable tracings were obtained, and the highest values were retained for analysis.30 Measurements were expressed in absolute values and as a percentage of the predicted value in a healthy Japanese population.31 Based on these results, the severity was classified according to Global Strategy for the Diagnosis Management and prevention of Chronic Obstructive Pulmonary Disease (GOLD).1
Dyspnea
The modified Medical Research Council (mMRC) dyspnea scale was used to assess functional limitation due to dyspnea. This scale is a valid method of categorising people with chronic lung disease in terms of their functional disability. This simple scale comprises five statements, which describe disability ranging from none (Grade 0) to almost complete incapacity (Grade 4). The participant selects the statement, which best reflects their level of limitation in activities of daily life due to breathlessness.32
Activities of Daily Living (ADL)
This was evaluated using the Nagasaki University Respiratory Activities of Daily Living questionnaire.33 This questionnaire consists of 10 ADL items and an additional score for continuous walking distance. Each of the 10 ADL items rates movement speed, dyspnea, and flow rate of supplemental oxygen, if used, from 0 to 3 points, and the continuous walking distance is scored from 0 to 10 points (≤50m, 0 points; 51–200m, 2 points; 201–500m, 4 points; 501m-1km, 8 points; > 1km, 10 points). The maximum total score is 100 points.
Exercise Performance
This was assessed using the 6-minute walk test (6MWT) in accordance with published guidelines.34 The test measures the maximum distance a person can walk in 6 minutes (6-minute walk distance, [6MWD]), on a flat, hard surface. It is a self-paced test with standardized encouragement given each minute. The higher 6MWD from two attempts was recorded.
Sample Size
The sample size was calculated using G*Power and based on a previous study in which the prevalence of pain was 72% in individuals with COPD14 and 34% in healthy control participants,13 for a t-test with an alpha of 0.05 and power of 0 0.80. A sample size of 61 participants in each group was required.
Data Analysis
Data were assessed for normality using the Shapiro–Wilk test. Data are expressed as the mean ± standard deviation or absolute number and percentage. Between-group differences (ie, COPD vs Control group; COPD with pain vs COPD without pain) were assessed using Mann–Whitney U-test, with categorical data analyzed using Chi Square test. Statistical analysis was performed using IBM SPSS statistics version 25.0 for Windows (IBM SPSS Statistics version 25.0, IBM, Chicago, IL, USA). The level of statistical significance was set at <0.05%.
Results
A total of 78 people with COPD and 78 community-dwelling individuals were screened for eligibility. Of these, 74 participants with COPD (COPD group) and 70 community-dwelling individuals (Control group) were recruited to the study. Data from 11 participants were not included in the analyses (6 COPD group, 5 Control group) due to incomplete or missing data (Figure 1). The demographics of the final sample (68 COPD group, 65 Control group) are presented in Table 1. There was a higher percentage of males in the COPD group (76% vs 32% in the Control group, p<0.001). Compared with the Control group, weight and body mass index (BMI) were lower (both p<0.05) and the prevalence of pain was higher in the COPD group (85% vs 51%, p<0.001).
 |
Table 1 Participant Demographics |
 |
Figure 1 Flow diagram of study participants. Abbreviation: COPD, chronic obstructive pulmonary disease. |
Figure 2 provides a comparison of the pain locations reported by the two groups. Pain was more commonly experienced in the neck/shoulders, chest, upper back, and upper limbs in the COPD group (p<0.001). A greater proportion of the Control group reported low back pain (p<0.05).
 |
Figure 2 Pain location. Abbreviations: COPD, chronic obstructive pulmonary disease; UL, upper limb; LL, lower limb. |
Table 2 compares the characteristics of the COPD and control participants who experienced pain. The number of pain locations was higher in the COPD group (p<0.001). The scores for helplessness, magnification and the total score on the PCS-J were higher in the COPD group compared to the Control group (all p<0.05). Sixteen (28%) participants with COPD and three (9%) participants in the Control group reported a high level of pain catastrophizing (p<0.05). Kinesiophobia (TSK-J total score >37) was present in 67% of the COPD group versus 42% in the Control group (p<0.05). There were no between-group differences in the mean scores for anxiety or depression, however, a higher proportion in the COPD group reported scores for anxiety and depression above the cut off for borderline abnormal (both p<0.05). Daily step count was significantly lower in the COPD group, being on average half that measured in the Control group (p<0.05).
 |
Table 2 Comparison of Characteristics Between the COPD and Control Group with Chronic Pain |
The clinical impact of pain in people with COPD is shown in Table 3. No differences in gender, BMI, spirometry, anxiety and depression, and duration of sleep were evident in those with chronic pain compared to those without pain. In contrast, those with pain had more severe COPD (GOLD grade) and higher levels of dyspnea (mMRC grade) (both p<0.001) and a higher proportion were using long-term oxygen therapy (LTOT, p<0.05). Further, the COPD group with pain were more restricted in ADL (NRADL, p<0.05), had worse exercise tolerance (6MWD, p<0.01) and a lower daily step count per day (mean difference −848 steps) (p<0.01).
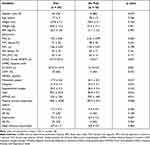 |
Table 3 Clinical Impact of Pain in Participants with COPD |
Discussion
To our knowledge, this is first study to undertake a chronic pain multifaceted evaluation of people with COPD. We found a higher prevalence of chronic pain among people with COPD compared to community-dwelling older people of a similar age with no history of lung disease. The commonly reported pain locations in our COPD sample were neck/shoulder, upper back, thorax and upper limbs, in contrast to low back pain which was the most frequent site of pain experienced by the community-dwelling individuals. Further, in those with COPD, the pain was associated with higher levels of catastrophizing, kinesiophobia, anxiety and depression, and reduced physical activity. The COPD participants who experienced pain were characterized by more severe disease, lower exercise capacity and physical activity, and had greater restrictions in ADL compared to those with COPD without chronic pain.
In the current study, the high (85%) prevalence of chronic pain in people with COPD was within the range (45–96%) reported in a systematic review.3 Our finding was also similar to the 81% prevalence of chronic pain reported by HajGhanbari et al,35 in a COPD sample of a similar age and disease severity (mean age; 72 years, FEV1 predicted; 43%). In addition, our study was similar to previous studies that reported a higher prevalence of pain in COPD patients compared to the general population.12,13,15 As a possible cause, HajGhanbari et al12 described that there might be systemic inflammation, central adaptation associated with dyspnea, and musculoskeletal disorders including restricted chest wall movement due to hyperinflation. Another systematic review36 reported that pain prevalence ranged from 32% to 60% and that the variation in prevalence could be explained by heterogeneity in the participants’ characteristics. Another contributor to the variation in prevalence is the specific definition of pain used in the studies. The same systematic review36 stated that a relatively high prevalence was found in some studies that used a low threshold for reporting pain, for example where pain was considered to be present in all participants who shaded pain on a body chart. We used a low threshold for reporting pain. In contrast, in other studies pain was required to be moderately severe or extremely severe for at least half of the time to meet the definition of pain.36 However, this study was not measured inflammatory markers or indicator of hyperinflation and could not clear about possible etiologies. The further research is required to determine the contributing factors to the etiology of pain in people with COPD.
The commonly reported pain locations such as neck/shoulder, chest and upper back in our COPD sample were consistent with previous studies.4,12,13,35 These were the most frequently reported sites as a result of a meta-analysis.36 In the previous studies, these were speculated that the pain was associated with cough, osteoporosis, gastroesophageal reflux disease or musculoskeletal disorders such as vertical deformity and costotransverse joint arthropathy of the chest wall caused by hyperinflation, excessive use of respiratory assist muscles due to abnormal breathing patterns.12,13,37,38 In the current study, the proportion of upper limbs was higher than the control group. It was different from a previous study. However, the cause of pain locations was not investigated in our study and further research is required to determine the contributing factors to the etiology of pain in people with COPD.
Responses to the questionnaires assessing pain catastrophizing and pain-related fear suggest that pain had a more profound effect on cognitive function in the COPD group compared to the control group. In the current study, the percentage (28%, 16/57) of the COPD group with pain who had a high level of pain catastrophizing (total PCS ≥30), was significantly higher than in the Control group with pain (9%, 3/33). This finding is consistent with a previous study where a greater percentage (24%, 7/29) of people with COPD had a high level of pain catastrophizing when compared to an age and gender-matched sample of healthy controls (11%, 2/19).15 People with pain may have catastrophic thinking that their pain will significantly affect their lives, and such thinking has been associated with disability among with chronic medical condition particularly chronic pain.39 Catastrophizing is an exaggerated negative cognitive toward noxious stimuli and experiences, characterized by rumination about those experiences, magnification of their threat value, and perceived inability to control them.40 In the previous studies with pain, catastrophizing has been found to be more strongly correlated with disability than the pain itself41,42 and has contributed to a fear avoidance model of disability.43 As well as this mechanism, pain in people with COPD could lead to more catastrophic thinking. Our COPD participants experienced pain-related fear as indicated by their scores on the TSK-J, a finding similar to that seen in other studies of people with chronic pain.6 People who experience pain may be concerned that physical activity may elicit or worsen their pain and in turn this may lead to progressive disability.6 Anxiety and depression are also associated with pain-exacerbating activities, such as avoidance of physical activity.36 One study that used the TSK in COPD participants with chronic pain reported that 93% of the sample had a high degree of kinesiophobia which was strongly associated with dyspnea perception and depression.16 A cardinal symptom of COPD is dyspnea, and mood disturbance (ie anxiety and depression) is a common comorbidity.1 Pain-related fear may relate to dyspnea, or to the presence of anxiety or depression in those with COPD.
The level of physical activity in the COPD group was half that of the Control group. Moreover, physical activity in both groups was less than the average step count reported for Japanese people aged over 70 (male; 5016 steps/day, female; 4225 steps/day).44 Contributing to the finding of lower levels of physical activity could be the effect of pain on cognitive function, anxiety and depression associated with pain-exacerbating activities, and the avoidance of physical activity leading to a downward spiral of increased disability.39 However, given that many studies have reported high levels of anxiety and depression and low physical activity in people with COPD,1 our finding may be a function of having COPD.
Our COPD participants with pain had worse respiratory function, higher levels of dyspnea and a higher proportion were using LTOT compared to COPD participants without pain. An earlier study reported that chronic pain in people with COPD was associated with greater dyspnea and that each symptom could amplify perception of the other.45 The lower 6MWD and greater limitation in daily life in the COPD participants with pain could be due to the combined effects of chronic pain and dyspnea on physical function and ADL. The association between pain and reduced physical activity is important, given the detrimental effects of a sedentary lifestyle on survival in COPD.46 However, this finding should be interpreted with caution due to the modest sample size of those without pain and the possibility in the group with pain that worse airflow limitation and a higher percentage receiving LTOT contributed to the lower 6MWD and as a consequence a greater reduction in physical activity.
The management of COPD prioritizes approaches that have been demonstrated to improve symptoms such as dyspnea.1 However, previous studies reported that dyspnea is more prevalent than pain.3,47 Therefore, dyspnea that precedes pain may be overlooked. Moreover, Lee et al9 reported that pulmonary rehabilitation did not relieve the sensation of pain or the patients’ response to COPD with pain. A better understanding of the characteristics of chronic pain and its influence on symptoms and physical activity will help inform approaches for managing chronic pain in people with COPD. Given this observation, it is necessary to assess the symptoms of chronic pain as well as dyspnea in people with COPD, and especially in those with more severe disease and establish chronic pain and dyspnea coping strategies that are essential for optimizing COPD management. There are evidences that the affective dimensions of dyspnea, such as distress and unpleasantness, are processed in the same areas of the brain that process fear, anxiety, and pain.5,48 In addition, the guidelines for chronic pain strongly recommend psychological treatments, specifically psycho-educational relaxation techniques, self-monitoring and other hand-based techniques, and cognitive-behavioral therapy as psychological approaches.49 Based on these results, our finding suggests the need for these interventions also in patients with COPD who have pain.
The strengths of our study include the systematically multifaceted assessment of chronic pain, which has been lacking in previous studies, the inclusion of a group without lung disease and assessment of the clinical impact of pain in those with COPD. This study has some limitations. Firstly, the COPD group had a high proportion of males in contrast to the Control group that was mostly female, consistent with the gender of those who attend community-based exercise classes within Japan. However, in the general population, the prevalence of pain in females is higher than in males.44 Secondly, community-dwelling participants did not undergo spirometry, so we cannot exclude the presence of undiagnosed lung disease. Thirdly, neither group was investigated for comorbidities such as osteoporosis and heart disease. Lastly, this study did not include an assessment of quality of life.
Conclusion
This study demonstrated that compared to community-dwelling participants, chronic pain is more prevalent in people with COPD. The pain was more commonly located in the neck/shoulder, upper back, thorax and upper limbs in those with COPD, whereas pain in the lower back was the commonest site in the community-dwelling sample. The presence of pain in COPD can have a greater impact on cognitive function and, combined with dyspnea, has the potential to lead to a negative spiral that adversely affects mood, exercise tolerance, physical activity, and ADL. Our results indicate that management of people with COPD may need to focus more attention, assessment and intervention such as psychological approaches of chronic pain, in addition to symptoms such as dyspnea, with the aim of implementing strategies to minimize pain impact and thus improve well-being.
Data Sharing Statement
Data used to support the findings of this study are included within the manuscript in Tables 1–3 and Figure 2. Raw data are available from the corresponding author upon reasonable request.
Acknowledgments
The authors were grateful to the study participants, physiotherapists who acquired data for the study from Department of Rehabilitation, Hozenkai Tagami Hospital; Department of Rehabilitation, Kirigaoka Tsuda Hospital; Department of Rehabilitation; Utsunomiya Medical Clinic and Special Elderly Nursing Home Keijyuen.
Author Contributions
TT, MO and RK contributed significantly to conception and design, or analysis and interpretation of data; and have been involved in drafting and reviewing the manuscript. SJ contributed significantly to analysis and interpretation data and has been involved in drafting and revised it critically for important intellectual content. All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work. All named authors had given final approval of the version to be published.
Funding
The study design, collection and analysis of the data, and preparation of the manuscript were not funded by any public, commercial, or not-for-profit sectors.
Disclosure
The authors report no conflicts of interest in this work.
References
1. GOLD. Global strategy for the diagnosis m, and prevention of chronic obstructive pulmonary disease; 2021. Available from: https://goldcopd.org/wp-content/uploads/2020/11/GOLD-REPORT-2021-v1.1-25Nov20_WMV.pdf.html.
2. Lewthwaite H, Williams G, Baldock KL, Williams MT. Systematic review of pain in clinical practice guidelines for management of COPD: a case for including chronic pain? Healthcare. 2019;7(1):15. doi:10.3390/healthcare7010015
3. Lee AL, Harrison SL, Goldstein RS, Brooks D. Pain and its clinical associations in individuals with COPD: a systematic review. Chest. 2015;147(5):1246–1258. doi:10.1378/chest.14-2690
4. Lohne V, Heer HC, Andersen M, Miaskowski C, Kongerud J, Rustoen T. Qualitative study of pain of patients with chronic obstructive pulmonary disease. Heart Lung. 2010;39(3):226–234. doi:10.1016/j.hrtlng.2009.08.002
5. von Leupoldt A, Sommer T, Kegat S, et al. Dyspnea and pain share emotion-related brain network. Neuroimage. 2009;48(1):200–206. doi:10.1016/j.neuroimage.2009.06.015
6. Raja SN, Carr DB, Cohen M, et al. The revised International Association for the Study of Pain definition of pain: concepts, challenges, and compromises. Pain. 2020;161(9):1976–1982. doi:10.1097/j.pain.0000000000001939
7. Quartana PJ, Campbell CM, Edwards RR. Pain catastrophizing: a critical review. Expert Rev Neurother. 2009;9(5):745–758. doi:10.1586/ern.09.34
8. Andenaes R, Momyr A, Brekke I. Reporting of pain by people with chronic obstructive pulmonary disease (COPD): comparative results from the HUNT3 population-based survey. BMC Public Health. 2018;18(1):181. doi:10.1186/s12889-018-5094-5
9. Lee AL, Butler SJ, Varadi RG, Goldstein RS, Brooks D. The impact of pulmonary rehabilitation on chronic pain in people with COPD. COPD. 2020;17(2):165–174. doi:10.1080/15412555.2020.1733952
10. HajGhanbari B, Garland SJ, Road JD, Reid WD. Pain and physical performance in people with COPD. Respir Med. 2013;107(11):1692–1699. doi:10.1016/j.rmed.2013.06.010
11. Esteban C, Quintana JM, Aburto M, et al. Impact of changes in physical activity on health-related quality of life among patients with COPD. Eur Respir J. 2010;36(2):292–300. doi:10.1183/09031936.00021409
12. HajGhanbari B, Holsti L, Road JD, Darlene Reid W. Pain in people with chronic obstructive pulmonary disease (COPD). Respir Med. 2012;106(7):998–1005. doi:10.1016/j.rmed.2012.03.004
13. Bentsen SB, Rustoen T, Miaskowski C. Prevalence and characteristics of pain in patients with chronic obstructive pulmonary disease compared to the Norwegian general population. J Pain. 2011;12(5):539–545. doi:10.1016/j.jpain.2010.10.014
14. Borge CR, Wahl AK, Moum T. Pain and quality of life with chronic obstructive pulmonary disease. Heart Lung. 2011;40(3):e90–101. doi:10.1016/j.hrtlng.2010.10.009
15. Lee AL, Goldstein RS, Brooks D. Chronic pain in people with chronic obstructive pulmonary disease: prevalence, clinical and psychological implications. Chronic Obstr Pulm Dis. 2017;4(3):194–203. doi:10.15326/jcopdf.4.3.2016.0172
16. Vardar-Yagli N, Calik-Kutukcu E, Saglam M, Inal-Ince D, Arikan H, Coplu L. The relationship between fear of movement, pain and fatigue severity, dyspnea level and comorbidities in patients with chronic obstructive pulmonary disease. Disabil Rehabil. 2019;41(18):2159–2163. doi:10.1080/09638288.2018.1459886
17. Negewo NA, McDonald VM, Baines KJ, et al. Peripheral blood eosinophils: a surrogate marker for airway eosinophilia in stable COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:1495–1504. doi:10.2147/COPD.S100338
18. Bollmeier SG, Hartmann AP. Management of chronic obstructive pulmonary disease: a review focusing on exacerbations. Am J Health Syst Pharm. 2020;77(4):259–268. doi:10.1093/ajhp/zxz306
19. Nakamura M, Nishiwaki Y, Ushida T, Toyama Y. Prevalence and characteristics of chronic musculoskeletal pain in Japan. J Orthop Sci. 2011;16(4):424–432. doi:10.1007/s00776-011-0102-y
20. Margolis RB, Tait RC, Krause SJ. A rating system for use with patient pain drawings. Pain. 1986;24(1):57–65. doi:10.1016/0304-3959(86)90026-6
21. Haefeli M, Elfering A. Pain assessment. Eur Spine J. 2006;15(Suppl 1):S17–24. doi:10.1007/s00586-005-1044-x
22. Matsuoka H, Sakano Y. Assessment of cognitive aspect of pain: development, reliability, and validation of Japanese version of pain catastrophizing scale. Jpn J Psychosom Med. 2007;47(8):95–102. Japanese. doi:10.15064/jjpm.47.2_95
23. Iwaki R, Arimura T, Jensen MP, et al. Global catastrophizing vs catastrophizing subdomains: assessment and associations with patient functioning. Pain Med. 2012;13(5):677–687. doi:10.1111/j.1526-4637.2012.01353.x
24. Roelofs J, Goubert L, Peters ML, Vlaeyen JW, Crombez G. The Tampa scale for kinesiophobia: further examination of psychometric properties in patients with chronic low back pain and fibromyalgia. Eur J Pain. 2004;8(5):495–502. doi:10.1016/j.ejpain.2003.11.016
25. Vlaeyen JWS, Kole-Snijders AMJ, Boeren RGB, van Eek H. Fear of movement/(re) injury in chronic low back pain and its relation to behavioral performance. Pain. 1995;62(3):363–372. doi:10.1016/0304-3959(94)00279-N
26. Kikuchi N, Matsudaira K, Sawada T, Oka H. Psychometric properties of the Japanese version of the Tampa Scale for Kinesiophobia (TSK-J) in patients with whiplash neck injury pain and/or low back pain. J Orthop Sci. 2015;20(6):985–992. doi:10.1007/s00776-015-0751-3
27. Shima S, Shikano T, Kitamura T, Asai M. A new self-rating scale for depression. Clin Psychiatry. 1985;27:717–723. Japanese.
28. Schneider PL, Crouter SE, Lukajic O, Bassett DR
29. Doi Y, Minowa M, Uchiyama M, et al. Psychometric assessment of subjective sleep quality using the Japanese version of the Pittsburgh Sleep Quality Index (PSQI-J) in psychiatric disordered and control subjects. Psychiatry Res. 2000;97(2–3):165–172. doi:10.1016/s0165-1781(00)00232-8
30. Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi:10.1183/09031936.05.00034805
31. Japanese Respiratory Society. Guidelines for the Diagnosis and Treatment of COPD.
32. Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest. 1988;93(3):580–586. doi:10.1378/chest.93.3.580
33. Takigawa N, Tada A, Soda R, et al. Comprehensive pulmonary rehabilitation according to severity of COPD. Respir Med. 2007;101(2):326–332. doi:10.1016/j.rmed.2006.03.044
34. Holland AE, Spruit MA, Troosters T, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J. 2014;44(6):1428–1446. doi:10.1183/09031936.00150314
35. HajGhanbari CY, Garland SJ, Garland RJ, et al. The relationship between pain and comorbid health conditions in people with chronic obstructive pulmonary disease. Cardiopulm Phys Ther J. 2014;25(1):29–35. doi:10.1097/01823246-201403000-00007
36. van Dam van Isselt EF, Groenewegen-Sipkema KH, Spruit-van Eijk M, et al. Pain in patients with COPD: a systematic review and meta-analysis. BMJ Open. 2014;4(9):e005898. doi:10.1136/bmjopen-2014-005898
37. Bordoni B, Marelli F, Morabito B, Castagna R. Chest pain in patients with COPD: the fascia’s subtle silence. Int J Chron Obstruct Pulmon Dis. 2018;13:1157–1165. doi:10.2147/COPD.S156729
38. Chen YW, Coxson HO, Coupal TM, et al. The contribution of thoracic vertebral deformity and arthropathy to trunk pain in patients with chronic obstructive pulmonary disease (COPD). Respir Med. 2018;137:115–122. doi:10.1016/j.rmed.2018.03.007
39. Boersma K, Linton SJ. Psychological processes underlying the development of a chronic pain problem: a prospective study of the relationship between profiles of psychological variables in the fear-avoidance model and disability. Clin J Pain. 2006;22(2):160–166. doi:10.1097/01.ajp.0000159582.37750.39
40. Sullivan MJL, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess. 1995;7(4):524–532. doi:10.1037/1040-3590.7.4.524
41. Crombez G, Vlaeyen JW, Heuts PHTG, Lysens R. Pain-related fear is more disabling than pain itself: evidence on the role of pain-related fear in chronic back pain disability. Pain. 1999;80(1–2):329–339. doi:10.1016/S0304-3959(98)00229-2
42. Helsen K, Goubert L, Peters ML, Vlaeyen JW. Observational learning and pain-related fear: an experimental study with colored cold pressor tasks. J Pain. 2011;12(12):1230–1239. doi:10.1016/j.jpain.2011.07.002
43. Vlaeyen JWS, Linton SJ. Fear-avoidance and its consequences in chronic musculoskeletal pain: a state of the art. Pain. 2000;85(3):317–332. doi:10.1016/S0304-3959(99)00242-0
44. Ministry of Health Law. National health and nutrition survey. Available from: https://www.mhlw.go.jp/content/10900000/000687163.pdf.
45. Cai B, Oderda GM. The association between pain and depression and some determinants of depression for the general population of the United States. J Pain Palliat Care Pharmacother. 2012;26(3):257–265. doi:10.3109/15360288.2012.703292
46. Garcia-Aymerich J, Lange P, Benet M, Schnohr P, Anto JM. Regular physical activity modifies smoking-related lung function decline and reduces risk of chronic obstructive pulmonary disease: a population-based cohort study. Am J Respir Crit Care Med. 2007;175(5):458–463. doi:10.1164/rccm.200607-896OC
47. Janssen DJ, Spruit MA, Uszko-Lencer NH, Schols JM, Wouters EF. Symptoms, comorbidities, and health care in advanced chronic obstructive pulmonary disease or chronic heart failure. J Palliat Med. 2011;14(6):735–743. doi:10.1089/jpm.2010.0479
48. Carrieri-Kohlman V, Donesky-Cuenco D, Park SK, Mackin L, Nguyen HQ, Paul SM. Additional evidence for the affective dimension of dyspnea in patients with COPD. Res Nurs Health. 2010;33(1):4–19. doi:10.1002/nur.20359
49. Williams AC, Eccleston C, Morley S. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst Rev. 2020;11:CD007407. doi:10.1002/14651858.CD007407.pub4
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


