Back to Journals » Infection and Drug Resistance » Volume 16
Clinical and Pathological Features of Hydroa Vacciniforme-Like Lymphoproliferative Disorder Along with Risk Factors Indicating Poor Prognosis
Authors Chang L , Zhang C, Lu J , Shen J, Hamal K, Liu D
Received 7 January 2023
Accepted for publication 6 March 2023
Published 16 March 2023 Volume 2023:16 Pages 1545—1559
DOI https://doi.org/10.2147/IDR.S402040
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Li Chang,1,2 Chaoyin Zhang,1 Jingjing Lu,1 Jiahui Shen,1 Krishna Hamal,1 Donghua Liu1,2
1Department of Dermatology and Venereology, the First Affiliated Hospital of Guangxi Medical University, Nanning, People’s Republic of China; 2Guangxi Key Laboratory of AIDS Prevention and Treatment, Nanning, People’s Republic of China
Correspondence: Donghua Liu, Department of Dermatology and Venereology, the First Affiliated Hospital of Guangxi Medical University, No. 6, Shuang Yong Road, Nanning, Guangxi Province, 530021, People’s Republic of China, Tel +86 771-5356752, Email [email protected]
Purpose: To examine the clinical and pathological features, laboratory markers, therapeutic options and risk factors indicating poor prognosis of hydroa vacciniforme-like lymphoproliferative disorder (HVLPD).
Patients and Methods: Seven patients with HVLPD had their clinical and pathological data collected. Immunohistochemical staining, Epstein-Barr virus-encoded RNA (EBER) in situ hybridization experiments, T-cell receptor (TCR) gene rearrangement, RT-PCR tests and the Elisa assay were carried out.
Results: The main clinical manifestations were papulovesicular lesions and ulcers on the face, neck, or trunk. Five cases had systemic symptoms. Three of the deceased patients had significant facial edema, deep body necrosis, and ulceration. The pathological results demonstrated that lymphocytes infiltrated blood vessels and sweat glands in addition to the dermis and subcutaneous tissues. All patients tested positive for CD3 and EBER. Six cases tested positive for TCRβF1, but none tested positive for TCRδ. TCRγ monoclonal rearrangement, strongly positive expression of TIA-1 and a Ki67 proliferation index of 40% occurred in 3 fatal cases. When compared to the survival group, the plasma EBV DNA in the deceased group was considerably higher (P< 0.05). IFN-γ and TNF-α cytokine levels in patients were higher than in the control group, particularly in the deceased group (P< 0.05). The skin lesions on all patients recovered quickly underwent conservative care. Nonetheless, 3 patients passed away as the disease progressed in its latter stages.
Conclusion: In our cases, the main infiltrating cells were T cells and the dominant lymphocyte subclass was αβT cells. A significant increase in lgE level, plasma EBV DNA, IFN-γ, and TNF-α cytokine levels, decreased hemoglobin level, strongly positive expression of TIA-1, high Ki67 proliferation index, and positive TCR gene rearrangement are all indicators of a poor prognosis.
Keywords: hydroa vacciniforme-like lymphoproliferative disorder, hydroa vacciniforme, Epstein-Barr virus, Epstein-Barr virus-encoded RNA, risk factors
Introduction
Mostly prevalent in eastern Asia1 and Latin America,2 hydroa vacciniforme-like lymphoproliferative disorder (HVLPD) is a rare lymphoproliferative condition that is closely linked to chronic Epstein-Barr virus (EBV) infection. It is characterized by monoclonal proliferation of natural killer (NK) or T lymphocytes (T cells), with a risk of progressing to malignant lymphoma.3,4 Its sensitivity to UVB or UVA has been thought to represent a significant pathogenic mechanism.5 HVLPD is comparable to hydroa vacciniforme (HV), one of the rarest photosensitive reactions. It mainly occurs in children, but can also be seen in adults. Recurrent vesiculopapules in sun-exposed parts of the skin lead to ulcers and hemorrhagic scabs as the predominant signs. The outcome is an acne-like scar. Avoiding the sun can help, and for some people, it can go away on its own during puberty.6,7 After a prolonged illness and numerous relapses, some unusual or severe HV patients may develop systemic disease with symptoms such as persistent fever, weight loss, facial edema, sun unexposed skin lesions, lymphadenopathy, hepatosplenomegaly, leukopenia, etc. In severe cases, fatal EBV-positive T-cell or NK cell lymphoma may develop.8 Therefore, HVLPD was classified as an HV-like lymphoma (HVLL) containing the T cell receptor (TCR) clonal rearrangement gene in the 2008 WHO classification of hematopoietic and lymphoid malignancies.9 Some people, however, can heal themselves or recover following therapy, while others develop lymphoma as a result of prognostic differences across patients. As a result, in the 2016 WHO updated classification of lymphoid malignancies, HVLL was reclassified as HVLPD.10 According to the WHO’s reclassification, HVLPD frequently presented as atypical cases with variable clinical and pathological manifestations, indicating that while clinical manifestations of the disease included invasion activity, atypical tumor cell infiltration might not be visible in histopathology.11 Therefore, HVLPD is often misdiagnosed in clinics due to its complexity and rarity. In addition, due to the lack of relevant studies on pathogenesis, disease assessment, and prognosis assessment, there is no standardized treatment plan in clinical practice.
Because EBV can attack different lymphocyte subsets, the immunohistochemical results of CD3, CD4, CD8, CD20, CD56, TIA-1, TCRβF1, TCRδ, and Ki67 can be used to identify lymphocyte subsets infected with EBV and assess cell proliferation. Then, it can help further accurate diagnosis and treatment in the clinic. EBV infection of T /NK cells also leads to clonal proliferation and adverse progression of HVLPD, which can be detected by TCR gene rearrangement.12,13 Previous studies have shown that TCR gene rearrangement is considered a useful tool for distinguishing between classical HV and severe HV.14 Due to EBV infection, patients may have different levels of EBV DNA in their plasma and Peripheral Blood Mononuclear Cell (PBMC),15,16 and Epstein-Barr virus-encoded RNA (EBER) in situ hybridization of tissues may be positive. In addition, the degree of EBV infection may also be related to the prognosis, but the relevant cases are rarely reported at present. EBV infection causes a distinct adaptive immune response in T and B cells, and the immune system secretes both pro-inflammatory (IFN-γ and TNF-α) and anti-inflammatory (IL-4, IL-10, and IL-13) cytokines, which play key roles.17 Plasma IgE level is known to be a marker of TH2 polarization, and when EBV infection occurs, it will stimulate the secretion of T helper type 1 (Th1) and T helper type 2 (Th2) lymphocytes, resulting in increased IgE.18 Nevertheless, clinically, this is frequently disregarded, and inflammation-related factors are not adequately addressed or treated.
The goal of this study is to outline the clinical manifestations and pathological aspects of typical HVLPD cases with various disease conditions in order to increase clinicians’ and pathologists’ awareness of HVLPD. We can grasp the relationship between patients’ prognosis and their WBC, hemoglobin (HB), platelets (PLT), aspartate transaminase(AST), alanine transaminase (ALT), Immunoglobulin E (IgE) and other associated biochemical markers, as well as EBV DNA detection in plasma and PBMC samples, by statistically analyzing their detection values. CD3, CD56, and other immunohistochemical indicators were used to understand the main infiltrated lymphocyte subclass in patient tissues. The clonability of T cells in patients was studied by detecting TCR gene rearrangements. In addition, IFN-γ, TNF-α, IL-4, IL-10, and IL-13 inflammatory factors were measured in patients to better understand the types of inflammatory factors associated with EBV infection in HVLPD and their link to prognosis.
Materials and Methods
Clinical Data
Seven cases of HVLPD with biopsy confirmation were gathered and examined from 2019 to 2022 at the Department of Dermatology and Venereology of the First Affiliated Hospital of Guangxi Medical University. The diagnostic criteria for this study were based on the WHO 2016 classification of hematopoietic and lymphatic tumors.10 Patients with complete clinical data, a clear pathological diagnosis, adequate skin tissue samples, the availability of blood samples, and successful follow-up were included in the study. In addition, patients with aggressive NK-cell leukemia, extranodal NK/T-cell lymphoma, and peripheral T-cell lymphoma needed to be excluded. Age, sex, the location of the affected skin, the clinical characteristics of the skin lesions, systemic symptoms, extracutaneous involvement, clinical laboratory findings, histopathological findings were all clinical data that were gathered and examined. Samples of whole blood were taken. For their participation in the study, every patient signed a written informed consent form. The Ethics Committee of the First Affiliated Hospital of Guangxi Medical University granted ethical approval.
Immunohistochemical Staining
Immunohistochemical SP staining was used. The wax sections were dewaxed with xylene and hydrated with anhydrous ethanol. The sections were then placed in an EDTA antigen repair solution for thermal remediation, but TCRβF1 was digested with pepsin instead of thermal remediation. After washing with PBS buffer solution (Main ingredient Biotech), the cells were incubated for 10 minutes with 3% H2O2 sealing solution. Then the cells were incubated with goat serum sealer at room temperature for 30 minutes. Following a wash with PBS buffer solution, the primary antibody’s diluent was added, and the following antibodies were incubated overnight at 37°C: CD3 (dilution 1:4000, Proteintech), CD4 (antibody working fluid, ZSGB-BIO), CD8 (antibody working fluid, ZSGB-BIO), CD20 (dilution 1:100, Abcam), CD56 (antibody working fluid, ZSGB-BIO), TIA-1 (antibody working fluid, ZSGB-BIO), TCRβF1 (dilution 1:150, Invitrogen), TCRδ (dilution 1:150, Santa Cruz Biotechnology), Ki67 (antibody working fluid, ZSGB-BIO). Common immunohistochemistry secondary antibody was applied to the sections after washing with PBS buffer solution. The sections were cleaned with PBS buffer solution, then stained with non-alcoholic hematoxylin after being dried and treated with DAB for color development. Resin was then applied to seal the piece. CD3, CD4, CD8, CD20, CD56 and TCRδ were found in the cell membrane, while TIA-1 was found in the cytoplasm, TCR-F1 was found in the cytoplasm or membrane, and Ki67 was found in the nucleus. All antibodies tested had appropriate positive and negative controls. The positive rate was determined by selecting five areas viewed under a 200-fold microscope, and the average value was calculated.
EBER in situ Hybridization Experiment
The EBER kit was purchased from Beijing Zhongshan Jinqiao Company. After being dewaxed with xylene for 30 minutes, the wax sections were put in anhydrous ethanol for 10 minutes. A gastric protein working solution was added after air drying, and the mixture was then incubated at 37°C in a wet box for 30 minutes. We used ethanol gradient dehydration (75%, 95%, and 100% for two minutes each). Following drying, the samples were incubated overnight at 4° in a wet box with the EBER probe added, silicized cover slides covered, and rubber cement edges sealed. The cover glass was cleaned once the rubber cement was taken off. Anti-digoxin antibody that was HRP-labeled was added, and it was incubated in a 37° wet box for 30 min. For color rendering, a freshly configured DAB was used. As a positive control, EBV+ NK/T cell lymphoma was used, and PBS buffer was used instead of a probe as a negative control. The findings revealed brown nuclei, which indicated positive EBER.
TCR Gene Rearrangement
The DNA of wax block specimens from patients was extracted using the QIAamp DNA Mini Kit (Qiagen Company). Appropriate positive and negative controls were set up. Multiple amplification was performed using polymerase chain reaction (PCR) based on the BIOMED-2 protocol to analyze T-cell clonality. The primers (Sangon Biotech) were used to detect TCR-β, TCR-γ and TCR-δ chains. A 2% agarose gel was used to electrophorese PCR products, and an ultraviolet gel imager was used to inspect the results. The electrophoretic band is regarded as a monoclonal rearrangement of the fragment if it has sharp edges, is clear and brilliant, and is the same size as the positive fragment in the BIOMED-2 primer system. If the opposite is true, it is interpreted as a negative.
Detection of EBV DNA by RT-PCR
An EBV DNA testing kit was purchased from Shengxiang Biotechnology Co., Ltd. After counting the thawed PBMC suspension or thawing the plasma, 100 μL of the PBMC sample or the plasma sample was collected and placed in a 1.5 mL EP tube. After adding 100μL of the concentrated solution, the sample was added once more, and the mixture was centrifuged for 5 minutes at 12,000 rpm. After discarding the supernatant, 50 μL of nucleic acid releaser were added, blown, and mixed for 10 minutes at room temperature. A total of 40 μL of PCR-MIX and 10 μL of the standing liquid were each added to a PCR reaction tube. The tube was covered and centrifuged for 30 seconds at 2000 rpm. The PCR reaction tube was then inserted into the RT-PCR apparatus. Using the measured values of the quantitative reference sets A, B, C, and D, quantitative standard curves were created. PBMC and plasma of healthy subjects, and negative and positive criteria were utilized as controls.
Detection of Cytokine Levels in Plasma by Quantitative ELISA
The Elisa kit, purchased from Elabscience Reagents, collected plasma from 7 patients and plasma from 5 healthy individuals who were negative for EBV DNA by RT-PCR. 5 healthy individuals were the control group. The sandwich Elisa quantified the human IL-4, IL-10, IL-13, IFN-γ, and TNF-α cytokine levels in plasma according to the manufacturer’s protocol. OriginPro 9.1 is used to calculate the data.
Statistical Analysis
Statistical software, SPSS Version 25.0 (SPSS, Inc.), was used to evaluate the data. Clinical and experimental measurement data were collected and described by Median (First quartile-Third quartile), including laboratory indicators such as biochemical indexes, EBV DNA, IL-4, IL-10, IL-13, IFN-γ and TNF-α cytokine levels. The exact Fisher test was performed to compare categorical variables. Non-parametric test was performed to compare continuous variables. The correlation test was performed to compare the correlation between two samples. P<0.05 was considered statistically significant.
Results
Clinical Presentation
The clinical characteristics of 7 HVLPD patients were studied (Table 1), including 3 males and 4 females ranging in age from 1 to 45 years, with an average age of 19.71 years and a median age of 17 years. All patients had no history of severe mosquito bite allergy. Six of the seven patients were children or young adults (under the age of 35), and one was a middle-aged patient (over the age of 35). Five patients with a male-to-female ratio of 3:2 and a mean age of 23 years had systemic HVLPD with persistent fever involving lymph nodes, bone marrow, liver, or spleen. The other two patients had typical HVLPD, with an average age of 11.5 years and a male-to-female ratio of 1:1. On the face and neck of every patient, erythema of various diameters, papulovesicular lesions, and ulcers of various depths can be detected. Scabs or atrophic scars may develop following the rupture of papulovesicular lesions (Figure 1a). Five of the patients (71%) had skin lesions on the trunk and lower extremities in the sun exposed area and the sun-protected area, manifested as papular blisters, scabs, erosion, ulcers, or sunken scars (Figure 1b and c). Three patients (43%) presented with severe skin lesions at a later stage, such as significant facial edema (Figure 1d), deep ulceration, and necrosis, accompanied by significant systemic symptoms, such as fever and enlargement of the liver, spleen, and lymph nodes. Two patients showed significant periorbital swelling. In laboratory indicators, WBC decreased in three patients (43%), two of whom died. All three patients with decreased hemoglobin died. Platelet elevation occurred in 4 patients, 2 of whom died. Elevated AST and ALT were found in 3 patients, 2 of whom were dead. Except for one patient with a missing IgE value, 5 of the remaining 6 patients (83%) had a severe IgE increase, and all 3 of the deceased patients had an IgE increase than the survival group (P=0.05) (Table 2).
 |
Table 1 Clinical Manifestations, Laboratory Indicators, Treatment and Follow-Up of HVLPD Patients |
 |
Table 2 Comparison of Experimental Indexes Between the Deceased Group and the Survival Group in HVLPD Patients |
Pathological Manifestation
The skin biopsy tissues of the seven patients revealed local necrosis and epidermal blister formation, infiltration of dermal lymphocytes and neutrophils, and erythrocyte extravasation, which primarily surrounded skin appendices (such as eccrine glands), as well as invasion of blood vessels and subcutaneous adipose tissue. The nucleus displayed mild to moderate atypia, the cell size was normal with sporadic atypia, and the nucleolus was not readily visible. The pathological features of cases 1, 2, 4, and 5 were represented. In both cases 2 (Figure 2a) and 1 (Figure 2b), the subcutaneous adipose septum was observed, with a large number of lymphoid cells infiltrating in some adipose lobules, mild pleomorphism of lymphoid cells, deep staining of individual nuclei, and infiltration of eosinophils. In case 1, the lymphocytes at the site were mildly anomalous. In case 4 (Figure 2c), diffuse infiltration of medium-sized lymphocytes and plasmacytoid lymphocytes was observed in the shallow depth of the dermis, along with slight atypical lymphocytes, significant erythrocyte extravasation, and local follicular structure formation. In Case 5 (Figure 2d), nodular infiltration of medium-sized lymphoid cells and eosinophils was observed around the vessels and appendages in the deep layer of the dermis; some lymphoid nuclei were deeply stained, erythrocyte extravasation was significant, and lymphoid cells invaded sweat glands and vascular walls.
Immunohistochemical Staining
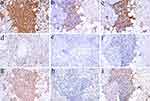
All patients had a strong positive expression of CD3 (Figure 3a) and a mild positive expression of CD4 (Figure 3b), and only four patients showed a scattered positivity for CD56 (Table 3). And three patients showed negative expression of CD56 (Figure 3e). With the exception of case 7, which expressed CD8 negatively, all other patients had positive CD8 expression (Figure 3c), and the positive rate for CD8 was noticeably higher than that for CD4 in the sections of the same case. With the exception of case 7, which lacked a sample, the remaining 6 patients underwent tests for CD20, TIA-1, TCRβF1, and TCRδ. The 6 patients showed focal positivity or scattered positivity for CD20 (Figure 3d). The results of TIA-1 (Figure 3f) and TCRβF1 (Figure 3g) were positive, and the results of TCRδ (Figure 3h) were negative. TIA-1 was strongly positive in all three patients who died. The range of the Ki67 (Figure 3i) proliferation index in each case was between 5% and 40%, with the three patients who passed away having a Ki67 index of 40%.
 |
Table 3 Immunohistochemistry of HVLPD Patients |
EBER in situ Hybridization and TCR Gene Rearrangement
EBER in situ hybridization revealed diffuse nuclear staining of infiltrating lymphoid cells in wax block slices from seven patients, including six with strong positive results (Figure 2e) and one with weak positive results. Furthermore, EBER was strongly positive in all three patients who died, with positive lymphocytes infiltrating subcutaneous tissues and surrounding appendages and individual nuclei showing atypia (Figure 2f). TCR gene rearrangement was performed on wax block specimens from 5 patients, of which 3 patients had monoclonal TCRγ rearrangement and 2 patients had no TCR gene rearrangement. All patients with monoclonal TCRγ rearrangement had systemic HVLPD and were deceased.
Detection of EBV DNA by RT-PCR
Plasma samples were collected from seven patients, and because PBMC was missing in case number one, only PBMC samples were collected from six patients. The EBV DNA of plasma and PBMC were detected, and the PBMC EBV DNA was uniformly used in 106 cells. Plasma EBV DNA expression was positive in 5 patients and negative in 2 patients. Plasma EBV DNA expression was significantly higher in the deceased group compared to the survival group (P=0.032). There was no significant difference between the deceased group and the survival group in PBMC EBV DNA detection (P>0.05). There was no correlation between the PBMC EBV DNA and plasma EBV DNA (P>0.05) (Table 4).
 |
Table 4 EBV DNA of Plasma and PBMC in HVLPD Patients |
Detection of Cytokine Levels in Plasma by Quantitative ELISA
The plasma IFN-γ and TNF-αcytokine levels of 7 patients were higher than those of the control group, and the difference was statistically significant (P<0.05) (Figure 4). There was no significant difference in IL-4, IL-10 and IL-13 cytokine levels in the patient group compared with the control group (P>0.05). IFN-γ, TNF-α, IL-4, IL-10, and IL-13 cytokine levels was not significantly different between the deceased group and the survival group (P>0.05) (Table 5). However, in the comparison between the deceased group and the control group, the levels of IFN-γ and TNF-α cytokine levels in the deceased group were significantly higher than the control group (P=0.025) (Table 6). But there was no significant difference in IL-4, IL-10, and IL-13 cytokine levels between the deceased group and the control group (P>0.05).
 |
Table 5 Comparison of Cytokine Levels Between the Deceased Group and the Survival Group of HVLPD Patients |
 |
Table 6 Comparison of Cytokine Levels Between the Deceased Group and the Control Group of HVLPD Patients |
Treatment and Follow-Up
All seven patients underwent conservative care, which included antiviral drugs like acyclovir and recombinant human interferon α-2b as well as glucocorticoids like prednisone acetate, immunomodulators like thalidomide and cyclosporine, and hepatoprotective and gastromucosal protective medications. Lesions improved immediately following treatment, but some patients continued to deteriorate over time. Patients were contacted for follow-up. The most current follow-up was on November 5, 2022, and it ranged from 1 to 44 months. About 42.86% (3/7) of the patients passed away. After receiving conservative treatment, about 42.86% (3/7) of the patients improved and developed no new skin lesions. Patients with the disease survived in 14.28% (1/7) of cases. The three individuals who passed away all had systemic HVLPD and died either from hemophagocytic syndrome or a progressive disease.
Discussion
HVLPD is a rare lymphoproliferative disease associated with EBV. In the early stages of the disease, HVLPD patients present with skin disease in sun-exposed areas, characterized by vesiculopapular skin lesions, ulcers, and scars with chronic hyperplasia, similar to the lesions of classic HV.19,20 However, some individuals go through a prolonged recurrence process in which the illness spreads to parts of the skin that are not exposed to sunlight. Recurrent skin lesions in patients might develop into systemic symptoms such as fever, hepatosplenic lymphadenopathy, hemophagocytic syndrome, and even lymphoma.21,22 In our research, there were more female patients than male patients. All of the patients had skin lesions that appeared as erythema, papulovesicular lesions, ulcers, and scabs of various sizes on exposed skin, such as the head, face, and neck, and five patients had skin lesions on the trunk and lower limbs in the sun protected area. This might be as a result of repeated UV exposure reactivating EBV in latent lymphocytes.14 The emergence of HVLPD is closely associated with ultraviolet light. The malignant transformation of HVLPD has been linked to ultraviolet radiation, and sunlight can both cause and exacerbate skin lesions.5 Furthermore, 5 patients developed systemic symptoms, and 3 of them ultimately developed severe hematological problems and died. This prognosis was comparable to that described in Japan,22 but our study’s cases were more serious than those in the United States and the United Kingdom, where the risk of conversion to systemic disease is low and mortality is low.7 This may be due to the regional prognostic variations of HVLPD, with Asian populations having a worse prognosis and a faster rate of disease progression. The prognosis is also somewhat impacted by the fact that HVLPD is more common in Asia.12 Our mean age was greater than what is frequently reported in studies (19.71 vs 5 or 8 or 11.6),7,23 since the patients in our study comprised both adults and children. It has been reported that adults have a worse prognosis than children. In our study, there were five adult patients, and two of the three patients who passed away were adult patients. As a result, the high percentage of adults could be one factor contributing to the poor prognosis in our case study.7 Facial edema was another important clinical feature in this study, and more importantly, all three patients who died showed this clinical feature. Therefore, this suggests that facial edema may be associated with a poor prognosis in HVLPD patients. Previous studies also supported the hypothesis that facial edema is usually associated with systemic or severe HVLPD and can be used as a clinical indicator of poor prognosis in patients associated with this disease.24 In addition, in our study, 3 patients with systemic HVLPD had undergone bone marrow aspiration, completed a bone marrow diagnosis, and evaluated T cell atypia. Only one patient had significantly reduced myelodysplasia and no neoplastic features, and that patient progressed quickly and died briefly. The other two patients showed no significant abnormalities and one of them survived and the other died. The abnormality of the bone marrow puncture can indicate the severity of the patient’s disease. It is necessary to do a bone marrow puncture at the appropriate time to understand the condition of the patient’s hematopoietic system.
Hemoglobin levels were considerably lower in the deceased group than in the survival group, according to the laboratory report (P<0.05), demonstrating that low hemoglobin levels are a key indicator of a bad prognosis. Leukocyte, platelet, AST, ALT, fever, and hepato-splenic lymph node enlargement were not significantly different between the deceased group and the survival group (P>0.05), which may have something to do with the small number of cases we had. IgE in deceased group was higher than that in survival group, and the difference was approximately statistically significant (P=0.05). In addition to the one patient who had no lgE data, lgE levels dramatically increased in five other patients, and in the three patients who died, lgE levels reached 1000 IU/mL, which may be because of the inflammatory reaction caused by the EBV infection.25 This shows the necessity of paying attention to the level of lgE in clinical practice and may be a significant indicator of how the disease is progressing. The patient’s condition should be reevaluated for more intensive treatment if the IgE increase is very significant. In contrast, all of the patients in the deceased group had fever, spleen enlargement, increased platelets, AST, ALT, and lgE, suggesting that these factors may be a significant contributor to the development of the disease and its clinical manifestations. This is consistent with what has been reported in the literature that hemocytopenia (>2 series) and liver dysfunction are risk factors for poor outcomes in lymphoproliferative disorders caused by the EBV.26 This recommends that during clinical follow-up, the changes in blood routine and liver function should be observed and prompts action taken.
The patients in our study had histopathological characteristics that were typical of HVLPD, including lymphocyte infiltration of the deep dermis and subcutaneous adipose lobules and occasional mild lymphocytic atypia. Additionally, eosinophil infiltration is frequent, which may be due to the fact that EBV infection promotes the secretion of inflammatory factors after cell polarization, which increases eosinophil activation,25 and then promotes the rise of IgE. All patients had a strong positive expression of CD3 and a mild positive expression of CD4. In addition to a case of negativity, the CD8 expression of other patients was positive. With the exception of case 7, which lacked a sample, six patients had a focal positive expression of CD20. Of all the patients, only four showed a scattered positivity for CD56. This indicated that in our study, T cells were mainly infiltrated in the tissues of HVLPD patients, and only a small number of tissues had NK cells and B cells infiltrated, which was consistent with previous research reports.22,27 With the exception of case 7, all of the other patients had positive CD4 and CD8 expressions, but CD8 positivity was more pronounced than CD4 positivity, showing that HVLPD patients were more susceptible to the infiltration of killer T cells when infected with EBV, which would improve tissue immune response and increase resistance to EBV infection.28 TIA-1 was positive in all 6 patients involved in the study and strongly positive in 3 deceased patients. This may be related to TIA-1 promoting apoptosis, necrosis, and disease progression, and the positive cytotoxic T cell protein further indicates the degree of pathological damage in the deceased patients.29 The positive degree of TCRβF1 can reflect the infiltration of αβT cells, and the positive degree of TCRδ can reflect the infiltration of γδT cells.30 The total positivity of TCRβF1 and total negativity of TCRδ also indicate that αβT cells are the dominant lymphocyte subclass infiltrated in our cases’ tissues. In addition, the range of the Ki67 proliferation index in each case was between 5% and 40%, with the three patients who passed away having a Ki67 index of 40%. This implies that the clinical invasive activity of HVLPD may be correlated with the cell proliferation marker Ki67.24
All 7 patients tested positive for EBER in situ hybridization in skin tissue sections, demonstrating that cells harboring the EBV would be recruited to the lesions.31 Patients with a poor prognosis are often strongly EBER-positive. The 3 patients in our study who passed away all had EBERs that were strongly positive, suggesting that the strength of an EBER’s positivity may be a good indicator of the severity of an Epstein-Barr virus infection. T-cell monoclonality is rare in typical HV, so TCR gene rearrangement may be useful in detecting atypical HV and adverse progression such as lymphoma, especially in combination with atypical or invasive clinical manifestations.3 In addition, Xie et al found that all patients with monoclonal TCR rearrangement died, suggesting that TCR gene rearrangement can be used as a prognostic indicator and auxiliary diagnostic tool.24 This is similar to the results of our study, in which patients with monoclonal TCR rearrangement all died while patients without TCR rearrangement had a good prognosis. And the three patients who died had systemic HVLPD.
Our study found that the plasma EBV DNA levels in dead cases were typically higher than those in living HVLPD patients. This finding was consistent with reports in the literature and suggested that a high plasma EBV DNA level would make it easier for patients to develop systemic HVLPD and give them a worse prognosis.19,24,32 The lack of a statistically significant difference in EBV DNA of PBMC between the deceased group and the survival group may have been caused by the fact that all patients had high EBV DNA of PBMC and that there were not a sufficient number of patients to make a difference. Furthermore, there was no correlation between the EBV DNA in patients’ PBMC and in patients’ plasma (P>0.05). Plasma EBV DNA is substantially more sensitive than PBMC to discriminate between the illness activation state and treatment response, according to relevant literature data.33 Therefore, the assessment of EBV infection should be combined with the EBER in situ hybridization test and EBV DNA detection in plasma or PBMC, but priority should be given to the plasma EBV DNA in the assessment of the disease. Plasma IFN-γ and TNF-α cytokine levels in all HVLPD patients were greater than those in the control group (P<0.05), showing that EBV infection is associated with a series of pro-inflammatory cytokine responses in HVLPD. IFN-γ and TNF-α are pro-inflammatory cytokines that promote nitric oxide (NO) synthesis in macrophages, are related with the T cell response, and also signal the severity of symptoms.34 IFN-γ and TNF-α cytokine levels were higher in the deceased group than in the control group (P<0.05), showing that they can reflect the severity of the disease and indicate a poor prognosis. As a result, it is critical to pay attention to the rise of these two markers in clinical practice, as well as timely focused treatment. IL-4, IL-10 and IL-13 cytokine levels of HVLPD patients showed no significant statistical significance compared with the control group (P>0.05), indicating that HVLPD produced less anti-inflammatory cytokines and more pro-inflammatory cytokines in immunity. In addition, in HVLPD cases, the difference between the survival group and the deceased group was not statistically significant, possibly because IFN-γ, TNF-α, IL-4, IL-10 and IL-13 cytokine levels in the two groups of patients had certain values and were higher than those of control group, so the comparison was meaningless. In addition, it is also related to the insufficient number of cases in this study.
At present, there is not a definite treatment protocol for HVLPD. All of our patients received conservative therapy, each with a unique therapeutic outcome, including glucocorticoids, immunomodulatory drugs, and antiviral medications. After treatment, three patients developed no new skin lesions; one patient survived the condition, but the other three passed away from systemic symptoms and the disease’s quick progression in the final stages. Additionally, there has been a recent advancement in the study of HVLPD treatment. Chinese researchers recommended conservative treatment for the majority of Chinese patients and advised against using chemotherapy as the first choice of treatment after examining 41 cases of HVLPD.27 However, studies in the United States have shown that haematopoieticstem cell transplantation (HSCT) can be used for HVLPD patients who have failed or relapsed after immune regulation and other treatments, which may cure HVLPD patients.35
This study has some limitations in terms of experimental samples. Due to the rarity of the disease, the sample size of the current study is very limited. Therefore, larger samples are needed to further confirm the results according to the current experimental results, technical basis and design ideas of this study.
Conclusion
In conclusion, clinical signs, pathological characteristics, TCR gene rearrangement, EBER in situ hybridization, and EBV DNA detection must all be taken into account while making the diagnosis of HVLPD according to our findings. Fever, hepatosplenomegaly, lymphadenopathy, decreased hemoglobin level, a significant increase in lgE level, plasma EBV DNA, IFN-γ, and TNF-α cytokine levels, strong positive expression of TIA-1, high Ki67 proliferation index, and positive TCR gene rearrangement are all indicators of a poor prognosis. Patients’ treatments should be dependent on the course of their disease as well as test markers. And HSCT should be prioritized and enhanced. However, because HVLPD cases around the globe are not only uncommon but also have a high rate of misdiagnosis, missed diagnosis, and malignant transformation, large-sample studies are lacking. As a result, HVLPD must continue to draw the attention of clinicians and pathologists, as well as improve its diagnostic and treatment levels through ongoing, in-depth study.
Abbreviations
HVLPD, hydroa vacciniforme-like lymphoproliferative disorder; TCR gene rearrangement, T-cell receptor gene rearrangement; EBV, Epstein-Barr virus; NK, natural killer; T cells, T lymphocytes; HV, hydroa vacciniforme; HVLL, hydroa vacciniforme-like lymphoma; Th1 lymphocytes, T helper type 1 lymphocytes; Th2 lymphocytes, T helper type 2 lymphocytes; EBER, Epstein-Barr virus-encoded RNA; PBMC, Peripheral Blood Mononuclear Cell; HB, hemoglobin; PLT, platelets; AST, aspartate transaminase; ALT, alanine transaminase; IgE, Immunoglobulin E.
Data Sharing Statement
The datasets used and/or analysed during the current study are available from the corresponding author.
Ethics Approval and Informed Consent
This study involved anonymous use of patients’ clinical data, pathological data, tissue wax blocks and blood samples, and patients were not identified during data collection and experiment. The study does not affect patient health or privacy. The study was conducted in accordance with the ethical standards of the Declaration of Helsinki and has been approved by the Ethics Committee of the First Affiliated Hospital of Guangxi Medical University, numbered as follows:NO.2022-S007-01.
Consent for Publication
The study has confirmed that details of all images, clinical data and experimental data are publishable, and the purpose and content of the study have been detailed to those who have provided consent. If requested, corresponding author may provide copies of signed consent forms to the journal editorial office.
Acknowledgments
The authors wish to thank the patients and their families for making this study possible. We would like to express our gratitude to all participants involved in this study. In addition, we would also like to thank our families and the people who helped us for their support.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Han B, Hur K, Ohn J, et al. Hydroa vacciniforme-like lymphoproliferative disorder in Korea. Sci Rep. 2020;10(1):19294. doi:10.1038/s41598-020-76345-2
2. Eminger LA, Hall LD, Hesterman KS, et al. Epstein-Barr virus: dermatologic associations and implications: part II. Associated lymphoproliferative disorders and solid tumors. J Am Acad Dermatol. 2015;72(1):21–34; quiz 35–6. doi:10.1016/j.jaad.2014.07.035
3. Quintanilla-Martinez L, Ridaura C, Nagl F, et al. Hydroa vacciniforme-like lymphoma: a chronic EBV+ lymphoproliferative disorder with risk to develop a systemic lymphoma. Blood. 2013;122(18):3101–3110. doi:10.1182/blood-2013-05-502203
4. Sundram U. Cutaneous lymphoproliferative disorders: what’s new in the revised 4th edition of the World Health Organization (WHO) classification of lymphoid neoplasms. Adv Anat Pathol. 2019;26(2):93–113. doi:10.1097/PAP.0000000000000208
5. Gupta G, Man I, Kemmett D. Hydroa vacciniforme: a clinical and follow-up study of 17 cases. J Am Acad Dermatol. 2000;42(2 Pt 1):208–213. doi:10.1016/S0190-9622(00)90127-0
6. Chen CC, Chang KC, Medeiros LJ, et al. Hydroa vacciniforme and hydroa vacciniforme-like lymphoproliferative disorder: a spectrum of disease phenotypes associated with ultraviolet irradiation and chronic Epstein-Barr virus infection. Int J Mol Sci. 2020;21(23):9314. doi:10.3390/ijms21239314
7. Cohen JI, Manoli I, Dowdell K, et al. Hydroa vacciniforme-like lymphoproliferative disorder: an EBV disease with a low risk of systemic illness in whites. Blood. 2019;133(26):2753–2764. doi:10.1182/blood.2018893750
8. Chen CC, Chang KC, Medeiros LJ, et al. Hydroa vacciniforme and hydroa vacciniforme-like T-cell lymphoma: an uncommon event for transformation. J Cutan Pathol. 2016;43(12):1102–1111. doi:10.1111/cup.12801
9. Campo E, Swerdlow SH, Harris NL, et al. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood. 2011;117(19):5019–5032. doi:10.1182/blood-2011-01-293050
10. Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–2390. doi:10.1182/blood-2016-01-643569
11. Kim WY, Montes-Mojarro IA, Fend F, et al. Epstein-Barr virus-associated T and NK-cell lymphoproliferative diseases. Front Pediatr. 2019;7:71. doi:10.3389/fped.2019.00071
12. Wang X, Wang P, Wang A, et al. Hydroa Vacciniforme-like Lymphoproliferative disorder in an adult invades the liver and bone marrow with clear pathological evidence: a case report and literature review. BMC Infect Dis. 2021;21(1):17. doi:10.1186/s12879-020-05697-x
13. Guo N, Chen Y, Wang Y, et al. Clinicopathological categorization of hydroa vacciniforme-like lymphoproliferative disorder: an analysis of prognostic implications and treatment based on 19 cases. Diagn Pathol. 2019;14(1):82. doi:10.1186/s13000-019-0859-4
14. Nahhas AF, Oberlin DM, Braunberger TL, et al. Recent developments in the diagnosis and management of photosensitive disorders. Am J Clin Dermatol. 2018;19(5):707–731. doi:10.1007/s40257-018-0365-6
15. Miyake T, Yamamoto T, Hirai Y, et al. Survival rates and prognostic factors of Epstein-Barr virus-associated hydroa vacciniforme and hypersensitivity to mosquito bites. Br J Dermatol. 2015;172(1):56–63. doi:10.1111/bjd.13411
16. Miyake T, Iwatsuki K, Hirai Y, et al. The AIM of the measurement of Epstein-Barr virus DNA in hydroa vacciniforme and hypersensitivity to mosquito bites. J Med Virol. 2020;92:3689–3696. doi:10.1002/jmv.25811
17. Van Tong H, Brindley PJ, Meyer CG, et al. Parasite infection, carcinogenesis and human malignancy. EBioMedicine. 2017;15:12–23. doi:10.1016/j.ebiom.2016.11.034
18. Spellberg B, Edwards JE. Type 1/type 2 immunity in infectious diseases. Clin Infect Dis. 2001;32(1):76–102. doi:10.1086/317537
19. Iwatsuki K, Satoh M, Yamamoto T, et al. Pathogenic link between hydroa vacciniforme and Epstein-Barr virus-associated hematologic disorders. Arch Dermatol. 2006;142(5):587–595. doi:10.1001/archderm.142.5.587
20. Kimura H, Ito Y, Kawabe S, et al. EBV-associated T/NK-cell lymphoproliferative diseases in nonimmunocompromised hosts: prospective analysis of 108 cases. Blood. 2012;119(3):673–686. doi:10.1182/blood-2011-10-381921
21. Sabattini E, Bacci F, Sagramoso C, et al. WHO classification of tumours of haematopoietic and lymphoid tissues in 2008: an overview. Pathologica. 2010;102(3):83–87.
22. Magaña M, Massone C, Magaña P, et al. Clinicopathologic features of hydroa vacciniforme-like lymphoma: a series of 9 patients. Am J Dermatopathol. 2016;38(1):20–25. doi:10.1097/DAD.0000000000000385
23. Garzón E, Dávila-Rodríguez JJ. Hydroa vacciniforme-like lymphoproliferative disorder in Ecuadorian children: a case series. Indian J Dermatol Venereol Leprol. 2021;1–5. doi:10.25259/IJDVL_847_19
24. Xie Y, Wang T, Wang L. Hydroa vacciniforme-like lymphoproliferative disorder: a study of clinicopathology and whole-exome sequencing in Chinese patients. J Dermatol Sci. 2020;99(2):128–134. doi:10.1016/j.jdermsci.2020.06.013
25. Andrea M, Susanna B, Francesca N, et al. The emerging role of type 2 inflammation in asthma. Expert Rev Clin Immunol. 2021;17(1):63–71. doi:10.1080/1744666X.2020.1860755
26. Paik JH, Choe JY, Kim H, et al. Clinicopathological categorization of Epstein-Barr virus-positive T/NK-cell lymphoproliferative disease: an analysis of 42 cases with an emphasis on prognostic implications. Leuk Lymphoma. 2017;58(1):53–63. doi:10.1080/10428194.2016.1179297
27. Liu Y, Ma C, Wang G, et al. Hydroa vacciniforme-like lymphoproliferative disorder: clinicopathologic study of 41 cases. J Am Acad Dermatol. 2019;81(2):534–540. doi:10.1016/j.jaad.2019.01.011
28. Reina-Campos M, Scharping NE, Goldrath AW. CD8(+) T cell metabolism in infection and cancer. Nat Rev Immunol. 2021;21(11):718–738. doi:10.1038/s41577-021-00537-8
29. Au WY, Weisenburger DD, Intragumtornchai T, et al. Clinical differences between nasal and extranasal natural killer/T-cell lymphoma: a study of 136 cases from the International Peripheral T-Cell Lymphoma Project. Blood. 2009;113(17):3931–3937. doi:10.1182/blood-2008-10-185256
30. Vega F, Medeiros LJ. A suggested immunohistochemical algorithm for the classification of T-cell lymphomas involving lymph nodes. Hum Pathol. 2020;102:104–116. doi:10.1016/j.humpath.2020.05.006
31. Mai ZM, Lin JH, Ngan RK, et al. Solar ultraviolet radiation and vitamin D deficiency on Epstein-Barr virus reactivation: observational and genetic evidence from a nasopharyngeal carcinoma-endemic population. Open Forum Infect Dis. 2020;7(10):ofaa426. doi:10.1093/ofid/ofaa426
32. Qiu LH, Li YY, Zheng YX, et al. EBV-DNA拷贝数在EBV+淋巴瘤患者中的临床意义[Clinical significance of EBV-DNA copy number in EBV positive lymphoma patients]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2021;29(6):1785–1789. Chinese. doi:10.19746/j.cnki.issn.1009-2137.2021.06.016
33. Berth M, Vanheule G, Depuydt C, et al. Serum Epstein-Barr virus (EBV) viral load can be a complementary sensitive test in primary Epstein-Barr virus infection. J Clin Virol. 2011;50(2):184–185. doi:10.1016/j.jcv.2010.11.002
34. Budiningsih I, Dachlan YP, Hadi U, et al. Quantitative cytokine level of TNF-α, IFN-γ, IL-10, TGF-β and circulating Epstein-Barr virus DNA load in individuals with acute Malaria due to P. falciparum or P. vivax or double infection in a Malaria endemic region in Indonesia. PLoS One. 2021;16(12):e0261923. doi:10.1371/journal.pone.0261923
35. Pillai V, Tallarico M, Bishop MR, et al. Mature T- and NK-cell non-Hodgkin lymphoma in children and young adolescents. Br J Haematol. 2016;173(4):573–581. doi:10.1111/bjh.14044
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.