Back to Journals » OncoTargets and Therapy » Volume 13
Clinical and Molecular Characterization of Incidentally Discovered Lower-Grade Gliomas with Enrichment of Aerobic Respiration
Authors Wang QW, Wang YW, Wang ZL, Bao ZS , Jiang T, Wang Z, You G
Received 6 February 2020
Accepted for publication 9 April 2020
Published 25 September 2020 Volume 2020:13 Pages 9533—9542
DOI https://doi.org/10.2147/OTT.S248623
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Sanjay Singh
Qiang-Wei Wang,1,* Yi-Wen Wang,2,* Zhi-Liang Wang,1 Zhao-Shi Bao,3 Tao Jiang,3– 5 Zheng Wang,3 Gan You3
1Beijing Neurosurgical Institute, Capital Medical University, Beijing, People’s Republic of China; 2Huadong Medical Institute of Biotechniques, Nanjing, People’s Republic of China; 3Department of Neurosurgery, Beijing Tiantan Hospital, Capital Medical University, Beijing, People’s Republic of China; 4China National Clinical Research Center for Neurological Diseases, Beijing, People’s Republic of China; 5Center of Brain Tumor, Beijing Institute for Brain Disorders, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Gan You
Department of Neurosurgery, Beijing Tiantan Hospital, Capital Medical University, No. 119 South Fourth Ring West Road, Fengtai District, Beijing 100070, People’s Republic of China
Tel/Fax +86-10-59976785
Email [email protected]
Purpose: Incidentally discovered diffusely infiltrating lower-grade gliomas (incidental LGGs, iLGGs) are defined as gliomas occasionally found in patients without tumor-related symptoms. At present, very few in-depth research studies on incidental LGGs were reported. We aimed to find out the inherent difference between iLGGs and LGGs with tumor-related symptoms.
Patients and Methods: We enrolled 2486 all-grade gliomas and screened 1594 lower-grade gliomas for further analysis. Medical records were retrospectively reviewed for iLGGs. Clinical and mRNA sequencing data were collected for in-depth analysis.
Results: We found that with increasing grade, the proportion of incidental glioma patients decreased obviously. In 1594 patients who underwent craniotomy for LGG, 80 (5%) patients were discovered incidentally. Grade II patients (88%) and patients bearing 1p/19q co-deletion in their tumors (23%) were more likely to be diagnosed as iLGGs. Regular radiological screening (48%) and trauma (24%) were the main complaint for brain imaging for iLGGs. Kaplan–Meier survival analysis indicated that iLGGs patients lived a significantly longer survival and Cox regression analysis revealed that iLGGs were an independent indicator of better prognosis. Subsequent gene set enrichment analysis and differential expression analysis based on the gene expression profile revealed that mitochondrial aerobic respiration process was enriched in iLGGs. Moreover, we found that iLGGs tended to generate energy by unique mitochondrial aerobic respiration.
Conclusion: These results provided a primitive exploration of iLGGs, which may potentially assist clinical neurosurgeons with personalized management of iLGGs.
Keywords: glioma, lower-grade gliomas, incidental, survival, aerobic respiration
Introduction
Glioma is the most prevalent and fatal primary cancer originated from the central nervous system (CNS).1,2 Patients with lower-grade gliomas (LGGs, WHO Grade II/III) have a median overall survival of 7 years while patients with glioblastomas (GBM, WHO Grade IV) only have a median overall survival of 15 months, despite treatment with surgery, radiation and/or chemotherapy.3,4 Although patients with LGG survive much longer than those with GBM, nearly all LGGs would eventually progress to secondary GBM.5 The Statistics of Surveillance, Epidemiology and End Results (SEER) program showed that overall survival of LGG patients has had no significant improvement since 1973.6
Most patients who suffered from glioma came to their doctors with initial symptoms such as headache, vomiting, seizure and so on. However, a small amount of glioma patients came to their doctors with few clinical manifestations. Incidental lower-grade gliomas (iLGGs) are LGGs without tumor-related symptoms. Incidental detection may result from regular screening or complaint of trauma, dizziness, atypical headaches,7,8 etc. With the development and popularization of imaging technology, the discovery rate and diagnosis rate of iLGGs increased greatly.9 However, management of iLGGs is still one of the most controversial areas in clinical neuro-oncology because of the poor understanding and less relevant literature.10
Heretofore, only a few studies described incidental gliomas of WHO Grade II7,8,11 and the number of cases in these studies was limited. To further explore the characteristics of iLGGs, we retrospectively reviewed 1594 LGG patients treated in two hospitals and analyzed the clinical and molecular features of iLGGs. Our study is conducive to comprehensive understanding of iLGGs and provides enlightenment for the clinical personal management of iLGGs. This is the first integrative study characterizing incidental gliomas of WHO Grade II and III molecularly and clinically.
Patients and Methods
Clinical Characteristics of Patients
We retrospectively reviewed patients with all-grade gliomas who underwent surgery in Beijing Tiantan Hospital and Beijing Sanbo Brain Hospital between January 2006 and May 2018. Written informed consent was provided for all patients and our research was approved by the Beijing Tiantan Hospital Capital Medical University Institutional Review Board (IRB KY2013-017-01).12 Clinical information (including age at diagnosis, gender, presenting symptoms, extent of resection, etc.) was obtained from electronic medical records including resident admit notes, discharge notes, operative report, etc. Meanwhile, the glioma grade and molecular pathological diagnosis (including IDH, and 1p/19q co-deletion status) were established by two neuropathologists according to 2016 WHO CNS tumor guidelines.13 It was common to employ a pyrosequencing technique to detect IDH mutation in CGGA.14 “Incidental” gliomas are detected in brain imaging that was checked for complaint not owing to the tumor, such as screening, trauma, dizziness, headache without related occupied effect or intracranial hypertension, or research purposes. Lower-grade gliomas, ranging from WHO Grade II to III, were included for further research.
Survival and Follow-Up
Postoperative follow-up was achieved via subsequent visit or telephone. The period starting from resection to death or the last follow-up was overall survival (OS) by definition. Progression-free survival (PFS) started from resection to progression in imaging.
Gene Set Enrichment Analysis (GSEA) and Gene Ontology (GO)
We collected mRNA sequencing data of 190 samples of lower-grade glioma from the Chinese Glioma Genome Atlas (CGGA) database, generated with the Illumina HiSeq platform.15 GSEA software (http://software.broadinstitute.org/gsea/index.jsp)16 was run for gene set enrichment analysis. We filtered differently expressed genes (fold change >2, FDR <0.05) between 17 iLGG and 173 niLGG patients in the CGGA dataset. Then, up-regulated genes in iLGGs were chosen to perform gene ontology (GO) analysis in DAVID. (https://david.ncifcrf.gov/)
Statistical Analysis
R language (version 3.5.1, http://www.r-project.org) was the main software environment for our statistical operation and graphics.17 Difference in clinicopathological characteristics between iLGG and non-incidental lower-grade glioma (niLGG) patients was assessed with Student’s t-test or chi-squared test. The Kaplan–Meier estimate was performed in survival analysis with two-sided log rank test. We further evaluate the prognostic role of clinicopathological factors with univariate and multivariate Cox regression analysis. The rest of the figures were drawn with some R packages, such as survminer, gglpot2 and Hmisc. It was considered to be statistically significant when a p value was less than 0.05.
Results
Incidentally Discovered Gliomas Were More Common in Lower Grade Tumors
Two thousand four hundred and eighty-six glioma patients were enrolled into our research totally, who were treated in Beijing Tiantan Hospital and Beijing Sanbo Brain Hospital between January 2006 and May 2018. In our cohort, 928 (37.3%) patients were diagnosed with WHO Grade II glioma, 666 (26.8%) patients with Grade III and 892 (35.9%) patients with Grade IV (Table 1). Among them, we found 70 (7.54%) incidental gliomas (iGs) in Grade II glioma, 10 (1.50%) iGs in Grade III glioma and 14 (1.57%) iGs in Grade IV glioma. With the increase of grade, the proportion of incidental glioma patients decreased obviously (Figure S1). Early tumor symptoms of higher-grade tumor patients are more susceptible to be noticed, leading to incidental gliomas being less common in these tumors.
 |
Table 1 Distribution of Incidental Gliomas in All Grades of Glioma |
Characteristics of Patients with Incidental Lower-Grade Gliomas
To further study incidental gliomas, we focused on 1594 lower-grade patients (WHO Grade II/III). The main characteristics of these patients are shown in Figure 1 and summarized in Table 2. Eighty patients (5.0%) meeting the criteria were included for iLGGs. In these iLGGs, the median age was 41 (from 14 to 63) years. Forty-three (54%) patients were male. Between iLGGs and niLGGs, there was no significant difference in the tumor location (p>0.05) and extent of tumor resection (p>0.05). Among iLGGs, 28 patients were IDH mutant, and there was no significant difference from niLGGs (p>0.05). Compared with niLGGs, there were more Grade II patients (88%, p<0.001) and 1p/19q co-deletion (23%, p<0.05) in iLGGs, and only a small percentage of patients (21%, p<0.001) received postoperative chemotherapy.
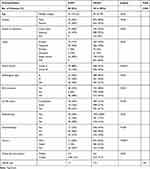 |
Table 2 Baseline Patient Characteristics |
 |
Figure 1 The main clinical and pathological characteristics of 1594 lower-grade patients (80 iLGGs and 1514 niLGGs). |
Radiological screening (48%, n=38) was the top complaint of iLGGs, followed by trauma (24%, n=19), dizziness (19%, n=15), headaches (6%, n=5) and others (4%, n=3) (Table 3). Dizziness and headaches were chronic and did not increase gradually. Moreover, nausea or vomiting because of tumor affect did not accompany, and there was no mass effect like edema in imaging.
 |
Table 3 The Discovery Mode of 80 iLGGs |
Incidental Lower-Grade Glioma Patients Had a Better Prognosis
To explore the survival difference between two groups of patients, we performed Kaplan–Meier survival analysis. In the two groups, survival data were available for 54 and 1299 patients, respectively. As shown in Figure 2A and D, iLGG patients lived a significantly longer survival (OS and PFS) than niLGG patients (p<0.05). While in WHO grade II (Figure 2B and E) and grade III (Figure 2C and F), Kaplan–Meier curves showed a similar pattern. Considering the effect of molecular characteristics and surgery on the prognosis of patients, we divided LGG into groups according to IDH (mutant and intact, Figure S2A), 1p/19q (co-deletion and intact, Figure S2B) and extent of resection (total and subtotal, Figure S2C), and found that the survival trend of the iLGGs was better in all groups.
To further assess the prognostic value of grouping, we conducted multivariate Cox regression analysis, adjusting previous widely accepted prognostic and predictive factors (gender, age, resection, grade). As shown in univariate analysis, age, grade and group were associated with survival (Table 4, p<0.01). After adjusting with age and grade, group was still associated with survival significantly (p<0.01). These results indicated that iLGGs served as an independent prognostic factor.
 |
Table 4 Univariate- and Multivariate Cox Regression Analyses for OS |
Altered Functional Characteristics in iLGGs
In order to further investigate the altered functional characteristics in iLGGs, we performed gene set enrichment analysis (GSEA) between iLGGs and niLGGs. We have collected mRNA sequencing data of 190 lower-grade glioma samples from the CGGA database, comprising 17 iLGGs and 173 niLGGs. Surprisingly, GSEA results showed that GO terms related to mitochondrial aerobic respiration were enriched in iLGGs, including “mitochondrial respiratory chain complex assembly” (NES=1.637, p=0.019), “inner mitochondrial membrane protein complex” (NES=1.652, p=0.020), “respiratory chain” (NES=1.580, p=0.021) and “electron transport chain” (NES=1.702, p=0.077, Figure 3). In Figure S3, the heatmap shows the enrichment of Go terms related to mitochondrial aerobic respiration in iLGGs compared to niLGGs.
 |
Figure 3 Altered functional characteristics in iLGGs (A–D). GO terms related to mitochondrial aerobic respiration were enriched in iLGGs. |
Meanwhile, we compared the expression difference and calculated the fold change with FPKM value between iLGGs and niLGGs. Significantly altered genes (fold change >2 or <0.5, and FDR of Student t-test <0.05, Tables S1 and S2) were displayed in a volcano plot (Figure 4A). We chose the up-regulated genes (489 genes, fold change >2) for GO analysis in DAVID and detected enrichment in the biological process of mitochondrial respiratory chain complex I assembly (FDR=0.025, Benjamini=0.018), and mitochondrial electron transport, NADH to ubiquinone (FDR=0.039, Benjamini=0.014) (Figure 4B), which was consistent with aerobic respiration.
 |
Figure 4 Differential expression analysis between iLGGs and niLGGs. Volcano plot showed differently expressed genes (A). Bubble plot showed an upregulated biology process in iLGGs (B). |
Discussion
The incidental findings of brain imaging were defined as potential clinically relevant abnormalities that were not previously discovered, and these abnormalities were discovered incidentally, regardless of the purpose of the examination. iLGGs were usually diagnosed by investigating non-specific or unrelated symptoms, such as screening (48%), trauma (24%), dizziness (19%), headaches (6%) and others (4%). We found iLGGs accounting for 7.54% in our Grade II gliomas, a ratio similar to that reported in the literature (5%–6.8%).7,18 As more and more brain imaging techniques are used by clinicians and researchers, these incidental findings are occurring more frequently.19,20 Currently, there are no guidelines for the treatment of this group of patients and the proper management of iLGGs is controversial. Some advocate a traditional wait-and-see approach: surgical intervention until clinical symptoms or radiological evidence of growth manifest. However, numerous untreated iLGG patients have neuropsychological impairments, such as tiredness, attentional impairment, altered executive functions and working memory impairment.11 While some literature has reported that early resection of tumor may be beneficial to survival of incidental patients.7,21 For neurosurgeons and radiologists, it is a dilemma crossing medicine and ethics to determine the interventions after routine brain imaging. On the one hand, it may cause harm rather than symptom relief after receiving surgery; but on the other, patients who were treated with conservative observation may be exposed to the risk of tumor progression.
In this study, we retrospectively reviewed 1594 lower-grade gliomas and infiltered 80 incidental LGG patients for further study. We found that WHO Grade II and 1p/19q co-deletion were more common in iLGGs. Few iLGG patients received chemotherapy due to most iLGGs being resected totally. Survival analysis indicated that iLGG patients lived a significantly longer survival (OS and PFS) than niLGG patients and that iLGG was an independent indicator of better prognosis. Hence, our study verified survival benefit of the surgical management in incidental LGGs and as a management option for these people.
Aerobic glycolysis, also known as the “Warburg effect”, was defined as the process of converting glucose into lactic acid in an aerobic environment. Increasing activity of aerobic glycolysis characteristically exists in tumor.22 Aerobic glycolysis has the advantage of generating energy with high speed and providing biomass for synthesis of amino acids and fatty acids, meeting the need of rapid cell proliferation.23,24 Increased production of acids due to upregulation of glycolysis leads to microenvironmental acidosis, which promotes invasion by damaging nearby normal populations, degrading the extracellular matrix and promoting angiogenesis.25 Glioma also converts its energy metabolism into high levels of glycolysis to produce cellular ATP.26–28 However, functional analysis and differential expression analysis in our study revealed the enrichment of mitochondrial aerobic respiration in iLGG patients, contrary to symptomatic gliomas. This unique metabolic pattern in iLGGs was not conducive to the proliferation of tumor cells, resulting in slow growth of tumors, few symptoms and a better prognosis in patients.
In the tricarboxylic acid cycle, isocitrate dehydrogenase 1/2 (IDH1/2) catalyze isocitrate to α-KG. Mutations in Arg132 of IDH1 or Arg172 appear in over 80% of Grade II/III gliomas and secondary GBMs.29,30 The data of IDH mutant status were available for 34 iLGGs, including 28 (82%) IDH mutant iLGGs in our study. The coexistence of IDH mutation and aerobic respiration in iLGGs seem to be contradictory. But simultaneous mutations of IDH1 and IDH2 are rare in glioma cells and the common genotype is IDH1 mutation and IDH2 wild-type.31 Wild-type IDH2 could catalyze the oxidative decarboxylation of isocitrate in IDH1 mutant iLGGs as well. On the other hand, the main effect of IDH mutation is causing genome-wide methylation and globally increases the methylation of histones H3 and H4.32 And 85%–90% of GBMs do not harbor IDH mutations but favor higher rates of glycolysis for ATP generation.27,33,34 In conclusion, glioma glycolysis or aerobic respiration is not determined by IDH mutation status. Despite numerous IDH mutations in iLGGs, mitochondrial aerobic respiration is not affected.
Conclusions
In summary, we found iLGG patients had a significantly better survival and iLGG was a potential prognostic marker. Unlike other gliomas, iLGGs mainly adopted the metabolic mode of aerobic respiration. Our study extends the understanding of iLGGs and contributes to better clinical management of patients with this type of tumor.
Funding
This study was funded by National Natural Science Foundation of China (81902528); The National Key Research and Development Plan (No. 2016YFC0902500); Beijing Science and Technology Plan (Z141100000214009); Capital Medical Development Research Fund (2016-1-1072); National Natural Science Foundation of China (81903078); National Natural Science Foundation of China (81972337); and National Natural Science Foundation of China (81902528); National Natural Science Foundation of China (No. 81871013); Beijing Municipal Education Commission Science and Technology Plan General Project (No.1192050172); Beijing Tiantan Hospital Young Scientist Program (YSP201705); Key Program of Administration of Traditional Chinese Medicine, Zhejiang Province (No. 2018ZZ015); Science and Technology Department of Zhejiang Province (Grant No. 2007C33042); and Provincial Key R&D Program, Science and Technology Department of Zhejiang Province (Grant No. 2017C03018).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Lapointe S, Perry A, Butowski NA. Primary brain tumours in adults. Lancet. 2018;392(10145):432–446. doi:10.1016/S0140-6736(18)30990-5
2. Wang Y, Jiang T. Understanding high grade glioma: molecular mechanism, therapy and comprehensive management. Cancer Lett. 2013;331(2):139–146. doi:10.1016/j.canlet.2012.12.024
3. Van Meir EG, Hadjipanayis CG, Norden AD, Shu HK, Wen PY, Olson JJ. Exciting new advances in neuro-oncology: the avenue to a cure for malignant glioma. CA Cancer J Clin. 2010;60(3):166–193. doi:10.3322/caac.20069
4. Weller M, Cloughesy T, Perry JR, Wick W. Standards of care for treatment of recurrent glioblastoma–are we there yet? Neuro-Oncology. 2013;15(1):4–27. doi:10.1093/neuonc/nos273
5. Hu H, Mu Q, Bao Z, et al. Mutational landscape of secondary glioblastoma guides MET-targeted trial in brain tumor. Cell. 2018;175(6):1665–1678.e1618. doi:10.1016/j.cell.2018.09.038
6. Claus EB, Walsh KM, Wiencke JK, et al. Survival and low-grade glioma: the emergence of genetic information. Neurosurg Focus. 2015;38(1):E6. doi:10.3171/2014.10.FOCUS12367
7. Opoku-Darko M, Lang ST, Artindale J, Cairncross JG, Sevick RJ, Kelly JJP. Surgical management of incidentally discovered diffusely infiltrating low-grade glioma. J Neurosurg. 2018;129(1):19–26. doi:10.3171/2017.3.JNS17159
8. Potts MB, Smith JS, Molinaro AM, Berger MS. Natural history and surgical management of incidentally discovered low-grade gliomas. J Neurosurg. 2012;116(2):365–372. doi:10.3171/2011.9.JNS111068
9. Floeth FW, Sabel M, Stoffels G, et al. Prognostic value of 18F-fluoroethyl-L-tyrosine PET and MRI in small nonspecific incidental brain lesions. J Nucl Med. 2008;49(5):730–737. doi:10.2967/jnumed.107.050005
10. Keles GE, Lamborn KR, Berger MS. Low-grade hemispheric gliomas in adults: a critical review of extent of resection as a factor influencing outcome. J Neurosurg. 2001;95(5):735–745. doi:10.3171/jns.2001.95.5.0735
11. Cochereau J, Herbet G, Duffau H. Patients with incidental WHO grade II glioma frequently suffer from neuropsychological disturbances. Acta Neurochir. 2016;158(2):305–312. doi:10.1007/s00701-015-2674-3
12. Wang QW, Liu HJ, Zhao Z, et al. Prognostic correlation of autophagy-related gene expression-based risk signature in patients with glioblastoma. Onco Targets Ther. 2020;13:95–107. doi:10.2147/OTT.S238332
13. Louis DN, Perry A, Reifenberger G, et al. The 2016 world health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. doi:10.1007/s00401-016-1545-1
14. Wang Q, Wang Z, Bao Z, Zhang C, Wang Z, Jiang T. PABPC1 relevant bioinformatic profiling and prognostic value in gliomas. Future Oncol. 2020;16(1):4279–4288. doi:10.2217/fon-2019-0268
15. Li G, Wang Z, Zhang C, et al. Molecular and clinical characterization of TIM-3 in glioma through 1,024 samples. Oncoimmunology. 2017;6(8):e1328339. doi:10.1080/2162402X.2017.1328339
16. Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102(43):15545–15550. doi:10.1073/pnas.0506580102
17. Team RC. R: a language and environment for statistical computing. R foundation for statistical computing. Vienna, Austria; 2016.
18. Morris Z, Whiteley WN, Longstreth WT, et al. Incidental findings on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2009;339:b3016. doi:10.1136/bmj.b3016
19. Illes J, Rosen AC, Huang L, et al. Ethical consideration of incidental findings on adult brain MRI in research. Neurology. 2004;62(6):888–890. doi:10.1212/01.WNL.0000118531.90418.89
20. Mamourian A. Incidental findings on research functional MR images: should we look? AJNR Am J Neuroradiol. 2004;25(4):520–522.
21. Pallud J, Fontaine D, Duffau H, et al. Natural history of incidental World Health Organization grade II gliomas. Ann Neurol. 2010;68(5):727–733. doi:10.1002/ana.22106
22. Warburg O. Ueber den stoffwechsel der tumoren. London: Constable; 1930.
23. Yu L, Lu M, Jia D, et al. Modeling the genetic regulation of cancer metabolism: interplay between glycolysis and oxidative phosphorylation. Cancer Res. 2017;77(7):1564–1574. doi:10.1158/0008-5472.CAN-16-2074
24. Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–1033. doi:10.1126/science.1160809
25. Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4(11):891–899. doi:10.1038/nrc1478
26. Oudard S, Boitier E, Miccoli L, Rousset S, Dutrillaux B, Poupon MF. Gliomas are driven by glycolysis: putative roles of hexokinase, oxidative phosphorylation and mitochondrial ultrastructure. Anticancer Res. 1997;17(3c):1903–1911.
27. Agnihotri S, Zadeh G. Metabolic reprogramming in glioblastoma: the influence of cancer metabolism on epigenetics and unanswered questions. Neuro-Oncology. 2016;18(2):160–172. doi:10.1093/neuonc/nov125
28. Strickland M, Stoll EA. Metabolic reprogramming in glioma. Front Cell Dev Biol. 2017;5:43. doi:10.3389/fcell.2017.00043
29. Guo C, Pirozzi CJ, Lopez GY, Yan H. Isocitrate dehydrogenase mutations in gliomas: mechanisms, biomarkers and therapeutic target. Curr Opin Neurol. 2011;24(6):648–652. doi:10.1097/WCO.0b013e32834cd415
30. Chang C-M, Xu K, Shu H. The role of isocitrate dehydrogenase mutations in glioma brain tumors. In: Molecular Targets of CNS Tumors. London: In TechOpen; 2011:413–436.
31. Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360(8):765–773. doi:10.1056/NEJMoa0808710
32. Duncan CG, Barwick BG, Jin G, et al. A heterozygous IDH1R132H/WT mutation induces genome-wide alterations in DNA methylation. Genome Res. 2012;22(12):2339–2355. doi:10.1101/gr.132738.111
33. Oudard S, Arvelo F, Miccoli L, et al. High glycolysis in gliomas despite low hexokinase transcription and activity correlated to chromosome 10 loss. Br J Cancer. 1996;74(6):839–845. doi:10.1038/bjc.1996.446
34. Zhou Y, Zhou Y, Shingu T, et al. Metabolic alterations in highly tumorigenic glioblastoma cells: preference for hypoxia and high dependency on glycolysis. J Biol Chem. 2011;286(37):32843–32853. doi:10.1074/jbc.M111.260935
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

