Back to Journals » Cancer Management and Research » Volume 11
Clinical And Imageological Features Of Lung Squamous Cell Carcinoma With EGFR Mutations
Authors Gao X, Zhu J, Chen L, Jiang Y, Zhou X, Shuai J, Zhao Y
Received 12 July 2019
Accepted for publication 18 September 2019
Published 21 October 2019 Volume 2019:11 Pages 9017—9024
DOI https://doi.org/10.2147/CMAR.S223021
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Lu-Zhe Sun
Xuejuan Gao,1,* Junjie Zhu,2,* Linsong Chen,2,* Yan Jiang,2 Xiao Zhou,2 Jianwei Shuai,1,3 Yanfeng Zhao2
1Department of Physics, Xiamen University, Xiamen, People’s Republic of China; 2Department of Thoracic Surgery, Shanghai Pulmonary Hospital Affiliated to Tongji University, Shanghai, People’s Republic of China; 3State Key Laboratory of Cellular Stress Biology, Innovation Center for Cell Signaling Network, Xiamen University, Xiamen, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jianwei Shuai
Department of Physics, Xiamen University, Xiamen 361005, People’s Republic of China
Tel +86 139 5928 7814
Email [email protected]
Yanfeng Zhao
Department of Thoracic Surgery, Shanghai Pulmonary Hospital affiliated to Tongji University, Shanghai 200433, People’s Republic of China
Tel +86 130 1281 4641
Email [email protected]
Purpose: To analyze the distribution of epidermal growth factor receptor (EGFR) mutations; characterize the clinical and imageological features of lung squamous cell carcinoma (LSCC) in a large population of patients; and assess correlations between clinical and imageological characteristics and clinical outcomes of LSCC patients harboring EGFR mutations.
Patients and methods: Three pathologists retrospectively evaluated the morphological and immunohistochemical data of 2,322 patients with LSCC resected between February 2013 and December 2017. Data on the distribution of EGFR mutations and the clinical and imageological characteristics of the patients were retrospectively collected. Correlations between the EGFR mutation status and clinical outcomes were evaluated using univariate and multivariate analyses.
Results: EGFR mutations were found in 3.4% of patients with LSCC and predominantly in female and non-smoking patients. Tumor lesions in patients with EGFR-positive mutations were more irregularly shaped than those in patients with EGFR-negative mutations (P = 0.045). In non-smoking patients with LSCC, the proportion of marked spiculation was significantly higher in the EGFR-positive group than in the EGFR-negative group (P = 0.043). No significant difference in recurrence-free survival was noted between LSCC patients harboring EGFR-positive and those harboring EGFR-negative mutations. No difference in metastases was observed between the EGFR-positive and EGFR-negative cohorts.
Conclusion: Female gender, non-smoking habit, irregularly shaped tumor, and marked spiculation might predict the presence of EGFR mutations in LSCC. The administration of tyrosine kinase inhibitors to patients with LSCC after screening for EGFR mutations based on their clinical and imageological features would likely result in a population with a greater sensitivity to afatinib.
Keywords: EGFR mutation, lung squamous cell carcinoma, imageological feature, recurrence-free survival
Introduction
Lung squamous cell carcinoma (LSCC) represents the second commonest histological type of non-small cell lung cancer (NSCLC).1 Despite the decrease in the incidence of LSCC in recent years, LSCC accounts for an estimated 30% and 20% of lung cancers in men and women, respectively; approximately 2100,000 new cases are reported worldwide each year.2–4 LSCC is highly associated with cigarette smoking, and the majority of patients with LSCC are either current or former heavy smokers.5 Therefore, it is not surprising that the genomic mutational profiles of LSCC reflect genomic complexities and high overall mutational loads, which are expected in tobacco carcinogenesis.3
Epidermal growth factor receptor (EGFR) is the most commonly mutated proto-oncogene in non-squamous NSCLC, with typical mutation rates of approximately 10–15% in Caucasians and up to 50% in Asians.6–9 Targeting EGFR with tyrosine kinase inhibitors (TKIs) has become the cornerstone for the management of advanced non-squamous NSCLCs harboring activating mutations of the EGFR gene.
However, genomic alterations in LSCC have not been completely characterized so far. The most frequent somatic mutations and alterations in LSCC have been identified in TP53, PIK3CA, FGFR1, MET, and DDR2, and none of these biomarkers have been validated as predictive for the particular targeted therapies.3,10 Platinum-based chemotherapy continues to remain the first-line treatment for LSCC owing to the lack of an effective targeted therapy for this disease.11
Although activating mutations of EGFR are uncommon in LSCC, patients with the genetic mutations of this subtype might benefit from EGFR-TKI-targeted therapies with lower side effects and toxicities than those of chemotherapy, thus highlighting the benefit of EGFR mutation status identification in patients with LSCC.12
Cumulative epidemiologic studies have identified several clinicopathological factors such as gender, smoking habits, histology of adenocarcinoma (ADC), and ethnicity that may be associated with a high prevalence of EGFR mutations.13–15 In addition, other tumor imageological characteristics and biological parameters may have a predictive effect on the EGFR mutation status in lung ADC.15,16 Unfortunately, the distribution of EGFR mutations in LSCC is poorly investigated, and the imageological features related to EGFR mutations in LSCC remain unclear. Therefore, in this study, we aimed to analyze the distribution of EGFR mutations and the clinical and morphological features of a large population of LSCC patients who underwent therapeutic resection and adjuvant chemotherapy post-surgery. Additionally, we assessed the correlations between clinical and imageological characteristics and the clinical outcome of LSCC patients with EGFR mutations.
Methods
Patient Cohort
All patients with solitary LSCC who underwent surgical resection at the Shanghai Pulmonary Hospital, affiliated to the Tongji University in China, between February 2013 and December 2017 were examined. A total of 2,322 patients were included in the study. All tumors were classified according to the 2015 World Health Organization classification and staged according to the seventh edition of the TNM system. The TNM stages include three components: primary tumor (T), nodal status for metastasis (N), and metastasis at distant organs (M). Written informed consents were obtained from all the patients, and the study was approved by the Institutional Review Board at the Shanghai Pulmonary Hospital.
Histologic Evaluation And Confirmation
Hematoxylin and eosin (H&E)-stained sections of the tumor were blindly reviewed by three experienced pulmonary pathologists. Immunohistochemical (IHC) staining was performed to exclude mixed and inconspicuous ADC components. The lung tissue sections were deparaffinized three times with xylene and dehydrated through a graded series of ethanol. Endogenous peroxidase activity was quenched with 3% H2O2 in water for 10 min. Antigen retrieval was performed by heating the slides in 0.1 M sodium citrate (pH 6.0) for 10 min. The sections were then incubated with primary antibodies for 30 min at room temperature. Sections incubated with antibody diluents were used as negative controls. The sections were developed using the Dako EnVision™ visualization system (Dako Cytomation, CA, USA), and the following antibodies were used for IHC staining: ΔNP63 (p40; Calbiochem, Darmstadt, Germany) and cytokeratin 5/6 (CK5/6; Dako).
DNA Extraction And EGFR Mutation Analysis
The Amplification Refractory Mutation System was used for molecular diagnosis in this study. Between February 2013 and December 2015, genomic DNA was extracted from fresh tissues using the QIAamp DNA Tissue Kit (Qiagen, Hilden, Germany). Mutations in the EGFR gene were detected using the Amoy Diagnostics Kit (AmoyDx, Xiamen, China) according to the manufacturer’s instructions.17 Between January 2016 and December 2017, DNA was extracted from five serial slices of a 5-μm-thick paraffin section using the DNA Formalin-Fixed Paraffin-Embedded Tissue Kit (Qiagen, Hilden, Germany). EGFR mutations were detected using the ACCB Diagnostics Kit (ACCB, Beijing, China) according to the manufacturer’s protocol18 The test could detect mutations at a sensitivity of 1% in no less than 5 ng/μL of the DNA sample.
Clinical Assessment
Data regarding patient characteristics at the time of lung cancer diagnosis, including age, sex, smoking history, tumor size, pathological TNM stage, tumor morphology, location, histological type, internal structure, lobulation, margin, shape, spiculation, and texture were collected retrospectively. Recurrence-free survival (RFS) was calculated from the date of surgical resection until the date of confirmed recurrence from any cause. Patients who were alive at the time of analysis were censored at the last known date of follow-up. All patients were followed up for more than 12 months.
Statistical Analysis
We compared the clinicopathological features of EGFR-positive patients with those of EGFR-negative patients. The EGFR-negative patients were consecutively selected from our department between January 2016 and March 2016.19 RFS measurements were obtained from EGFR-negative patients who underwent surgery on the same day as EGFR-positive patients or at a time that was closest to that of EGFR-positive patients. No differences in clinicopathological features were detected between those two non-mutated populations (Table 1). Statistically significant differences in categorical variables between the groups were analyzed using the χ2 or Fisher’s exact test, as appropriate. RFS was estimated by the Kaplan-Meier method, and the log rank test was used for the univariate analysis. The Cox-proportional hazard model was used for the multivariate analysis. The covariates considered for the multivariate analysis were gender, age, TNM stage, tumor size, and smoking status. Two-sided P values of <0.05 were considered statistically significant. SPSS version 20.0 for Windows (IBM SPSS Statistics, Chicago, IL) was used for the statistical analyses.
 |
Table 1 The Clinicopathological Characteristics Of Two EGFR-Negative Populations |
Results
Confirmation Of LSCC By H&E Staining And IHC Analysis
The expression of CK5/6 and ΔNP63 (p40) is considered a classical hallmark of LSCC.20–22 In the present study, three pathologists retrospectively evaluated the H&E- and IHC-stained sections from the 2,322 patients with LSCC. The sections were strongly positive for CK5/6 and ΔNP63 (Supplementary Figure). Based on morphological and IHC data, all included tumors were confirmed to be LSCC.
Distribution Of The EGFR Mutation In LSCC
EGFR gene mutations were detected in 80 of the 2,322 samples (mutation rate, 3.4%). Exon 19 deletions (19-del) and the L858R mutations in exon 21 comprised 48.75% and 32.5% of all EGFR mutations, respectively, in LSCC patients. The uncommon EGFR mutations observed in the cohort included 5 (6.25%) cases of 20-ins mutations, 3 (3.75%) of T790M mutations, 3 (3.75%) of G719X mutations, and 1 (1.25%) of L861Q mutation. The dual T790M and L858R mutations were observed in 2 (2.5%) patients and dual G719X and L861Q mutations were found in 1 (1.25%) patient (Figure 1).
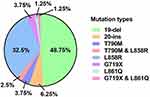 |
Figure 1 A comprehensive view of EGFR mutations in patients with LSCC (n = 80). |
Clinicopathological Characteristics
The clinicopathological characteristics of 80 EGFR-positive and 95 EGFR-negative cases are listed in Table 2. No differences in age, drinking, TNM stage, tumor size, and site were observed between the two groups. However, the proportion of females was higher in the EGFR-positive group than in the EGFR-negative group, with statistical significance notwithstanding (P = 0.064). The number of patients who never smoked was significantly higher in the EGFR-positive group than in the EGFR-negative group (P = 0.006).
 |
Table 2 The Clinicopathological Characteristics Of 175 Patients With LSCC |
Patient Imageological Characteristics
The imageological characteristics of 80 EGFR-positive and 95 EGFR-negative patients with LSCC, including the internal structure, margin, shape, and presence of lobulation and spiculation, are shown in Table 3. No differences in internal structure, margin, lobulation, and spiculation were detected between the two groups. Irregularly shaped lesions were more common in the EGFR-positive group than in the EGFR-negative group (P = 0.045).
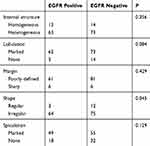 |
Table 3 The Imageological Characteristics Of 175 Patients With LSCC |
The imageological characteristics of EGFR-positive and EGFR-negative non-smoker patients with LSCC were examined because the majority of patients with EGFR-mutated LSCCs were generally non-smokers. As shown in Table 4, no differences in internal structure, margin, shape, and lobulation were detected between the two groups of non-smoker patients. The proportion of marked spiculation was significantly higher in the EGFR-positive group than in the EGFR-negative group (P = 0.043; Table 4; Figure 2).
 |
Table 4 The Imageological Characteristics Of Non-Smokers With LSCC |
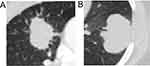 |
Figure 2 Imageological characteristics of non-smoking LSCC patients with (A) or without (B) EGFR mutations. |
Analysis Of RFS
No significant difference in RFS was identified between the EGFR-positive (45.9 months) and EGFR-negative (49.5 months) patients. The patients were then stratified into two subgroups based on their need for adjuvant therapy after surgery (Figure 3). The RFS of EGFR-positive patients in both the stage IA group (no specific treatment, 52.5 months vs. 47.9 months) and the stage IB-IIIA group (adjuvant therapy, 48.1 months vs. 42.4 months) was not significantly different from that in EGFR-negative patients.
Metastasis Of Patients
Of the 175 patients with LSCC, 127 with follow-up information were included in the analysis; among them, 56 were EGFR-positive and 71 had wild-type EGFR (WT). The incidence of brain metastases was 1.79% (1/56) in EGFR-positive patients and 1.41% (1/71) in EGFR WT patients (P = 0.038; hazard ratio [HR], 1.4). Furthermore, the incidences of bone, lung and multiple-site metastases were 3.57% (2/56) vs 1.41% (1/71), 5.36% (3/56) vs 1.41% (1/71), and 1.79% (1/56) vs 1.41% (1/71) in EGFR-positive and EGFR WT patients. No significant differences in single-site (brain, bone, and lung) and multiple-site metastases were observed between EGFR-positive and EGFR WT patients (Table 5).
 |
Table 5 The Metastatic Site And Number Of Patients With LSCC |
Discussion
The incidence of EGFR mutations, imageological features, and clinical outcomes were evaluated in a large cohort of postoperative patients with LSCC in the present study. EGFR mutations were detected with increased frequency in females and non-smokers. Moreover, the tumors in patients with EGFR-positive mutations were more irregularly shaped and demonstrated marked spiculation compared with those with EGFR-negative mutations. Thus, patients with active EGFR mutations might benefit from second-generation EGFR-TKIs treatments.23,24
Consistent with the findings of previous studies,25,26 the EGFR mutation rate in patients with LSCC in the current study was 3.4%; however, this value was much lower than that reported in patients with ADC.27 Exon 19-del and L858R mutations in exon 21 of the EGFR gene are the two commonest mutations that predict the favorable efficacy of EGFR-TKIs.28 The L858R mutation is reportedly the commonest type of mutation in patients with ADC, whereas exon 19-del is dominant in LSCC. In the present study, exon 19-del and EGFR L858R mutations accounted for 48.75% and 32.5% of all EGFR mutations, respectively, in patients with LSCC. The mutation rate of the EGFR L858R mutation in patients with LSCC was lower than that reported in patients with non-squamous NSCLC.29,30 In contrast, the uncommon EGFR mutations observed in the cohort included 5 (6.25%) cases of 20-ins mutations and 3 (3.75%) of T790M mutations. The frequency of uncommon mutations (20-ins and T790M) in LSCC was higher than that in NSCLC, which might partly explain the poor response to EGFR-TKIs.31
Evaluation of the clinicopathological characteristics of the patients revealed the absence of any association between EGFR mutation and the majority of clinicopathological characteristics. However, the proportion of females in the EGFR-positive group was higher than that in the EGFR-negative group, although statistical significance was not achieved (P = 0.064). In addition, EGFR mutations were more frequent in non-smokers than in previous smokers or current smokers (P = 0.006). Moreover, based on the logistic regression analysis, smoking was negatively correlated (odds ratio [OR], 0.68; 95% confidence interval [CI], 0.476–0.973; P = 0.035) with EGFR mutations (data not shown). This is consistent with the clinical characteristics of EGFR mutations in ADC and LSCC.13–15,32
No differences in tumor metastatic capability and patient outcome were noted; however, the imageological characteristics were different between EGFR-positive and EGFR-negative LSCC patients. The LSCC lesions were more irregularly shaped in the EGFR-positive group than in the EGFR-negative group (P = 0.045). After subgrouping of the non-smoker LSCC patients into EGFR-positive and EGFR-negative groups, significantly higher amounts of marked spiculation were detected in the EGFR-positive group than in the EGFR-negative group (P = 0.043). Nonetheless, the logistic regression analysis did not show any correlations between the imageological features and EGFR mutations.
Targeting EGFR with TKIs has become the cornerstone for the management of advanced non-squamous NSCLC harboring activating EGFR mutations; however, the relevance of EGFR inhibitors in LSCC is poorly defined. The role of erlotinib in the treatment of LSCC, irrespective of the EGFR mutation status, has been evaluated in several clinical trials, including the observational PEPiTA study,33 the BR.21 trial,34 the SATURN study,35 the TRUST study,36 the TITAN,37 and the DELTA trial.38 Data from these clinical trials demonstrated that as a maintenance treatment option, the therapeutic effects of erlotinib were similar to those of first- or second-line chemotherapy. Furthermore, the role of afatinib in the treatment of LSCC has been evaluated in several clinical trials.39 Shun Lu and his colleagues conducted a post hoc analysis of the data of patients in the LUX-Lung 8 trial conducted in mainland China.23 Compared with erlotinib, afatinib reduced the risk of disease progression or death in the Chinese subgroup by 30% (HR, = 0.70; 95% CI, 0.38–1.27), thereby indicating that afatinib may be considered as a feasible treatment option for Chinese patients with advanced LSCC following the progression of the disease after platinum-based chemotherapy. Yuri Taniguchi and his colleagues showed that the administration of afatinib to LSCC patients who were selected using the EGFR mutation test based on the underlying pulmonary disease and smoking status would likely result in a population with a greater sensitivity to afatinib.39
This study has several limitations. First, despite the inclusion of large-scale data compared with those of previous studies, the patients belonged to a single center, which can lead to a bias in the selection of patients. Second, this was a retrospective study, and we could not collect any data related to targeted therapy (although afatinib has been shown to be effective for LSCC patients harboring activating EGFR mutations); therefore, the efficacy of EGFR-TKIs was not evaluated in this study. The use of molecular testing for early-stage lung cancer patients based on the availability of high-quality resection samples will prove beneficial, particularly for those who present with disease progression, thus providing them with another option during treatment planning.40
The most important predictors of the presence of EGFR mutations in LSCC were female gender, non-smoking habit, irregularly-shaped lesions, and marked spiculation (imageological finding). The administration of EGFR-TKIs to patients with LSCC after screening for EGFR mutations on the basis of their clinical and imageological features would likely result in a population with a greater sensitivity to afatinib.
Acknowledgment
We would like to acknowledge the funding support of the National Natural Science Foundation of China (Grants No. 11675134, No. 11474345, No. 674043, No. 11874310 and No. 81572614).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Travis WD. Pathology of lung cancer. Clin Chest Med. 2011;32(4):669–692. doi:10.1016/j.ccm.2011.08.005
2. Klughammer B, Brugger W, Cappuzzo F, et al. Examining treatment outcomes with erlotinib in patients with advanced non-small cell lung cancer whose tumors harbor uncommon EGFR mutations. J Thorac Oncol. 2016;11(4):545–555. doi:10.1016/j.jtho.2015.12.107
3. Gandara DR, Hammerman PS, Sos ML, Lara PN
4. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
5. Khuder SA, Mutgi AB. Effect of smoking cessation on major histologic types of lung cancer. Chest. 2001;120(5):1577–1583. doi:10.1378/chest.120.5.1577
6. Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735–742. doi:10.1016/S1470-2045(11)70184-X
7. Rosell R, Moran T, Queralt C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361(10):958–967. doi:10.1056/NEJMoa0904554
8. Zhang GC, Lin JY, Wang Z, et al. Epidermal growth factor receptor double activating mutations involving both exons 19 and 21 exist in Chinese non-small cell lung cancer patients. Clin Oncol (R Coll Radiol). 2007;19(7):499–506. doi:10.1016/j.clon.2007.04.006
9. Shi Y, Au JS, Thongprasert S, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol. 2014;9(2):154–162. doi:10.1097/JTO.0000000000000033
10. Cancer Genome Atlas Research N. Comprehensive genomic characterization of squamous cell lung cancers. Nature. 2012;489(7417):519–525. doi:10.1038/nature11404
11. Einhorn LH. First-line chemotherapy for non-small-cell lung cancer: is there a superior regimen based on histology? J Clin Oncol. 2008;26(21):3485–3486. doi:10.1200/JCO.2008.17.2056
12. Tagliamento M, Genova C, Rijavec E, et al. Afatinib and erlotinib in the treatment of squamous-cell lung cancer. Expert Opin Pharmacother. 2018;19(18):2055–2062. doi:10.1080/14656566.2018.1540591
13. Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304(5676):1497–1500. doi:10.1126/science.1099314
14. Kosaka T, Yatabe Y, Endoh H, Kuwano H, Takahashi T, Mitsudomi T. Mutations of the epidermal growth factor receptor gene in lung cancer: biological and clinical implications. Cancer Res. 2004;64(24):8919–8923. doi:10.1158/0008-5472.CAN-04-2818
15. Liu Y, Kim J, Balagurunathan Y, et al. Radiomic features are associated with EGFR mutation status in lung adenocarcinomas. Clin Lung Cancer. 2016;17(5):441–448 e446. doi:10.1016/j.cllc.2016.02.001
16. Rizzo S, Petrella F, Buscarino V, et al. CT radiogenomic characterization of EGFR, K-RAS, and ALK mutations in non-small cell lung cancer. Eur Radiol. 2016;26(1):32–42. doi:10.1007/s00330-015-3814-0
17. Yang Z, Yang N, Ou Q, et al. Investigating novel resistance mechanisms to third-generation EGFR tyrosine kinase inhibitor osimertinib in non-small cell lung cancer patients. Clin Cancer Res. 2018;24(13):3097–3107. doi:10.1158/1078-0432.CCR-17-2310
18. Wang X, Gao Y, Wang B, et al. Analytic and clinical validation of an ultrasensitive, quantitative polymerase chain reaction assay for EGFR mutation analysis with circulating tumor DNA. Arch Pathol Lab Med. 2017;141(7):978–984. doi:10.5858/arpa.2016-0083-OA
19. Pan Y, Zhang Y, Li Y, et al. Prevalence, clinicopathologic characteristics, and molecular associations of EGFR exon 20 insertion mutations in East Asian patients with lung adenocarcinoma. Ann Surg Oncol. 2014;21(Suppl 4):S490–S496. doi:10.1245/s10434-013-3452-1
20. Calio A, Nottegar A, Gilioli E, et al. ALK/EML4 fusion gene may be found in pure squamous carcinoma of the lung. J Thorac Oncol. 2014;9(5):729–732. doi:10.1097/JTO.0000000000000109
21. Bishop JA, Teruya-Feldstein J, Westra WH, Pelosi G, Travis WD, Rekhtman N. p40 (DeltaNp63) is superior to p63 for the diagnosis of pulmonary squamous cell carcinoma. Mod Pathol. 2012;25(3):405–415. doi:10.1038/modpathol.2011.173
22. Rekhtman N, Ang DC, Sima CS, Travis WD, Moreira AL. Immunohistochemical algorithm for differentiation of lung adenocarcinoma and squamous cell carcinoma based on large series of whole-tissue sections with validation in small specimens. Mod Pathol. 2011;24(10):1348–1359. doi:10.1038/modpathol.2011.92
23. Lu S, Li W, Zhou C, et al. Afatinib vs erlotinib for second-line treatment of Chinese patients with advanced squamous cell carcinoma of the lung. Onco Targets Ther. 2018;11:8565–8573. doi:10.2147/OTT.S161506
24. Soria J-C, Felip E, Cobo M, et al. Afatinib versus erlotinib as second-line treatment of patients with advanced squamous cell carcinoma of the lung (LUX-Lung 8): an open-label randomised controlled phase 3 trial. Lancet Oncol. 2015;16(8):897–907. doi:10.1016/S1470-2045(15)00006-6
25. Shigematsu H, Gazdar AF. Somatic mutations of epidermal growth factor receptor signaling pathway in lung cancers. Int J Cancer. 2006;118(2):257–262. doi:10.1002/ijc.21496
26. Park SH, Ha SY, Lee JI, et al. Epidermal growth factor receptor mutations and the clinical outcome in male smokers with squamous cell carcinoma of lung. J Korean Med Sci. 2009;24(3):448–452. doi:10.3346/jkms.2009.24.3.448
27. Jia XL, Chen G. EGFR and KRAS mutations in Chinese patients with adenosquamous carcinoma of the lung. Lung Cancer. 2011;74(3):396–400. doi:10.1016/j.lungcan.2011.04.005
28. Rotow J, Bivona TG. Understanding and targeting resistance mechanisms in NSCLC. Nat Rev Cancer. 2017;17(11):637–658. doi:10.1038/nrc.2017.84
29. Makoto Maemondo MD
30. Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin–paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947–957. doi:10.1056/NEJMoa0810699
31. O’Kane GM, Bradbury PA, Feld R, et al. Uncommon EGFR mutations in advanced non-small cell lung cancer. Lung Cancer. 2017;109:137–144. doi:10.1016/j.lungcan.2017.04.016
32. Zhang Q, Zhu L, Zhang J. Epidermal growth factor receptor gene mutation status in pure squamous-cell lung cancer in Chinese patients. BMC Cancer. 2015;15:88. doi:10.1186/s12885-015-1584-3
33. Monnet I, Audigier-Valette C, Girard N, et al. Real-life effectiveness of erlotinib as second-line treatment of stage IIIB/IV squamous non-small cell lung cancer: results of the PEPiTA observational study. Lung Cancer. 2016;98:84–90. doi:10.1016/j.lungcan.2016.05.016
34. Tsao MS, Sakurada A, Cutz JC, et al. Erlotinib in lung cancer - molecular and clinical predictors of outcome. N Engl J Med. 2005;353(2):133–144. doi:10.1056/NEJMoa050736
35. Cappuzzo F, Ciuleanu T, Stelmakh L, et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2010;11(6):521–529. doi:10.1016/S1470-2045(10)70112-1
36. Reck M, van Zandwijk N, Gridelli C, et al. Erlotinib in advanced non-small cell lung cancer: efficacy and safety findings of the global phase IV Tarceva Lung Cancer Survival Treatment study. J Thorac Oncol. 2010;5(10):1616–1622. doi:10.1097/JTO.0b013e3181f1c7b0
37. Ciuleanu T, Stelmakh L, Cicenas S, et al. Efficacy and safety of erlotinib versus chemotherapy in second-line treatment of patients with advanced, non-small-cell lung cancer with poor prognosis (TITAN): a randomised multicentre, open-label, phase 3 study. Lancet Oncol. 2012;13(3):300–308. doi:10.1016/S1470-2045(11)70385-0
38. Kawaguchi T, Ando M, Asami K, et al. Randomized phase III trial of erlotinib versus docetaxel as second- or third-line therapy in patients with advanced non-small-cell lung cancer: Docetaxel and Erlotinib Lung Cancer Trial (DELTA). J Clin Oncol. 2014;32(18):1902–1908. doi:10.1200/JCO.2013.52.4694
39. Taniguchi Y, Matsumoto Y, Furukawa R, Ohara S, Usui K. The clinical features of squamous cell lung carcinoma with sensitive EGFR mutations. Int J Clin Oncol. 2018;23(3):452–457. doi:10.1007/s10147-017-1233-8
40. Lindeman NI, Cagle PT, Aisner DL, et al. Updated molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors: guideline from the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. J Thorac Oncol. 2018;13(3):323–358. doi:10.1016/j.jtho.2017.12.001
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

