Back to Journals » Neuropsychiatric Disease and Treatment » Volume 20
Clinical Analysis of Lacosamide Monotherapy in the Treatment of Self-Limited Epilepsy with Centrotemporal Spikes
Authors Feng J, Zhang L, Tang J, Zhang B, Xiao X, Shi X
Received 2 December 2023
Accepted for publication 22 February 2024
Published 4 March 2024 Volume 2024:20 Pages 459—467
DOI https://doi.org/10.2147/NDT.S452784
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
Jun Feng, Liya Zhang, Jihong Tang, Bingbing Zhang, Xiao Xiao, Xiaoyan Shi
Department of Neurology, Children’s Hospital of Soochow University, Suzhou, Jiangsu, 215025, People’s Republic of China
Correspondence: Liya Zhang; Jihong Tang, Email [email protected]; [email protected]
Objective: To evaluate the efficacy and safety of lacosamide (LCM) monotherapy in the treatment of self-limited epilepsy with centrotemporal spikes (SeLECTS).
Methods: In this study, 89 children with SeLECTS who were treated with LCM monotherapy in the Children’s Hospital Affiliated to Soochow University from June 2019 to June 2021 were included. Clinical seizures and spike wave index (SWI) on video EEG during slow-wave sleep were evaluated before and after treatment. The role of LCM monotherapy in improving SWI, controlling clinical seizures and improving cognition was analyzed, and corresponding adverse reactions were documented.
Results: There were 52 males and 37 females in this group, with an average age of 7.6 ± 2.1 years. The total effective rate was 93.83% and at 18 months of treatment, the cumulative control rate was 85.19%, the retention rate was 91.01% and the effective rate in terms of EEG spike index was 72.92%, all of which showed a high rate; there was no statistically significant difference in intelligence quotient before and after treatment (P > 0.05). In addition, it was found in the study that the earlier the age of onset, the less obvious the improvement in SWI after LCM treatment; the lower the baseline seizure frequency, the more significant the improvement in SWI after LCM treatment.
Conclusion: LCM monotherapy had curative effect and adverse reactions for SeLECTS, with no negative impact on cognition. These significant findings indicate that LCM is likely to become a widely prescribed ASM for the treatment of SeLECTS. Meanwhile, the onset age and baseline seizure frequency had certain value in judging prognosis and predicting curative effect.
Keywords: lacosamide, epilepsy, self-limited epilepsy with centrotemporal spikes, efficacy, safety
Introduction
Epilepsy is a common neurological disorder in children and adolescents, and about three-quarters of patients with epilepsy have onset in childhood, with a prevalence of about 0.3% to 0.9%, of which self-limited epilepsy with centrotemporal spikes (SeLECTS) is the most common focal epilepsy for children.1 The typical interictal EEG pattern is a high-amplitude spike or sharp wave with a prominent following slow wave appearing singly or in groups most commonly located at the midtemporal and central region. Epileptic discharges on EEG increased significantly after children with SeLECTS fell asleep. In a few children, spike and wave index (SWI) can reach 50% or even more than 80% during non-rapid eye movement sleep (NREM) period.2 Currently, there are a variety of antiseizure medications (ASMs) that can treat SeLECTS,3 but there is no consensus on which ASM to use for this epilepsy. However, several studies have investigated various ASMs in the treatment of SeLECTS, such as Levetiracetam and oxcarbazepine, which are suggested to be effective and well-tolerated drugs.4 But 25% to 30% of pediatric patients still have poor response to medical treatment, incomplete control of seizures, and even develop into drug-refractory epilepsy.5
In addition, some studies6–8 have shown that children with SeLECTS have a certain degree of cognitive impairment. Several studies have investigated the impact of treatment in cognitive profile, such as levetiracetam, which is suggested a nonworsening of the cognitive profile, on the contrary, cognitive scores also improved over time.9 To protect the learning ability of school-age child, it is particularly important to get timely and effective treatment and choose drugs that have no negative effects on cognition.
LCM is a third-generation antiseizure medication that was available clinically in 2008. LCM has a novel mechanism of action and anticonvulsant effects by selectively acting on the slow voltage-gated sodium channel inactivation. Unlike conventional sodium channel blockers, LCM does not affect fast inactivation.10,11 A large number of clinical studies have confirmed that LCM has good seizure control efficacy with less side effects in patients with epilepsy as adjunctive therapy or monotherapy. Based on the findings of three randomized controlled trials, LCM was initially approved as an adjunctive therapy for partial-onset (focal) seizures, whether with or without secondary generalization.10–13 The further open-label extensions showed that LCM had a sustained efficacy and good long-term safety.14 The efficacy and safety of lacosamide in treatment in patients with epilepsy is confirmed by a recent study conducted in Italy, that compared four third-generation ASM (lacosamide, brivaracetam, perampanel and eslicarbazepine) in a real-life setting.15 A retrospective study of 18 pediatric patients with SeLECTS in Japan showed that LCM monotherapy may be considered as a first-line drug for pediatric patients with possible SeLECTS. The drug showed meaningful efficacy with >80% of the children achieving seizure freedom finally and no severe adverse effects during the study period. However, limitations of the study16 include its relatively small sample size and lack of insight into cognition and EEG.
In China, the drug was approved by national medical products administration (NMPA) in 2018 for the adjunctive treatment of focal seizures in patients aged 4 years and order with epilepsy, and in 2021 for monotherapy indications in the same population. Although a number of studies have reported LCM monotherapy for epilepsy in Western countries, there is insufficient clinical experience and limited date in using LCM monotherapy in Chinese patients. Recently, Zhao et al found that LCM therapy is effective and safe for Uighur children with epilepsy in China, leading to a decrease in the seizure frequency.17 Another retrospective study from Children’s Hospital of Nanjing Medical University also found that LCM treatment used alone or with other ASMs in children with focal epilepsy can reduce the seizure frequency with mild adverse reactions reported. Based on the result, the reference range, ie, 2.0–7.0μg/mL is recommended for routine LCM monitoring in Chinese children with epilepsy.18 However, the clinical efficacy and safety of LCM monotherapy for SeLECTS in China have not been reported. Clinical studies with larger sample sizes are also needed to further confirm the effect of LCM for children with SeLECTS.
Based on the above, this study aimed to evaluate the efficacy and safety of LCM in Chinese children with SeLECTS and compare the changes of cognition and EEG during treatment.
Materials and Methods
This research was conducted in Children’s Hospital Affiliated to Soochow University. The protocol of this study was approved by the Ethics Committee of Children’s Hospital Affiliated to Soochow University, and it was conducted according to the Declaration of Helsinki.
Participants
This is a single-center retrospective study involving pediatric patients with SeLECTS treated with LCM monotherapy from 2019 to 2022 at Children’s Hospital Affiliated to Soochow University. All children diagnosed with SeLECTS were screened according to inclusion and exclusion criteria (Figure 1). The patient inclusion criteria were as follows: (i) The age range is from 4 to 14 years old; (ii) Experience typical seizures, which are closely related to sleep and appear as focal seizures, some of which may be followed by generalised seizures.; (iii) Frequent epileptic seizures, with one persistent state of epilepsy or more than two focal seizures in the past six months; (iv) Long-range video-EEGs show normal interictal background activity, spikes, sharp waves or their complex waves can be seen in the central and temporal regions, and abnormal waves during sleep are significantly increased, in line with the EEGs characteristics of SeLECTS; (v) Psychomotor development, neurological and neuroimaging examinations are all normal.
 |
Figure 1 Number of patients who were eligible for this study. |
The exclusion criteria were as follows: (i) Patients without seizures or with treatable causes, such as intracranial mass lesions or poisoning; (ii) Patients with atrioventricular block in ECGs; (iii) Allergic constitution; (iv) Patients with other serious diseases affecting efficacy or safety evaluation; (v) Except for atypical evolution of SeLECTS; (vi) Those who do not meet the above inclusion criteria. The study was reviewed and approved by the Medical Ethics Committee of Children’s Hospital of Soochow University and informed consent was obtained from the children’s guardian.
Treatment Protocol
All patients included in this study were newly diagnosed with SeLECTS and received LCM monotherapy. The initial dose of LCM was 2 mg/(kg·d), increased by 2 mg/(kg·d) every two weeks, and the maintenance dose was 4 to 8 mg/(kg·d) for children patients weighing ≥30 kg and <50 kg; the maintenance dose was 6 to 12 mg/(kg·d) for those weighing ≥11 kg and <30 kg, administered in two oral doses. Regular follow-up visits were performed at 1, 2, 4, 6, 9, 12, 15 and 18 months after treatment. Their efficacy was analyzed and judged by understanding the status of epileptic seizure and the SWI on EEG; at the same time, the impact of LCM on cognition and post-treatment adverse reactions was evaluated, and laboratory and device examination results, including liver and kidney function, blood electrolytes, hematology, urinalysis and ECG results, were analyzed.
Data Collection
General data, medical history, follow-up data, EEG findings, intelligence assessment and other clinical data were collected. EEG monitoring was performed for approximately 3 h on the day after sleep deprivation to record at least one full wake-sleep cycle. Intelligence was tested by age according to The Wechsler Preschool and Primary Scale of Intelligence-Fourth Edition (WPPSI-IV) (2 years, 6 months through 7 years, 7 months) or Wechsler Intelligence Scale for Children-Fourth Edition [WISC-IV] (6 years through 16 years). Due to the difference in the test structure between WPPSI-IV and WISC-IV, the mean value of visual spatial reasoning and fluid reasoning in WPPSI-IV replaced the perceptual reasoning of WISC-IV in order to compare the differences between them. Intelligence assessment was followed up and evaluated for least 1 year.
Definitions of Clinical Response
The criteria for judging the efficacy are as follows. The frequency of two or more than two seizures in half a year was used as the baseline seizure frequency. The post-treatment frequency of seizures and SWI were compared with the baseline data before treatment, that is, the efficacy was judged by (number of seizures – number of baseline seizures)/number of baseline seizures. (i) Control: The control rate is 100% and achieved clinical seizure freedom; (ii) Significantly effective: The control rate is 75% to 99% and clinical seizures were significantly reduced; (iii) Efficient: The control rate is 50% to 74%, and clinical seizures were reduced; (iv) Invalid: Control rate < 50%, and clinical seizures were slightly reduced. The total effective rate of antiepileptic treatment is the sum of the control rate, significantly effective rate and efficient rate, which is used as the main judgment standard. Video EEG was performed before LCM treatment, 6 months after treatment and one year after treatment. The response of EEG to treatment was divided into 3 categories: markedly effective: ≥50% reduction in SWI; effective: 25% ≤ reduction in SWI < 50%; ineffective: <25% reduction or increase in SWI.
Statistical Analysis
SPSS 26 statistical software was used for data analysis. All measurement data was expressed as 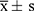 , with rates compared using a fourfold table χ2 test; the mean was compared by one-way ANOVA, and P < 0.05 indicated statistically significant difference.
, with rates compared using a fourfold table χ2 test; the mean was compared by one-way ANOVA, and P < 0.05 indicated statistically significant difference.
Result
Patients’ Characteristics
The age of the 89 children patients at visiting ranged from 4 to 14 years, with an average of 7.6 years, including 19 cases <6 years, 48 cases of 6 to 9 years, and 22 cases >9 years. Among them, there were 37 females and 52 males, and all 89 children had seizures related to sleep, with seizure forms: There were 36 cases of focal seizures, manifested as unilateral orofacial or ipsilateral limb twitching, salivation, throat vocalization, inability to speak, but clear consciousness; 28 cases of focal seizures generalized to generalized seizures, manifested as limb tonic-clonic seizures with loss of consciousness secondary to orofacial twitching; there were 25 cases of generalized tonic-clonic seizures when their parents discover. The seizure duration ranged from 1 to 7 min, and there were no patients with status epilepticus.
Efficacy Analysis
Efficacy for Seizure Control
The efficacy of LCM monotherapy in the treatment of SeLECTS is shown in Table 1. Among the 89 children patients, 3 had worsened seizures and withdrew from LCM treatment after 2 months of medication and switched to levetiracetam, 2 had first-degree atrioventricular block and atrial premature beat at 4 months of medication and switched to other antiepileptic drugs for safety reasons, and 3 had poor control at 6 months of medication and switched to other drugs, which was counted as failures. The remaining 81 children patients were included in the efficacy analysis. In 81 children patients with SeLECTS, the total effective cases reached 76 cases (93.83%) at 1, 2, 4, 6, 9, 12, 15 and 18 months of medication, and the cumulative control cases reached 69 cases (85.19%) at 18 months of medication, with a retention efficacy of 91.01%, all of which showed a high rate.
 |
Table 1 Efficacy of LCM in the Treatment of SeLECTS |
Efficacy for SWI
SWI ≥ 50% was indicated on first video EEG obtained from 48 out of 89 children with SeLECTS (85% not reached). The EEG results obtained from these 48 children at 6 months and 1 year after LCM treatment were analyzed. It was found that 6 months after LCM treatment, 19 children (39.58%) had ≥50% reduction in SWI, and the treatment was effective in 13 children (27.08%), with an overall effective rate of 66.67% (32/48); 1 year after LCM treatment, 25 children (52.08%) had ≥50% reduction in SWI, and the treatment was effective in 10 children (20.83%), with an overall effective rate of 72.92% (35/48).
Effect on Intelligence Quotient
Of 89 children, 42 underwent WPPSI-IV or WISC-IV before treatment and at the last follow-up, with an interval of at least 1 year. After self-control, it was found that there were no statistically significant differences in verbal comprehension index, perceptual reasoning index, working memory index and processing speed index, indicating that long-term treatment with LCM had no negative affect on cognition (P > 0.05), as shown in Table 2. However, in this study, it was found that the working memory index of children was somewhat increased after treatment with LCM, despite no notable statistical difference (P = 0.079), and it was clinically observed that 13 children were found to concentrate more than before, have improved memory, and have improved academic record after controlling SWI, indicating that LCM treatment can improve cognition to some extent.
 |
Table 2 Effect of LCM Monotherapy on Intelligence Quotient of Children with SeLECTS |
Predictors of Improvement in SWI
Children were divided into effective group (n=35) and ineffective group (n=13) according to the improvement in SWI after 1 year of treatment. Two groups were compared in terms of age of onset, degree of first seizure, pre-treatment basic intelligence test results, and baseline seizure frequency. It was found that among the children with SWI ≥50% to <85%, the earlier the onset age, the less obvious the improvement in SWI after LCM treatment; the lower the baseline seizure frequency, the more significant the improvement in SWI after LCM treatment, as shown in Table 3.
 |
Table 3 Predictors of the Efficacy of LCM Treatment on EEG |
Adverse Reactions
A total of 29 patients (32.58%) experienced adverse reactions in the whole group, manifested as dizziness, nausea, vomiting, diplopia, and somnolence (Table 4), these adverse reactions are mild and transient, which can be tolerated with prolonged medication; they can also be alleviated after appropriate adjustment of the dose or titration of the drug rate, and basically disappear when followed up for 2 months. Two of the 89 pediatric patients had ECGs showing first degree atrioventricular block and premature beat atrial, respectively, the correlation with LCM was unclear, but due to concerns about medication safety, other antiepileptic drugs were switched. Laboratory tests showed no abnormalities in all children patients, and there were no withdrawals or dressing changes due to financial reasons.
 |
Table 4 Adverse Reactions of LCM Treatment in Children with SeLECTS |
Discussion
Self-limited epilepsy with centrotemporal spikes (SeLECTS) in children is the most common form of epileptic syndrome for children, with remission of seizures by adolescence and normal psychomotor development, which mostly onsets between the ages of 2 to 14 years, accounting for 15% to 24% of epilepsy in children.19 Seizures mostly appear during the time period before waking up or shortly after falling asleep, and seizures appear as focal seizures, with most children starting in the orofacial region and typically showing mouth twitching, laryngeal vocalization, increased saliva, and inability to actively vocalize, but maintaining clear consciousness, and some of these pediatric patients have secondary generalized tonic-clonic seizures, along with loss of consciousness.20 LCM is currently approved by the European Medicines Agency21 (EMA) and the Food and Drug Administration22 (FDA), which are indicated for the treatment of patients with focal epilepsy aged ≥2 years and ≥1 month respectively as monotherapy and adjunctive therapy.
LCM is a novel sodium channel blocker antiepileptic drug. Unlike traditional sodium channel blockers, lacosamide selectively acts on the slow inactivation of voltage-gated sodium channels, and also spares the rapid inactivation. This does not affect short-time high-frequency discharges involving normal brain function, so it plays a stabilizing role in the cell membrane of hyperexcitable neurons, which in turn achieves the purpose of controlling seizures, without affecting normal physiological functions.23,24 SeLECTS is a kind of focal epilepsy, while LCM, as a new antiepileptic drug, has high safety and efficacy.25 In this paper, LCM monotherapy is chosen for the treatment of SeLECTS in order to understand the clinical efficacy and safety of LCM.
The initial dose of LCM was 2 mg/(kg·d), increased by 2 mg/(kg·d) every other week, and titrated to the maintenance dose according to the body weight of the child. The total effective rate within 12 months was 94.12%, the cumulative control rate was 86.27%, and the retention rate was 89.47%, suggesting that LCM monotherapy has good efficacy and high retention rate for the treatment of SeLECTS. Studies have confirmed that the proportion of seizure-free patients after treatment with lacosamide for focal epilepsy in adults at 6 months is 90%, with high efficacy and safety.14 This study confirmed that the efficacy of LCM in children with focal epilepsy is comparable to that in adults, and it is easy for LCM to control SeLECTS, with a significant effect.
In addition, it has been shown in other study that26 long-term treatment of focal epilepsy with LCM does not negatively affect cognition and can even improve cognition, which is consistent with the results obtained from this study. After scoring the children using the Wechsler Intelligence Scale before treatment and at the last follow-up, it was found in this study that no statistical significance in verbal comprehension index, perceptual reasoning index, working memory index and processing speed index. All those demonstrated that LCM had no significant effect on cognition in children. However, there are 13 children with SeLECTS were found by their parents to concentrate more than before, have improved memory, and have improved academic record after improvement in SWI, indicating that LCM treatment can improve cognition to some extent. The high drug retention in this group was due to the fact that LCM monotherapy has few adverse reactions and significant effect in the treatment of SeLECTS without affecting cognition, which is conducive to improving the quality of life of children with SeLECTS, thus increasing their medication compliance.
By observing and comparing SWI on video EEG of 48 children with SeLECTS before and after LCM monotherapy, it was found that 35 children had significant reduction in SWI on EEG one year after treatment, with a total effective rate of up to 72.92%, but still lower than the total effective rate of clinical seizures, indicating that the normalization of EEG with SeLECTS was later than the termination of epileptic seizure, suggesting that the improvement of EEG by LCM was not as obvious as the improvement of seizures. This study found that the earlier the age of onset, the less obvious the improvement in SWI after LCM treatment; the lower the baseline seizure frequency, the more significant the improvement, which is of certain significance for the prediction of the efficacy of LCM.
In terms of safety, LCM has a low incidence of adverse reactions, which are mild and infrequent. The most common drug-related adverse reactions were dizziness, nausea, and diplopia. There were studies27 stated that the most common adverse drug reactions for LCM were dizziness (30.6% and 8.2% in the LCM group and placebo group, respectively), diplopia (10.5% and 1.9% in the two groups, respectively), and nausea (11.4% and 4.4% in the two groups, respectively). In addition, adverse reactions include liver enzymes abnormal, weight changes, rash, depression, and psychosis. An add-on treatment trial mainly enrolled Chinese and Japanese, and adverse reactions related to LCM included dizziness (the incidence of adverse reactions in the LCM group and placebo group was 22.9% and 8.2%, respectively), diplopia (4.4% and 0.5% in the two groups, respectively), vomiting (4.1% and 1.6% in the two groups, respectively), somnolence (8.8% and 2.2% in the two groups, respectively) and headache (4.1% and 3.3% in the two groups, respectively).28 Findings on drug tolerability and safety in the above two studies27,28 were similar. In this study, after LCM monotherapy treatment of SeLECTS, the incidence of adverse reactions was 32.58%. Adverse reactions were not serious, mainly manifested as dizziness (19.10%), nausea, vomiting (4.49%), diplopia (5.62%) and somnolence (3.37%). Similar to the above studies, by adjusting the dose and drug titration rate and prolonging medication time, the adverse drug reactions of LCM could be gradually reduced in a short time until disappear, indicating that LCM had good tolerance and safety. In this group, two patients had ECGs showing first degree atrioventricular block and premature beat atrial, respectively, and it was not possible to determine whether there was a correlation with LCM, but due to concerns about medication safety, other antiepileptic drugs were switched. No other adverse reactions, such as headache, rash, weight gain or loss, and liver enzymes abnormal etc., were observed, which may be related to the small number of cases.
There were still some limitations in our study. First of all, we had small sample size, short follow-up period and large patients heterogeneity, which may lead to statistical bias. Secondly, the study on cognition was limited due to the lack of a control group for any evaluation of development-associated changes. Therefore, further studies are needed in the future to further evaluate the effectiveness, tolerability and safety of LCM administration, with larger sample sizes and longer clinical observations.
Conclusion
LCM is a novel antiepileptic drug, which has mild adverse reactions that are dose-related, mostly involving the central nervous system and gastrointestinal tract which can gradually disappear in a short time, indicating its good tolerability and safety. It has a high effective rate in the treatment of epilepsy and considered to be an ideal treatment medicine for focal epilepsy. LCM monotherapy is effective and safe in the treatment of SeLECTS, without leading to significant abnormal behaviors and decreased cognition, which has high value of clinical application.
Statement of Ethics
The study protocol complied with the Declaration of Helsinki and was approved by the Ethics Committee of the Children’s Hospital Affiliated to Soochow University in November 2021 (protocol code 2021CS172). The procedures complied with institutional guidelines. Given the retrospective enrollment, patient consent for participation was waived by Children’s Hospital Affiliated to Soochow University. We promise that patient privacy data will not be available and published.
Acknowledgment
This project is supported by Science and Technology Development Plan of Suzhou with grant No. SKY2022007.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Iapadre G, Balagura G, Zagaroli L, et al. Pharmacokinetics and drug interaction of antiepileptic drugs in children and adolescents. Paediatr Drugs. 2018;20(5):429–453. doi:10.1007/s40272-018-0302-4
2. Xiao-Yan L. Clinical electroencephalography. Beijing. 2017;(335):342. Chinese.
3. Yang L, Gao L, Luan Y, et al. Different drug treat with central-temporal region spike wave of benign epilepsy in children. J Med Forum. 2017;38(9):21–23.
4. Coppola G, Franzoni E, Verrotti A, et al. Levetiracetam or oxcarbazepine as monotherapy in newly diagnosed benign epilepsy of childhood with centrotemporal spikes (BECTS): an open-label, parallel group trial. Brain Dev. 2007;29(5):281–284. doi:10.1016/j.braindev.2006.09.008
5. Verrotti A, Loiacono G, Coppola G, et al. Pharmacotherapy for children and adolescents with epilepsy. Expert Opin Pharmacother. 2011;12(2):175–194. doi:10.1517/14656566.2010.517194
6. Wickens S, Bowden SC, D’souza W. Cognitive functioning in children with self-limited epilepsy with centrotemporal spikes: a systematic review and meta-analysis. Epilepsia. 2017;58(10):1673–1685. doi:10.1111/epi.13865
7. Zanaboni MP, Pasca L, Bova SM, et al. WISC-IV intellectual profiles in Italian children with self-limited epilepsy with centrotemporal spikes. Epileptic Disord. 2023;25(2):160–172. doi:10.1002/epd2.20003
8. Moritz S, Conor G, Najma A, et al. Care and three-year outcomes of children with benign epilepsy with centro-temporal spikes in England. Epilepsy Behav. 2023;148:109465. doi:10.1016/j.yebeh.2023.109465
9. Operto FF, Pastorino GMG, Mazza R, et al. Cognitive profile in BECTS treated with levetiracetam: a 2-year follow-up. Epilepsy Behav. 2019;97:187–191. doi:10.1016/j.yebeh.2019.05.046
10. Ben-Menachem E, Biton V, Jatuzis D, Abou-Khalil B, Doty P, Rudd GD. Efficacy and safety of oral lacosamide as adjunctive therapy in adults with partial-onset seizures. Epilepsia. 2007;48(7):1308–1317. doi:10.1111/j.1528-1167.2007.01188.x
11. Curia G, Biagini G, Perucca E, Avoli M. Lacosamide: a new approach to target voltage-gated sodium currents in epileptic disorders. CNS Drugs. 2009;23(7):555–568. doi:10.2165/00023210-200923070-00002
12. Chung S, Sperling MR, Biton V, et al. Lacosamide as adjunctive therapy for partial-onset seizures: a randomized controlled trial. Epilepsia. 2010;51(6):958–967. doi:10.1111/j.1528-1167.2009.02496.x
13. Halasz P, Kalviainen R, Mazurkiewicz-Beldzinska M, et al. Adjunctive lacosamide for partial-onset seizures: effi cacy and safety results from a randomized controlled trial. Epilepsia. 2009;50(3):443–453. doi:10.1111/j.1528-1167.2008.01951.x
14. Baulac M, Rosenow F, Toledo M, et al. Efficacy, safety, and tolerability of lacosamide monotherapy versus controlled-release carbamazepine in patients with newly diagnosed epilepsy: a phase 3, randomised, double-blind, non-inferiority trial. Lancet Neurol. 2017;16(1):43–54. doi:10.1016/S1474-4422(16)30292-7
15. Roberti R, Di Gennaro G, Anzellotti F, et al. A real-world comparison among third-generation antiseizure medications: results from the COMPARE study. Epilepsia. 2023;65(2):456–472. doi:10.1111/epi.17843
16. Tohru O, Yuji F, Satoru S, et al. Lacosamide monotherapy for the treatment of childhood epilepsy with centrotemporal spikes. Brain Dev. 2022;44(6):380–385. doi:10.1016/j.braindev.2022.02.005
17. Zhao T, Li HJ, Ma L, et al. Safety, efficacy, and tolerability of lacosamide for the treatment of epilepsy in pediatric patients in Uygur, China. Epilepsy Behav. 2021;117:107814. doi:10.1016/j.yebeh.2021.107814
18. Yue L, Guo H-L, Zhang -Y-Y, et al. Plasma lacosamide monitoring in children with epilepsy: focus on reference therapeutic range and influencing factors. Front Pediatr. 2022;10. doi:10.3389/fped.2022.949783
19. Parisi P, Paolino MC, Raucci U, et al. Atypical forms of benign epilepsy with centrotemporal spikes (BECTS): how to diagnose and guide these children. A practical/scientific approach. Epilepsy Behav. 2017;6(75):165–169. doi:10.1016/j.yebeh.2017.08.001
20. Liu N, Zhou T, Zhu JP, et al. Clinical feature and treatment strategy of benign childhood epilepsy with centrotemporal spikes. Chin Pediatr Emerg Med. 2017;24(3):228–232.
21. European Medicines Agency. Lacosamide: summary of product characteristics [EB/OL].2022. Available from: https://www.ema.europa.eu/en/documents/product-information/vimpat-epar-product-information_en.pdf.
22. US FDA. Vimpat(lacosamide): highlights of prescribing information [EB/OL]. 2021. Available from: https://www.vimpat.com/vimpat-prescribing-information.pdf.
23. Beyreuther BK, Freitag J, Heers C, et al. Lacosamide: a review of preclinical properties. CNS Drug Rev. 2010;13(1):21–42. doi:10.1111/j.1527-3458.2007.00001.x
24. Rogawski MA, Tofighy A, White HS, et al. Current understanding of the mechanism of action of the antiepileptic drug lacosamide. Epilepsy Res. 2015;110:189–205. doi:10.1016/j.eplepsyres.2014.11.021
25. Farkas A, Steinborn B, Flamini JR, et al. Efficacy and tolerability of adjunctive lacosamide in pediatric patients with focal sei-zures. Neurology. 2019;93(12):e1212–e1226. doi:10.1212/WNL.0000000000008126
26. Meschede C, Witt JA, Rademacher M, et al. Evaluating the longer-term cognitive effects of adjunctive perampanel compared to lacosamide in a naturalistic outpatient setting. Seizure. 2018;58:141–146. doi:10.1016/j.seizure.2018.04.015
27. Biton V, Gil-nagel A, Isojarvi J, et al. Safety and tolerability of lacosamide as adjunctive therapy for adults with partial onset seizures: analysis of data pooled from three randomized, double-blind, placebo-controlled clinical trials. Epilepsy Behav. 2015;52(Pt.A):119–127. doi:10.1016/j.yebeh.2015.09.006
28. Hong Z, Inoue Y, Liao W, et al. Efficacy and safety of adjunctive lacosamide for the treatment of partial-onset seizures in Chinese and Japanese adults: a randomized, double-blind, placebo-controlled study. Epilepsy Res. 2016;127:267–275. doi:10.1016/j.eplepsyres.2016.08.032
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
