Back to Journals » Cancer Management and Research » Volume 10
Classification of positive surgical margins and tumor recurrence after nephron-sparing surgery for small renal masses
Authors Li G, Zhu DS, Lang ZQ, Wang AX, Li YH, Zhang RY, Niu YJ
Received 29 July 2018
Accepted for publication 31 October 2018
Published 3 December 2018 Volume 2018:10 Pages 6591—6598
DOI https://doi.org/10.2147/CMAR.S181843
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Rituraj Purohit
Gang Li,1,* Dong-Sheng Zhu,1,* Zhi-Qiang Lang,2 Ai-Xiang Wang,3 Yu-Hong Li,4 Ren-Ya Zhang,5 Yuan-Jie Niu1
1Department of Urology, Tianjin Institute of Urology, The Second Hospital of Tianjin Medical University, Tianjin 300211, China; 2Department of Pathology, Yuhuangding Hospital of Qingdao University, Yantai 264000, China; 3Department of Pathology, Tianjin Institute of Urology, Tianjin 300211, China; 4Department of Pathology, The People’s Hospital of Liaocheng, Liaocheng 252000, China; 5Department of Pathology, Affiliated Hospital of Jining Medical University, Jining 272029, China
*These authors are co-first authors
Background: The association of positive margin and local recurrence after nephron-sparing surgery (NSS) remains a notably controversial issue. The aim of the present study was to investigate the relationship between classification of positive surgical margins (PSMs) and tumor recurrence based pathological findings.
Methods: Clinical, pathological, and follow-up data of 600 small renal cancer patients who underwent NSS between November 2007 and November 2017 at four hospitals in China were analyzed retrospectively.
Results: Of the 600 reviewed patients, 20 had positive margins. During the follow-up period of 56 months, only three cases of tumor recurrence were identified. Pathological examination was performed, and subsequently a new classification criteria were proposed: 1) False PSMs, which could be further divided into three subtypes: i) no standard processing performed on pathological specimens (seven patients); ii) incidental incision into the tumor during operation, with the tumor bed free of tumor residues (four patients); iii) part of the tumor pseudocapsule was noted to be remained in the tumor bed, with no signs of tumor residue (four patients). 2) True PSMs with two subtypes: i) a large number of residual tumor cells at the surgical margin (three patients); ii) incision of satellite tumor nodules detected around a large tumor (two patients).
Conclusion: Taken together, PSMs in NSS were rarely found. Based on the pathological examination findings, PSMs can be divided into false positive and true positive. This being said, PSMs were determined to be poor predictors for local recurrence, with no predominant association with true tumor remnants in the majority of our evaluated cases. Through the key findings of our study, we concluded that PSMs should be carefully analyzed and treated on a case-by-case basis.
Keywords: small renal masses, positive surgical margins, nephron-sparing surgery, recurrence
Background
Renal cell carcinoma (RCC) represents 2%–3% of all cancers. Over the last 20 years, reports have indicated an annual increase of ~2% in its incidence on a worldwide scale.1 Studies have highlighted an increased detection of kidney tumors by ultrasound (US) and computed tomography (CT) as key components linked to the increased number of RCC cases. These masses are usually smaller at an early stage.2 Currently, surgery remains the optimal therapeutic approach for RCC, while nephron-sparing surgery (NSS) has become the golden standard of care for clinically localized RCC when technically feasible, particularly for cases of small renal masses.3 However, positive surgical margins (PSMs) at times may occur, which poses a significant dilemma for both surgeons and patients. Owing to the fact that PSMs rates are relatively low,4–7 few investigations have been conducted into their mechanism, with little existing literature and research done on the pathological fate of the renal unit. The majority of studies emphasize the outcomes of their respective observations. The aim of our study was to analyze the relationship between the classification of PSMs and tumor recurrence after NSS in cases of small renal masses.
Methods
The current study was conducted in a retrospective fashion, collecting the data from four hospitals in China. Our study was conducted in strict accordance with the respective approval of the institutional review boards of the Second Hospital of Tianjin Medical University, Yuhuangding Hospital of Qingdao University, People’s Hospital of Liao Cheng, and the Affiliated Hospital of Jining Medical University, as well as the principles of the Declaration of Helsinki. All participating patients signed written informed consent documents prior to enrollment into our study. The records of patients who underwent open partial nephrectomy or laparoscopic partial nephrectomy for a small renal tumor (≤4 cm) and limited to the kidney (T1a) between December 2007 and December 2017 were reviewed accordingly. A total of 600 cases were collected, including 432 males and 168 females between the ages of 39 and 82 years, with a mean age of 56 years. There were 314 tumors in the right kidney and 286 in the left. All surgical specimens were examined by at least two experienced urological pathologists. The pathological findings were grouped into PSMs and negative surgical margins (NSMs), with the PSM group subsequently subgrouped into the true PSMs and false PSMs. The false PSMs were placed into three categories: 1) specimen was not stained using link, sorted by no standard processing means; 2) incidentally incised into the tumor during operation, with the tumor bed confirmed to be free of any tumor residues; 3) part of tumor pseudocapsule was found to be still in the tumor bed, with no evidence of tumor residue. The true PSMs had two subtypes: 1) a large number of residual tumor cells at the surgical margin; 2) incision of satellite tumor nodules around the large tumor. The relapse rate after NSS was subsequently compared between patients with PSMs and NSMs, true PSMS and NSMs, and false PSMs and NSMs.
Fisher’s exact test was employed to evaluate the proportions of the three groups. All P-values were two sided, while a P-value of <0.05 was considered to be indicative of statistical significance. All statistical analyses were performed using SPSS v. 17 statistical software (SPSS Inc., Chicago, IL, USA).
Results
There were a total of 600 cases collected for the current survey from four centers, including 20 cases with positive margins on final pathological examination. The average positive margin rate was found to be 3.3%. All patients with or without PSMs were followed up for a median duration of 56 months. The follow-up was specific to each institution’s practice with a physical examination and a CT scan of the abdomen usually included. There were 20 patients with recurrence events (3 with PSMs and 17 with NSMs). None of the patients developed metastatic progression, with only one case of in situ recurrence detected, which was connected with NSMs (Table 1). On the basis of the analytical observations of the sections for pathological examination, we raised a set of new classification criteria: 1) false PSMs, which could be further divided into three subtypes: i) no standard processing was performed on the pathological specimens, leading to false positives (Type A; Figure 1); ii) incidental incision into the tumor during the operative procedure, with the tumor bed noted to be free of any tumor residues (Type B; Figures 2 and 3); iii) part of tumor pseudocapsule remained in the tumor bed, with no sign of tumor residue (Type C; Figure 4). 2) True PSMs, which could be further subdivided into two subtypes: i) a large number of residual tumor cells at the margin, as well as the tumor bed (Type D; Figure 5); ii) incision of satellite tumor nodules in the vicinity of a large tumor, resulting in positive margins (Type E; Figure 6). The pathological and clinical data of 20 cases with PSMs were summarized (Table 2). Five patients had true PSMs on final pathological examination, with two cases of tumor recurrence. Fifteen patients were detected to have false PSMs, with one case of recurrence. The relapse rates after NSS between patients with PSMs and NSMs, true PSMS and NSMs, and false PSMs and NSMs were determined using Fisher’s exact test, P=0.0252, 0.0094, and 0.3727, respectively (Figures 7–9).
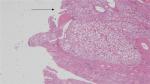  | Figure 1 Surgical margin without ink stain. |
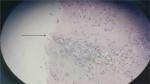  | Figure 2 A small amount of tumor cells at the margin of incision. |
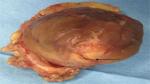  | Figure 3 Accidental tumor incision during NSS. Abbreviation: NSS, nephron-sparing surgery. |
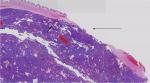  | Figure 4 Part of tumor pseudocapsule remains in the tumor bed, but there are no tumor residues. |
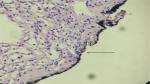  | Figure 5 A large number of residual tumor cells at the margin. |
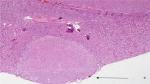  | Figure 6 Incision of satellite tumor nodules detected around the large tumor. |
  | Table 2 Pathological classification and recurrence characteristics Note: True PSMs obviously increases the tumor recurrence rate. Abbreviation: PSMs, positive surgical margins. |
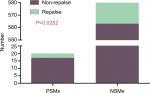  | Figure 7 PSMs significantly increases the tumor recurrence rate (P=0.02). Abbreviations: NSMs, negative surgical margins; PSMs, positive surgical margins. |
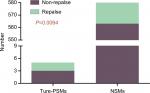  | Figure 8 True PSMs significantly increases the tumor recurrence rate (P=0.009). Abbreviations: NSMs, negative surgical margins; PSMs, positive surgical margins. |
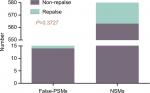  | Figure 9 False PSMs does not increase the tumor recurrence rate (P=0.37). Abbreviations: NSMs, negative surgical margins; PSMs, positive surgical margins. |
Discussion
Small renal cancer is commonly defined as the existence of a mass with a dimension of 4 cm or less detected on abdominal imaging.8 Surgery remains the optimal therapeutic approach for cases of RCC. More recently, NSS has been suggested to be a standard for treating small renal tumors. Both open nephron-sparing and laparoscopic nephron-sparing surgical methods, along with the long-term oncological results, have not been found to be worse than radical nephrectomy, which are also accompanied by a lower risk of developing renal insufficiency or contralateral renal disease in the future.9 Compared to open surgery, laparoscopic partial nephrectomy is accompanied by a greater set of difficulties and requires a more sophisticated set of surgical skills. However, due to the rapid development of technological and surgical skills, more and more laparoscopic partial nephrectomy procedures have been conducted in recent years. The primary objective of oncological surgery is to completely remove the tumor while leaving an NSM. However, in the event that a partial nephrectomy procedure is performed, PSMs may occasionally occur. In recent years, there have been a number of articles that have suggested that the oncological outcomes between renal tumor enucleation and NSS are similar; however, the positive margins of enucleation appear to be higher than the NSS.10 There is no doubt that resection of one kidney or the removal of a tumor with an adequate normal parenchyma margin is almost always a guarantee of an NSM. However, it may increase the risk of developing renal insufficiency in the future, ultimately highlighting the notable significance associated with the confirmation of an NSM during NSS.
Consistent with the conclusion of the current study, PSMs after NSS have been reported to be somewhat of a rare event, with studies indicating that the overall rates of PSMs range between 0% and 7% in literature of recent years,11 0% and 7% after open NSS, 0.7% and 4% after laparoscopic NSS, and 3.9% and 5.7% after robot-assisted NSS.12 Tabayoyong et al13 performed the analysis of the National Cancer Database data of 11,587 patients who were treated with partial nephrectomy from 2010 to 2011, concluding that the PSM rate of laparoscopic and robotic approaches was higher than that of open partial nephrectomy. Meanwhile, the greater majority of researchers believe that PSMs have no significant impact on both the overall survival and cancer-specific survival of patients undertaking by NSS.4 However, controversial conclusions in literature regarding the recurrence of PSMs in NSS have been noted. Although Bernhard et al14 reported that the ipsilateral recurrence rate was ~3.2% after NSS, bilateral tumors, tumor size >4 cm, and PSMs have been positively correlated with recurrence. The majority of research findings indicated that the presence of PSMs did not increase the risk of tumor recurrence.15–17 A study reported that after a 10-year follow up, there was no significant difference between patients with PSMs and patients with no tumor residues (P=0.97).15 In other words, PSMs were not associated with both local recurrence and distant recurrence. However, investigators holding differing views have challenged this notion. A retrospective study reviewing 1,240 patients found PSMs in 97 patients, during a median follow-up of 33 months, and the results obtained revealed that PSMs were associated with a greater risk of relapse following multivariable analysis (P=0.03).18 The summarized versions of these documents are illustrated in Table 3. Unlike the greater majority of scholar conclusions, we found that PSMs increase the risk of tumor relapse when compared with NSMs (P=0.0252). However, the majority of patients with PSMs did not fall victim to tumor recurrences; thus, we conclude that cautious observation should be maintained.
Snarskis et al proposed a new scoring system based on tumor invasion of pseudocapsule (i-Cap). The tumors were completely within the pseudocapsule, and the pseudocapsule integrity was defined as i-Cap score of 1. The tumors invaded the pseudocapsule, but did not break the pseudocapsule defined as i-Cap of 2. i-Cap score of 3 was assigned to tumors that had broken through the pseudocapsule and extended into surrounding healthy parenchyma. During their studies, they collected data from 267 patients, with scores of 1, 2, and 3, respectively, accounting for 24.2%, 53.8%, and 22%.19 On the basis of this scoring system, we also evaluated 20 of our own patients with PSMs (Table 4) and found that the proportion of patients with a score of 3 was higher in our study.
  | Table 4 Clinical data of 20 patients with PSMs Abbreviations: F, female; i-Cap, invasion of pseudocapsule; M, male; NA, not applicable; PSMs, positive surgical margins. |
After vigilant evaluation of the pathological findings and clinical data, we speculated that arguments may arise due to the lack of appropriate pathological classifications. Based on our observations, no articles regarding the PSMs of NSS and the prognostic analysis exist. We are the first to present a systematic summary and classification. Following specimen collection and delivery to a pathologist, ink stains of the surgical margin is the standard method for sample processing, followed by slicing it into sections. If the margin of the sample has tumor cells, it is deemed to be truly positive. However, some pathologists choose not to ink stain the specimen in many cases, which may lead to false positives. Meanwhile, surgeons receive the specimen without adherence to standard procedure or cut the specimen inappropriately, which may result in false positives, as well. Furthermore, accidental tumor incision during NSS may also cause false PSMs. In this study, we found seven patients of this type and one patient with recurrence during follow-up. We suggest that surgical specimens should be treated in accordance with the recommendations of The International Society of Urological Pathology Consensus. When extracting specimens, caution should be exercised to ensure not to use tweezers or forceps, aiming to maintain the original form of all submission, while the ink should be used to mark the margin prior to pathological incision.20
The second subtype of false PSMs indicated a small amount of tumor cells at the margin of incision with none or very few tumor cells left at the NSS bed. However, surgeons often employ argon beam coagulation to achieve satisfactory renal parenchymal hemostasis after tumor excision in the NSS bed, which will also destroy the potential cancer cells.21 The application of absorbable gelatin sponge or other hemostatic materials is more common in NSS bed, with some scholars suggesting that these materials could lead to a direct ablation of cancer cells through an inflammatory reaction or immunological response with cytotoxic capacity.22 Moreover, clamping of the renal artery may induce renal ischemia, owing to the high metabolic requirements, and cancer cells may be extirpated without their required nourishment.15 For these given reasons, the residual tumor cells will be cleared. Thus, we conclude that this situation has no influence on cancer relapse.
If a renal tumor has clear signs of a pseudocapsule, NSS could lead to the rupture of the tumor pseudocapsule, leaving part of the pseudocapsule in the tumor bed, resulting in a pathological diagnosis with positive margin. In reality, the entire mass had been resected successfully. We classified this as the third subtype of true PSMs. The traditional recommendation of a surgical margin width is at least 1 cm of normally appearing renal tissue around the mass to ensure complete tumor removal and get a negative margin.23 Some scholars have subsequently suggested that, as long as the tumor is completely resected, a margin of <5 mm is safe and effective in treating small renal mass by NSS, exhibiting excellent preservation of renal function and favorable long-term progression-free survival without increasing the risk of local recurrence.24 However, accumulating data have shown that margin thickness was not correlated with tumor progression and relapse, while also indicating that it could avoid the loss of healthy issue.25 This being said, we suggest the enucleation of the tumor, particularly tumors with a pseudocapsule. Considerations were made in relation to the incidental cutting of the renal mass pseudocapsule during an operation, even with complete resection of the tumor, with no tumor cells left in the tumor bed. However, when pathologists receive a tumor sample with ruptured pseudocapsule, they may present a pathological finding of PSMs. Yet, with no tumor cell residue, there is no need for further treatment. Thus, we disagree with the notion that this situation influences tumor recurrence.
Comparing the tumor recurrence rate of false PSMs and NSMs, we obtained a P-value of 0.3727, obviously, indicating that false PSMs had no impact on tumor recurrence.
Then, we discuss the true PSMs: true PSMs significantly increase the tumor recurrence rate (P=0.0094). Some tumors have no pseudocapsules, and surgeons are often unable to visually recognize the correct tumor margins. There could be a wide range of tumor cells at the margin, with a greater possibility of residual tumor cell, leading to the increased possibility of local recurrence. This positive margin would require increased clinical emphasis, especially in dealing with high-grade tumors, which would require radical resection as soon as possible. We would recommend secondary NSS rather than the application of radical nephrectomy. Analysis of our postoperative recurrence data revealed one case of the aforementioned type.
Besides the subtype one, some satellite tumor nodules are within the vicinity of the main renal tumor, and incision of satellite tumor nodules often occurs during NSS. This statue was identified as the second category of true PSMs, because the satellite tumor nodules cannot be detected by preoperative imaging, as well as gross examination during operation. Adequate considerations were made regarding the relative ease involved with the incision of satellite tumor nodules, resulting in the occurrence of PSMs. In our study, we found a single case of this type, which relapsed during follow-up. In the event of this particular PSMs occurrence, we recommend radical nephrectomy.
Based on the aforementioned analyses, although the pathological findings were PSMs, it appears that most PSMs are not equal to true tumor residue, and this may be a reason for the low rate of tumor recurrence during our follow-up. Therefore, we were more inclined to conduct a closer follow-up in lieu of a more aggressive surgical approach, after excluding the first and second case of true PSMs as mentioned above. We strongly recommend that urologists and pathologists actively communicate to further clarify which type of positive margins exists after the detection of PSMs.
There were certain limitations faced during our study. First, this was a retrospective study, which could lead us to be vulnerable to potential biases. With the possibility that the level of evidence was not strong enough due to ethical issues, prospective studies cannot be started. Second, our data were collected from four different centers, with no realistic method of centralizing or proofreading all specimens, which may of course had certain variations among them, such as the level of medical care, the surgical techniques, and pathological assessment, in addition to the fact that some centers did not dye the suspicious margins, which could lead to a misdiagnosis. Third, due to the low positive margins, the samples were small, leading to a higher heterogeneity of the study. Fourth, no data relating to the association of tumor grade and clinical stage with PSMs were included in the study. Finally, our follow-up duration was relatively short.
Conclusion
Regardless of the aforementioned limitations, our study does shed light on the relationship between the classification of PSMs and tumor recurrence after NSS for small renal masses. We recommend that PSMs be vigilantly analyzed and treated on an individual basis. However, the key findings of our study present evidence suggesting radical surgery or active surveillance when PSMs are found after NSS.
Ethics approval and consent to participate
This study was approved by ethical committee review board of the Second Hospital of Tianjin Medical University, Tianjin, China; ethical committee review board of Yuhuangding Hospital of Qingdao University, Yantai, China; ethical committee review board of the People’s Hospital of Liao Cheng, Liaocheng, China; and ethical committee review board of the Affiliated Hospital of Jining Medical University, Jining, China. All participants received written informed consent.
Data sharing statement
The data supporting the results of this research are available from the corresponding author at reasonable request.
Acknowledgment
This work was supported by National Natural Science Foundation of China (grants 81472682 and 81772756), Natural Science Foundation of Tianjin (17JCZDJC35300, 15JCZDJC35400, and 15JCYBJC27200), and Tianjin Municipal Natural Science Foundation (grant 17JCYBJC26000).
Author contributions
YJN, GL, and DSZ conceived and directed the project. DSZ and GL contributed to the writing of the manuscript. GL, DSZ, ZQL, AXW, YHL, and RYZ analyzed the data. All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49(6):1374–1403. | ||
Kato M, Suzuki T, Suzuki Y, Terasawa Y, Sasano H, Arai Y. Natural history of small renal cell carcinoma: evaluation of growth rate, histological grade, cell proliferation and apoptosis. J Urol. 2004;172(3):863–866. | ||
Ljungberg B, Cowan NC, Hanbury DC, et al; European Association of Urology Guideline Group. EAU guidelines on renal cell carcinoma: the 2010 update. Eur Urol. 2010;58(3):398–406. | ||
Bensalah K, Pantuck AJ, Rioux-Leclercq N, et al. Positive surgical margin appears to have negligible impact on survival of renal cell carcinomas treated by nephron-sparing surgery. Eur Urol. 2010;57(3):466–473. | ||
Breda A, Stepanian SV, Liao J, et al. Positive margins in laparoscopic partial nephrectomy in 855 cases: a multi-institutional survey from the United States and Europe. J Urol. 2007;178(1):47–50. | ||
Kieran K, Montgomery JS, Daignault S, Roberts WW, Wolf JS. Comparison of intraoperative parameters and perioperative complications of retroperitoneal and transperitoneal approaches to laparoscopic partial nephrectomy: support for a retroperitoneal approach in selected patients. J Endourol. 2007;21(7):754–759. | ||
Patard JJ, Pantuck AJ, Crepel M, et al. Morbidity and clinical outcome of nephron-sparing surgery in relation to tumour size and indication. Eur Urol. 2007;52(1):148–154. | ||
Gill IS, Aron M, Gervais DA, Jewett MA. Clinical practice. Small renal mass. N Engl J Med. 2010;362(7):624–634. | ||
Fergany AF, Hafez KS, Novick AC. Long-term results of nephron sparing surgery for localized renal cell carcinoma: 10-year followup. J Urol. 2000;163(2):442–445. | ||
Wang L, Hughes I, Snarskis C, et al. Tumor enucleation specimens of small renal tumors more frequently have a positive surgical margin than partial nephrectomy specimens, but this is not associated with local tumor recurrence. Virchows Arch. 2017;470(1):55–61. | ||
Kwon EO, Carver BS, Snyder ME, Russo P. Impact of positive surgical margins in patients undergoing partial nephrectomy for renal cortical tumours. BJU Int. 2007;99(2):286–289. | ||
Marszalek M, Carini M, Chlosta P, et al. Positive surgical margins after nephron-sparing surgery. Eur Urol. 2012;61(4):757–763. | ||
Tabayoyong W, Abouassaly R, Kiechle JE, et al. Variation in surgical margin status by surgical approach among patients undergoing partial nephrectomy for small renal masses. J Urol. 2015;194(6):1548–1553. | ||
Bernhard JC, Pantuck AJ, Wallerand H, et al. Predictive factors for ipsilateral recurrence after nephron-sparing surgery in renal cell carcinoma. Eur Urol. 2010;57(6):1080–1086. | ||
Yossepowitch O, Thompson RH, Leibovich BC, et al. Positive surgical margins at partial nephrectomy: predictors and oncological outcomes. J Urol. 2008;179(6):2158–2163. | ||
Peycelon M, Hupertan V, Comperat E, et al. Long-term outcomes after nephron sparing surgery for renal cell carcinoma larger than 4 cm. J Urol. 2009;181(1):35–41. | ||
Marszalek M, Meixl H, Polajnar M, Rauchenwald M, Jeschke K, Madersbacher S. Laparoscopic and open partial nephrectomy: a matched-pair comparison of 200 patients. Eur Urol. 2009;55(5):1171–1178. | ||
Shah PH, Moreira DM, Okhunov Z, et al. Positive surgical margins increase risk of recurrence after partial nephrectomy for high risk renal tumors. J Urol. 2016;196(2):327–334. | ||
Snarskis C, Calaway AC, Wang L, et al. Standardized reporting of microscopic renal tumor margins: introduction of the renal tumor capsule invasion scoring system. J Urol. 2017;197(1):23–30. | ||
Trpkov K, Grignon DJ, Bonsib SM, et al; Members of the ISUP Renal Tumor Panel. Handling and staging of renal cell carcinoma: the International Society of Urological Pathology Consensus (ISUP) conference recommendations. Am J Surg Pathol. 2013;37(10):1505–1517. | ||
Eggener S. Editorial comment on: positive surgical margin appears to have negligible impact on survival of renal cell carcinomas treated by nephron-sparing surgery. Eur Urol. 2010;57(3):472. | ||
Lam JS, Bergman J, Breda A, Schulam PG. Importance of surgical margins in the management of renal cell carcinoma. Nat Clin Pract Urol. 2008;5(6):308–317. | ||
Uzzo RG, Novick AC. Nephron sparing surgery for renal tumors: indications, techniques and outcomes. J Urol. 2001;166(1):6–18. | ||
Li QL, Cheng L, Guan HW, Zhang Y, Wang FP, Song XS. Safety and efficacy of mini-margin nephron-sparing surgery for renal cell carcinoma 4-cm or less. Urology. 2008;71(5):924–927. | ||
Sutherland SE, Resnick MI, Maclennan GT, Goldman HB. Does the size of the surgical margin in partial nephrectomy for renal cell cancer really matter? J Urol. 2002;167(1):61–64. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


