Back to Journals » Journal of Pain Research » Volume 16
Circum-Psoas Block versus Supra-Inguinal Fascia Iliaca Block for Postoperative Analgesia in Patients Undergoing Total Hip Arthroplasty: A Randomized Clinical Trial
Authors Zheng J, Mi Y, Liang J , Li H , Shao P, Wen H, Wang Y
Received 13 August 2023
Accepted for publication 14 November 2023
Published 20 November 2023 Volume 2023:16 Pages 3961—3970
DOI https://doi.org/10.2147/JPR.S435159
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jinlei Li
Junwei Zheng,1,2 Yan Mi,3 Jinghan Liang,1 Huili Li,1 Peiqi Shao,1 Hong Wen,1 Yun Wang1
1Department of Anesthesiology, Beijing Chaoyang Hospital, Capital Medical University, Beijing, People’s Republic of China; 2Department of Anesthesiology, Renmin Hospital, Hubei University of Medicine, Shiyan, Hubei Province, People’s Republic of China; 3Department of Anesthesiology, Tumour Hospital, Zhengzhou University, Zhengzhou, Henan Province, People’s Republic of China
Correspondence: Yun Wang; Hong Wen, Department of Anesthesiology, Beijing Chaoyang Hospital, Capital Medical University, Beijing, 100020, People’s Republic of China, Tel +86-10-85231330, Fax +86-10-65077808, Email [email protected]; [email protected]
Purpose: Total hip arthroplasty (THA) is often associated with moderate to severe pain. The present study compared the efficacy of circum-psoas block (CPB) with supra-inguinal fascia iliaca block (SIFIB) for postoperative analgesia in patients undergoing THA.
Patients and Methods: In this randomized trial, sixty-four patients undergoing THA were allocated randomly to the CPB group or SIFIB group with 40 mL of 0.3% ropivacaine. The primary outcome was dynamic pain score at 6 h postoperatively. Secondary outcomes included dynamic pain scores at 12, 24 and 48 h; static pain scores; sensory and motor block; opioid consumption; time to first opioid request; length of hospital stay; patient satisfaction; and adverse events.
Results: CPB patients showed significantly lower dynamic pain scores at 6 (3.11 ± 0.66 vs 4.47 ± 0.74, respectively; P = 0.000), 12 (2.52 ± 0.73 vs 3.53 ± 0.85, respectively; P = 0.000) and 24 h (2.30 ± 0.57 vs 2.87 ± 0.71, respectively; P = 0.001) after surgery, as well as lower static pain scores at 6 and 12h (P = 0.001 and P = 0.033 respectively) than SIFIB patients. Lower opioid consumption was observed in the CPB group at 24 and 48 h (P = 0.000, both) than in the SIFIB group. Patients in the CPB group reported improved quadriceps strength at 6 and 12 h (P = 0.000, both), as well as better muscle strength of hip flexion at 6, 12 and 24 h (P = 0.000, P = 0.000 and P = 0.025 respectively). Compared with SIFIB, CPB was associated with increased sensory block coverage at 6, 12 and 24 h (P = 0.000, P = 0.000, and P =0.022, respectively).
Conclusion: CPB has a greater potential to alleviate postoperative pain and improve recovery in THA patients than SIFIB.
Keywords: total hip arthroplasty, lumbar plexus, sacral plexus, regional anesthesia
Introduction
Total hip arthroplasty (THA) is a common surgical procedure, with more than 280,000 THA procedures performed annually in the United States.1 Patients undergoing THA often experience moderate to severe pain, which can reduce their overall quality of life and even develop into persistent pain.2 Consequently, efforts are made to facilitate enhanced recovery profiles to reduce postoperative pain in THA patients. At present, multimodal analgesia approaches have been used for pain management after THA, including oral analgesia, epidural analgesia, and peripheral nerve block.3–6 However, pain management after THA is a challenging goal to achieve, and an ideal analgesic regimen for THA has yet to be defined.
Hebbard et al first proposed ultrasound-guided supra-inguinal fascia iliaca block (SIFIB) in 2011.7 As part of a multimodal analgesic approach, it is now frequently used for THA.8,9 With the cranial spread of local anesthetic (LA), SIFIB has been suggested to effectively block the femoral nerve (FN), the obturator nerve (ON) and the lateral femoral cutaneous nerve (LFCN).10 However, the SIFIB approach often leads to quadricep weakness11,12 and fails to block the subcostal nerve, iliohypogastric nerve, ilioinguinal nerve, and sacral nerves, which are considered to be highly important for postoperative analgesia after THA.13–15 In addition, Bendtsen et al argued that the ON did not cross the fascia iliaca compartment,16 thus compromising the effectiveness of the obturator nerve block caused by SIFIB.
Recently, Li et al reported a new circum-psoas block (CPB) technique.17 With this approach, sensory block coverage can be achieved from the T8-11 to S1-3 dermatomes with little effect on quadriceps strength. This may help to provide more widespread analgesia coverage than the SIFIB approach in patients with THA.
We thus conducted this study to assess whether CPB is associated with superior pain control and reduced opioid consumption compared to SIFIB among patients undergoing THA. The primary outcome was dynamic pain score at 6 h after surgery.
Materials and Methods
The current trial was approved by the Ethics Committee Board of Beijing Chaoyang Hospital, Capital Medical University on October 11, 2021, with reference number 2021-ke-583 and registered at http://www.chictr.org.cn (ChiCTR2100053421) on November 20, 2021. The study adhered to the Consolidated Standards of Reporting Trials (CONSORT) guidelines and Helsinki declaration.
Participants
We enrolled patients between December 31, 2021, and September 10, 2022, who would undergo elective THA with posterolateral approach under spinal anesthesia (Figure 1). The study protocol was explained to eligible patients, and they provided written informed consent to our team. The study included participants aged 18–90 with a body mass index (BMI) of 18–30 kg/m2 and American Society of Anesthesiologists (ASA) physical status class I to III. Patients with puncture site infection, anticoagulant status, allergy to LA, serious cardiovascular disease and blood disease, chronic opioid consumption or alcohol abuse, myasthenia gravis or Parkinson’s disease or pregnancy, previous hip or spinal surgery history, narrow interspace between the transverse process of L5 and the sacral ala (X-ray film anteroposterior: less than 1 cm), cognitive dysfunction before surgery and inability to cooperate with pain assessment were excluded.
 |
Figure 1 The CONSORT study flowchart. Abbreviations: CONSORT, Consolidated Standards of Reporting Trials; PCA, patient-controlled analgesia. |
Randomization and Blinding
Sixty-four eligible participants were randomly assigned to receive either SIFIB or CPB by computerized sequence generation. Randomization and group allocation were performed at a 1:1 intergroup ratio, and allocation information was placed in opaque sealed envelope. Two investigators blinded to the study design opened the sealed envelope just before surgery and prepared a syringe containing 40 mL of 0.3% ropivacaine that was labeled with the randomization number of the patient receiving the block. Afterward, a skilled anesthesiologist performed the ultrasound-guided nerve block according to the allocation. All other researchers and outcome assessors were masked to the allocation of the groups until the study was completed.
Intraoperative Management
In the preoperative holding area, all patients received celecoxib 200 mg and acetaminophen 650 mg orally. After entering the operating room, all patients were monitored with standard ASA monitors (including pulse oximetry, noninvasive blood pressure, and electrocardiography), and peripheral venous access was obtained. Then, an arterial catheter was inserted into the radial artery for haemodynamic monitoring.
After premedication with 1–2 mg midazolam, patients were placed in a lateral decubitus position with the operative side up to receive spinal anesthesia. Hypobaric ropivacaine (2.5 mL of 0.5% ropivacaine) was administered intrathecally at the L3-4 interspace, which was injected over 30s. Patients remained for 15 minutes in a lateral decubitus position to achieve a more intense block of the operative side. Spinal anesthesia was considered to be successful if sensory block to T10 level was acquired,18 as evaluated by loss of cold sensation. For both blocks, a 100-mm, 22-gauge needle (Tuoren, Henan, China) was inserted with strict antiseptic protocols. All procedures were performed under ultrasonographic guidance (KONICA Minolta, Tokyo, Japan) by trained regional anesthesia specialists (YW, JZ, and HL), who performed CPB and SIFIB in more than 25 patients in the preliminary study.
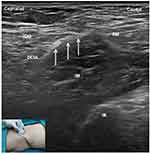
For SIFIB, patients were placed supine after 15 minutes of spinal anesthesia. A linear transducer was used to obtain the image of the anterior superior iliac spine in the sagittal position. The internal oblique muscles, iliacus and sartorius can be identified by sliding the probe medially (Figure 2). After identifying the “bow-tie sign”, the needle was inserted in a caudad-cephalad direction. Using an in-plane approach, the fascia iliaca was penetrated and hydrodissected from the iliacus muscle with 0.9% saline (2–5 mL). Upward movement of the deep circumflex artery after injection was a sign of a successful injection beneath the fascia iliaca. Following repeated negative aspiration, 40 mL of 0.3% ropivacaine was injected into the fascia iliaca compartment.
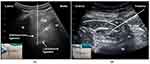
For CPB, the procedure was performed with lateral decubitus after 15 minutes of spinal anesthesia. A curved array transducer was placed in a paramedian sagittal plane to identify the sacrum and the transverse process of the L5 vertebral body of the surgical side. In this position, the probe was rotated 90 degrees to obtain the paramedian transverse plane of the lumbosacral region until the intertransverse ligament, and the lumbosacral ligament could be seen in the sonogram between the iliac bone and laminae of L5 (Figure 3A). Using an out-of-plane approach, the needle was inserted to penetrate the erector spinae, the intertransverse ligament, and the lumbosacral ligament. An endpoint of injection was an observation of the needle tip penetrating the lumbosacral ligament and a distinct loss of resistance.19 Injection of 2–5 mL of 0.9% saline was used to confirm the correct position of the needle tip. An additional 20 mL of 0.3% ropivacaine was injected with repeated negative aspiration. After that, the curved array transducer was placed cranial to the iliac crest in a transverse plane and slid to identify the shamrock sign. A puncture needle was then inserted in a posterolateral to anteromedial direction with an in-plane approach until the needle tip penetrated the transversalis fascia covering the psoas major (PM) muscle, and a loss of resistance was obtained (Figure 3B). The distribution of fluid around the antero-lateral margin of the PM muscle beneath the transversalis fascia was used as an indicator of successful penetration of the transversalis fascia following injection of 2–5 mL of 0.9% saline. With intermittent negative aspiration, an additional 20 mL of 0.3% ropivacaine was injected. In this study, all surgical interventions were performed by the same team of orthopaedic surgeons from our institution, and the duration of surgery was recorded.
Postoperative Pain Management
After surgery, patients were provided with a patient-controlled analgesia (PCA) pump, which was programmed to deliver 2 μg of intravenous sufentanil on demand with a lockout interval of 15 min. In the surgical ward, in addition to PCA, they also received regular doses of celecoxib 200 mg orally every 12 h and acetaminophen 650 mg orally every 8 h. Rescue analgesics (administered if patients could not tolerate the pain) consisted of oxycodone 5 mg administered orally in the ward.
Assessment of Outcomes
The primary outcome was dynamic (with passive 90° flexion of the hip) pain score at 6 h after surgery. Pain intensity was evaluated using a visual analogue scale (VAS) ranging from 0 (no pain) to 10 (extreme pain).
Secondary outcomes included opioid consumption at 24 and 48 h (expressed as intravenous morphine milligram equivalents (MME) using a conversion factor of 100020 and 1.521 for intravenous sufentanil and oral oxycodone, respectively); dynamic pain scores at 12, 24 and 48 h; static (at rest) pain scores, sensory block, motor block at 6, 12, 24 and 48 h; first opioid request; length of hospital stay (the time from randomization to hospital discharge); patient satisfaction (0 = “unsatisfied”, 10 = “extremely satisfied”); and incidence of adverse events (including nausea, vomiting, dizziness, urinary retention, respiratory depression, vascular puncture, nerve injury, and local anesthetic toxicity).
Sensory block was evaluated by using the standardized stimuli of ice and compared with the contralateral side. Rating was categorized as normal sensation and any changes in sensation (eg, no sensation or decreased sensation). The test was conducted at the anterior axillary line to determine the blocked areas of thoracic spinal nerves.22 Lumbosacral nerves (L1-S3), FN and LFCN were measured at different dermatomes according to a previous study.23
Motor block was evaluated by measuring the muscle strength of hip flexion, knee extension, and ankle flexion. The following criteria were used to score muscle strength: 0, no muscle contraction; 1, muscle contraction without joint movement; 2, joint movement but no gravity resistance; 3, movement against gravity; 4, gravity and partial against resistance; and 5, normal strength.
Statistical Analysis
Based on a previous study, the median minimal clinically important difference (median MCID) for dynamic pain score was absolute 1.8 after THA.24 This study was powered to detect a difference of 2 in dynamic pain scores21 between patients undergoing CPB versus those receiving SIFIB, assuming a SD of 2.5. Using a two-sample t test, a sample of 52 participants (26 in each group) would have approximately 80% power to detect a clinically significant reduction in pain score with a significance level alpha of 0.05. Taking into account a dropout rate of approximately 20%, a total of 64 patients were selected with 32 patients per group.
Statistical analysis was performed using the SPSS V.19 software package (IBM, Armonk, New York). For continuous data, normality was first assessed with the Shapiro–Wilk normality test. Data with a normal distribution were reported as mean [standard deviation (SD)] and analyzed with Student’s t test, while nonnormal data and ordinal data were expressed as median [interquartile range (IQR)] and analyzed with the Mann‒Whitney U-test. Categorical data were compared with the chi-squared (χ²) test. When the expected count for any of the aforementioned categorical data was less than five, Fisher’s exact test was used. A two-sided P value of <0.05 was considered as statistically significant.
Results
The flowchart according to the CONSORT statement is shown in Figure 1. In the present study, 67 patients were enrolled, and three were excluded due to refusal to participate. Consequently, we randomly assigned 64 patients to either the CPB or SIFIB groups, each with 32 patients. One SIFIB group patient withdrew from PCA analgesia due to dizziness. The block failed in one CPB group patient due to poor ultrasound image quality. Finally, 62 patients completed the study and were analyzed (n = 31 in each group). The demographic parameters, surgical side and surgical duration are presented in Table 1.
 |
Table 1 Demographic and Clinical Characteristics |
Primary Outcome
As shown in Table 2, patients receiving CPB had lower dynamic pain scores than those receiving SIFIB at 6 hours after surgery (3.11 ± 0.66 vs 4.47 ± 0.74, respectively), reaching statistical significance (P = 0.000).
 |
Table 2 Postoperative Pain Scores, Opioid Consumption, and Number of Affected Dermatomes |
Secondary Outcomes
In the CPB group, dynamic pain scores were significantly lower at 12 h and 24 h (P = 0.000 and P = 0.001 respectively), as well as static pain scores at 6 h and 12 h (P = 0.001 and P = 0.033 respectively) after surgery (Table 2). The VAS scores of the two groups were not significantly different at 48 h after surgery. Besides, the cumulative opioid requirements were lower in the CPB group at 24 h and 48 h (P = 0.000, both) than in the SIFIB group (Table 2). There were no differences in the proportion of patients who needed oxycodone at 24 (CPB 3.2% vs SIFIB 16.1%, P = 0.2) and 48 h (CPB 3.2% vs SIFIB 19.4%, P = 0.1).
Compared with SIFIB, CPB was associated with reduced motor block of the quadriceps at 6 and 12 h (P = 0.000, both), as evidenced by enhanced knee extension (Table 3). Furthermore, CPB significantly improved the muscle strength of hip flexion at 6, 12 and 24 h (P = 0.000, P = 0.000 and P = 0.025 respectively). Patients in the CPB group reported median (IQR) muscle strength with ankle flexion at 6 h of 5 [5 to 5] compared to 5 [5 to 5] in the SIFIB group (P = 0.088). The muscle strength of ankle flexion was not reported at 12, 24 and 48 h after surgery since it completely regressed at 12 h in all patients.
 |
Table 3 Postoperative Motor Block, Adverse Events, Length of Hospital Stay, and Satisfaction Score |
The presence of sensory blockade for different dermatomes and for the terminal nerves are shown in Figure 4. Compared with SIFIB, CPB was associated with significantly increased dermatomal coverage of sensory block at 6, 12 and 24 h (P = 0.000, P = 0.000 and P = 0.022 respectively).
As shown in Table 3, patient satisfaction scores were high between groups with no significant difference. There was no difference in length of hospital stay (median, IQR) – CPB group 6 [5 to 6] days, SIFIB 6 [5 to 7] days. No differences were found in the incidence of nausea, vomiting, dizziness, or urinary retention after surgery between the two groups. No patients in either group suffered clinically significant vascular injury, nerve injury, respiratory depression, or local anesthetic toxicity.
Discussion
This prospective randomized trial compared ultrasound-guided CPB with SIFIB in patients undergoing THA. We found that patients in the CPB group had significantly lower dynamic pain scores at 6 h with improved quadriceps strength when compared with SIFIB. These findings support the use of CPB as an effective approach to promote rehabilitation in THA patients.
Although Diwan et al initially described circumpsoas block25 by depositing LA between the anterior psoas fascia and the PM muscle, this method can only block nerves emerging from the lumbar plexus. Due to this, Li et al proposed another method for CPB in the previous study,17 which differed from Diwan’s method in terms of injection sites and dermatomal coverage. In the present study, we thus observed extensive sensory block coverage ranging from the T9-12 to S1-3 dermatomes after CPB.
Our primary outcome requires discussion, as a number of clinical studies15,26,27 have chosen opioid consumption as pain surrogate marker since the use of PCA devices became common practice. However, early mobilization with low level of pain intensity is essential for enhanced postoperative recovery. In our study, CPB was associated with significantly lower dynamic pain score compared with SIFIB (3.11 ± 0.66 vs 4.47 ± 0.74) at 6 h postoperatively. Although we did not detect a difference of 2 in dynamic pain score, a 31% decrease in dynamic pain score still echoes the median MCID for a relative pain reduction recommended by Laigaard et al.24 This encouraging result can be attributed to the extensive sensory block coverage of the CPB approach.
We additionally observed lower dynamic pain scores at 12 and 24 h, lower static pain scores, and less opioid consumption postoperatively in patients who received CPB compared with those who received SIFIB. However, the differences between CPB group and SIFIB group may be of limited clinical importance, as they failed to meet the median MCIDs for pain scores and opioid consumption.24 Despite all this, CPB can provide practitioners with alternative analgesic options for managing postoperative pain in patients undergoing THA. In our study, we found no differences in rescue analgesia need between the groups. The result may partly be due to the limited sample size. Furthermore, PCA pumps were used to provide immediate pain relief when pain gradually increased, thus reducing the number of patients requiring rescue analgesia in the SIFIB group.
We also found that CPB led to sensory blockage but had little effect on motor function. This observation may be due to the fact that we use relatively low concentration of ropivacaine. In addition, the nerves are located away from the inject site of CPB block, so ropivacaine hydrochloride could be dissipated during the spread of the LA near the target nerves, which may result in a lower concentration of LA acting on nerves than we actually used. Therefore, CPB is effective in improving the early recovery of motor function by preserving muscle strength. Notably, however, the muscle strength of ankle flexion did not differ between groups. Since CPB results in sensory block at level L5/S1, one should expect an inherent impairment of ankle mobilization as well. The lack of intergroup differences may be explained by the fact that ropivacaine has the property of separation between motor and sensory blockade at low concentrations; thus, our trial may have not been able to fully evaluate the changes in muscle strength using the current method.
CPB appears to be safe in the present study, as no block-related complications were observed. Despite being in a deep location, CPB is situated away from major blood vessels, which reduces the risk of complications. In addition, sonography can be used to identify the needle position and prevent vascular puncture. In our trial, CPB was performed with a relatively low concentration of LA, which produced effective analgesia with a wide margin of safety.
This study has certain limitations. First, SIFIB and CPB would require different positioning, so patients may not be blinded to the nerve block performed. The best way26 to guarantee blinding would have been to perform the sham block and therapeutic block in each patient. We decided not to perform a sham block because we considered patients in category 3b of Serious Harm and Morbidity.28 Second, spinal anesthesia may have the potential to last longer than six hours. However, we used a relatively low concentration of spinal anesthetic, and the median duration of analgesia would not have been expected to last more than 4.5 h.29 In addition, Hu et al also chose dynamic pain score at 6 h after THA as the primary outcome.13 Thus, the time point of 6 h after surgery was deemed reasonable for the primary outcome. Third, we could not evaluate the real-time effects of CPB or SIFIB by checking the dermatomes for the block-level range because we administered the blocks after spinal anesthesia. However, all the block procedures were administered by experienced anesthesiologists who had performed CPB and SIFIB in more than 25 patients before the study. Therefore, we believe that all blocks have been implemented successfully. Finally, this was a single-centre study, and the findings warrant further investigation before application in general practice.
Conclusion
Ultrasound-guided CPB led to better postoperative pain relief, lower opioid consumption, and slightly affected muscle strength compared to SIFIB. Thus, CPB may be a promising approach for analgesia in patients undergoing THA.
Data Sharing Statement
We all agree to share all the data for this article. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Funding
This study was supported by Clinical Research Incubation Project of Beijing Chaoyang Hospital, Capital Medical University (CYFH202205) and the Beijing Hospitals Authority Clinical Medicine Development of Special Funding Support (XMLX202106).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Gwam CU, Mistry JB, Mohamed NS, et al. Current epidemiology of revision total Hip arthroplasty in the United States: national Inpatient Sample 2009 to 2013. J Arthroplasty. 2017;32(7):2088–2092. doi:10.1016/j.arth.2017.02.046
2. Wylde V, Hewlett S, Learmonth ID, Dieppe P. Persistent pain after joint replacement: prevalence, sensory qualities, and postoperative determinants. Pain. 2011;152(3):566–572. doi:10.1016/j.pain.2010.11.023
3. Højer Karlsen AP, Geisler A, Petersen PL, Mathiesen O, Dahl JB. Postoperative pain treatment after total Hip arthroplasty: a systematic review. Pain. 2015;156(1):8–30. doi:10.1016/j.pain.0000000000000003
4. Soffin EM, YaDeau JT. Enhanced recovery after surgery for primary Hip and knee arthroplasty: a review of the evidence. Br J Anaesth. 2016;117:62–72. doi:10.1093/bja/aew362
5. Fillingham YA, Hannon CP, Kopp SL, et al. Regional Nerve Blocks in Primary Total Hip Arthroplasty: the Clinical Practice Guidelines of the American Association of Hip and Knee Surgeons, American Society of Regional Anesthesia and Pain Medicine, American Academy of Orthopaedic Surgeons, Hip Society, and Knee Society. J Arthroplasty. 2022;37(9):1697–1700. doi:10.1016/j.arth.2022.02.121
6. Anger M, Valovska T, Beloeil H, et al. PROSPECT guideline for total Hip arthroplasty: a systematic review and procedure-specific postoperative pain management recommendations. Anaesthesia. 2021;76(8):1082–1097. doi:10.1111/anae.15498
7. Hebbard P, Ivanusic J, Sha S. Ultrasound-guided supra-inguinal fascia iliaca block: a cadaveric evaluation of a novel approach. Anaesthesia. 2011;66(4):300–305. doi:10.1111/j.1365-2044.2011.06628.x
8. Desmet M, Vermeylen K, Van Herreweghe I, et al. A longitudinal supra-inguinal fascia iliaca compartment block reduces morphine consumption after total Hip arthroplasty. Reg Anesth Pain Med. 2017;42(3):327–333. doi:10.1097/AAP.0000000000000543
9. Carella M, Beck F, Piette N, et al. Effect of suprainguinal fascia iliaca compartment block on postoperative opioid consumption and functional recovery in posterolateral-approached total Hip arthroplasty: a single-blind randomized controlled trial. Reg Anesth Pain Med. 2022;47(9):547–553. doi:10.1136/rapm-2021-103427
10. Kim DH, Kim SJ, Liu J, Beathe J, Memtsoudis SG. Fascial plane blocks: a narrative review of the literature. Reg Anesth Pain Med. 2021;46(7):600–617. doi:10.1136/rapm-2020-101909
11. Behrends M, Yap EN, Zhang AL, et al. Preoperative fascia iliaca block does not improve analgesia after arthroscopic Hip surgery, but causes quadriceps muscles weakness: a randomized, double-blind trial. Anesthesiology. 2018;129(3):536–543. doi:10.1097/ALN.0000000000002321
12. Gasanova I, Alexander JC, Estrera K, Wells J, Sunna M, Minhajuddin A. Ultrasound-guided suprainguinal fascia iliaca compartment block versus periarticular infiltration for pain management after total Hip arthroplasty: a randomized controlled trial. Reg Anesth Pain Med. 2019;44(2):206–211. doi:10.1136/rapm-2018-000016
13. Hu J, Wang Q, Zeng Y, Xu M, Gong J, Yang J. The impact of ultrasound-guided transmuscular quadratus lumborum block combined with local infiltration analgesia for arthroplasty on postoperative pain relief. J Clin Anesth. 2021;73:110372. doi:10.1016/j.jclinane.2021.110372
14. Zheng T, Hu B, Zheng CY, Huang FY, Gao F, Zheng XC. Improvement of analgesic efficacy for total Hip arthroplasty by a modified ultrasound-guided supra-inguinal fascia iliaca compartment block. BMC Anesthesiol. 2021;21:1–8. doi:10.1186/s12871-021-01296-8
15. Xia Q, Ding W, Lin C, Xia J, Xu Y, Jia M. Postoperative pain treatment with transmuscular quadratus lumborum block and fascia iliaca compartment block in patients undergoing total Hip arthroplasty: a randomized controlled trial. BMC Anesthesiol. 2021;21(1):1–11. doi:10.1186/s12871-021-01413-7
16. Bendtsen TF, Pedersen EM, Moriggl B, et al. Anatomical considerations for obturator nerve block with fascia iliaca compartment block. Reg Anesth Pain Med. 2021;46(9):806–812. doi:10.1136/rapm-2021-102553
17. Li H, Shi R, Shao P, Wang Y. Evaluation of sensory loss obtained by circum-psoas blocks in patients undergoing total Hip replacement: a descriptive pilot study. J Pain Res. 2022;15:827–835. doi:10.2147/JPR.S354829
18. Almeida CR, Vieira L. Combination of a deep fascia iliaca block with ultra-low dose spinal anesthesia for Hip fracture surgery. Can J Anesth. 2022;69(3):402–404. doi:10.1007/s12630-021-02178-w
19. Strid JM, Pedersen EM, Al-Karradi SN, et al. Real-Time Ultrasound/MRI fusion for Suprasacral parallel shift approach to lumbosacral plexus blockade and analysis of Injectate spread: an exploratory randomized controlled trial. Biomed Res Int. 2017;2017:1873209. doi:10.1155/2017/1873209
20. Li H, Shi R, Shi D, Wang R, Liu Y, Wang Y. Anterior quadratus lumborum block at the lateral supra-arcuate ligament versus transmuscular quadratus lumborum block for postoperative analgesia in patients undergoing laparoscopic nephrectomy: a randomized controlled trial. J Clin Anesth. 2021;75:110561. doi:10.1016/j.jclinane.2021.110561
21. Polania Gutierrez JJ, Ben-David B, Rest C, Grajales MT, Khetarpal SK. Quadratus lumborum block type 3 versus lumbar plexus block in Hip replacement surgery: a randomized, prospective, non-inferiority study. Reg Anesth Pain Med. 2021;46(2):111–117. doi:10.1136/rapm-2020-101915
22. Marhofer D, Marhofer P, Kettner SC, et al. Magnetic resonance imaging analysis of the spread of local anesthetic solution after ultrasound-guided lateral thoracic paravertebral blockade: a volunteer study. Anesthesiology. 2013;118(5):1106–1112. doi:10.1097/ALN.0b013e318289465f
23. Bendtsen TF, Pedersen EM, Haroutounian S, et al. The suprasacral parallel shift vs lumbar plexus blockade with ultrasound guidance in healthy volunteers–a randomised controlled trial. Anaesthesia. 2014;69(11):1227–1240. doi:10.1111/anae.12753
24. Laigaard J, Pedersen C, Rønsbo TN, Mathiesen O, Karlsen APH. Minimal clinically important differences in randomised clinical trials on pain management after total Hip and knee arthroplasty: a systematic review. Br J Anaesth. 2021;126(5):1029–1037. doi:10.1016/j.bja.2021.01.021
25. Diwan S, Nair A, Gawai N, Shah D, Sancheti P. Circumpsoas block–an anterior myofascial plane block for lumbar plexus elements: case report. Braz J Anesthesiol. 2021. doi:10.1016/j.bjane.2021.04.015
26. Bravo D, Layera S, Aliste J, Jara Á, Fernández D, Barrientos C. Lumbar plexus block versus suprainguinal fascia iliaca block for total Hip arthroplasty: a single-blinded, randomized trial. J Clin Anesth. 2020;66:109907. doi:10.1016/j.jclinane.2020.109907
27. Brixel SM, Biboulet P, Swisser F, et al. Posterior quadratus lumborum block in total Hip arthroplasty: a randomized controlled trial. Anesthesiology. 2021;134(5):722–733. doi:10.1097/ALN.0000000000003745
28. Mcguirk S, Fahy C, Costi D, Cyna A. Use of invasive placebos in research on local anaesthetic interventions. Anaesthesia. 2011;66(2):84–91. doi:10.1111/j.1365-2044.2010.06560.x
29. Van Kleef JW, Veering BT, Burm AGL. Spinal anesthesia with ropivacaine: a double-blind study on the efficacy and safety of 0.5% and 0.75% solutions in patients undergoing minor lower limb surgery. Anesth Analg. 1994;78(6):1125–1130.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.