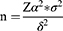Back to Journals » Journal of Inflammation Research » Volume 16
Circulating Inflammatory Factor Levels in the Early Phase of COVID-19 are Associated with the Progression of Respiratory Failure: A Single-Center Retrospective Study
Authors Xiang X, Zhang Z, Liu Y , Xu W, Gong J, Yu S , Zhang L, Jiang T
Received 12 July 2023
Accepted for publication 6 November 2023
Published 14 November 2023 Volume 2023:16 Pages 5249—5260
DOI https://doi.org/10.2147/JIR.S430221
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Monika Sharma
Xiaoli Xiang,1,2 Zhicheng Zhang,1 Ying Liu,1 Wenxuan Xu,1 Ju Gong,3 Sheng Yu,4 Lan Zhang,5 Tingwang Jiang1
1Department of Key Laboratory, Affiliated Changshu Hospital of Nantong University, Changshu, People’s Republic of China; 2Department of Ophthalmology, Affiliated Changshu Hospital of Nantong University, Changshu, People’s Republic of China; 3Department of Emergency Medicine, Affiliated Changshu Hospital of Nantong University, Changshu, People’s Republic of China; 4Department of Critical Care Medicine, Affiliated Changshu Hospital of Nantong University, Changshu, People’s Republic of China; 5Information Center, Affiliated Changshu Hospital of Nantong University, Changshu, People’s Republic of China
Correspondence: Tingwang Jiang, Department of Key Laboratory, Affiliated Changshu Hospital of Nantong University, No. 68 Haiyu Nan Road, Changshu, Jiangsu Province, People’s Republic of China, Tel +86 15995921425, Fax +86 512-52277250, Email [email protected]
Purpose: To evaluate the potential relationships between serum interleukin (IL)-2, IL-4, IL-6, IL-10, IL-17, interferon (IFN)-γ, and tumor necrosis factor (TNF)-α levels and occurrence of respiratory failure in patients with early-stage COVID-19 disease.
Patients and Methods: We analyzed clinical characteristics, laboratory parameters, and immunoinflammatory markers in 302 patients diagnosed with SARS-CoV-2 infection who required hospitalization at Changshu Hospital of Nantong University. IL-2, IL-4, IL-6, IL-10, IL-17, IFN-γ, and TNF-α levels in the peripheral blood of patients hospitalized five days after disease onset were measured using multiplex bead-based flow fluorescent immunoassay (MBFFI).
Results: Patients with respiratory failure had higher serum IL-4 [0 (0, 0.54) pg/mL], IL-6 [40.76 (12.33, 90.28) pg/mL], IL-10 [6.65 (4.12, 11.34) pg/mL], and IL-17 [9.48 (4.31, 12.13) pg/mL] levels than patients without respiratory failure (P=0.042, P< 0.0001, P=0.012, and P=0.036, respectively). Serum IL-2, IFN-γ, and TNF-α levels were not significantly different between the two groups. The occurrence of respiratory failure was positively correlated with sex (R=0.122, P=0.034), lactic acid (R=0.193, P=0.007), white blood cell count (R=0.121, P=0.038), erythrocyte distribution width (R=0.131, P=0.024), thyrocalcitonin (R=0.280, P< 0.0001), and D-dimer levels (R=0.214, P< 0.0001) but negatively correlated with oxygen partial pressure (R=− 0.208, P=0.004), oxygen saturation (R=− 0.220, P=0.002), lymphocyte count (R=− 0.129, P=0.026), and calcium (R=− 0.152, P=0.042). Among the immunoinflammatory biomarkers, the occurrence of respiratory failure was positively correlated with IL-4 (R=− 0.117, P=0.042), IL-6 (R=0.206, P< 0.0001), IL-10 (R=0.145, P=0.012), and IL-17 (R=0.121, P=0.036) levels.
Conclusion: Serum levels of pro-inflammatory cytokines IL-6 and IL-17 and anti-inflammatory cytokines IL-4 and IL-10 were significantly elevated in patients with respiratory failure and weakly positively correlated with the occurrence of respiratory failure. Further studies are required to explore these key immune mechanisms to help clinicians better manage acute complications, long-term sequelae, and possible future COVID-19 variants and be flexible in managing future epidemics and similar public health threats.
Keywords: COVID-19, respiratory failure, hypoxemia, biomarkers, cytokines, disease progression
Introduction
Coronavirus disease 2019 (COVID-19) is the most serious acute respiratory infectious disease globally in recent years,1 and the resulting pandemic has affected patients in various ways. The symptoms of COVID-19 are typically caused by viral replication and disappear within 1–2 weeks.2 Most patients had self-limiting infections and recovered without hospitalization, while 15–20% of them face excessive inflammation caused by a large number of cytokines, which is known as a cytokine storm and eventually leads to alveolar damage and respiratory failure (RF).3 Following acute infection, more serious events occur when myeloid cells such as monocytes and macrophages generate a cytokine storm with the swift release of IL-6, IL-1b, CXCL10, CCL7, and other inflammatory molecules.4 Thus, hyperinflammation is a major problem in COVID-19 cases.5 As the pathological damage caused by COVID-19 involves the immune system, any factor that regulates the immune response may likewise regulate the consequences of COVID-19.6 The pathological features of human severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) remain to be completely discovered; however its immune pathogenesis is known to be related to the feedback imbalance of the immune system. Extensive systemic inflammatory feedback has been found in severe COVID-19 cases, which is regarded as cytokine release syndrome (CRS).7 CRS is common in severe cases of COVID-19, and the increase of serum IL-6 is associated with RF, ARDS, and adverse clinical outcomes.8
In late November 2021, the SARS-CoV2 Omicron (B.1.1.529/21K) variant was detected in South Africa and was associated with rapidly increasing case numbers worldwide. The Omicron variant carries more than 50 mutations and does not appear to change its capacity to induce cytokine release.3 During the initial three-year period of the epidemic, the number of COVID-19 cases in China remained low, primarily due to stringent control measures.9,10 Upon the reconfiguration of China’s zero-COVID approach in December of 2022, the initial countrywide episode of Omicron cases arose shortly following the relaxation of non-pharmaceutical interventions. The peak of the outbreak was swiftly surpassed within one month, resulting in over 50,000 fatalities.11 To explore what changes in cytokines and clinical characteristics occurred in Changshu patients during this infection peak, we analyzed the laboratory indicators of 302 patients with laboratory-confirmed SARS-CoV2 Omicron variant at the time of admission in December 2022. Additionally, we evaluated serum interleukin (IL)-2, IL-4, IL-6, IL-10, IL-17, interferon (IFN)-γ, and tumor necrosis factor (TNF)-α levels in the peripheral blood of patients with COVID-19 to analyze the potential value of these indicators for predicting the risk of RF.
Materials and Methods
This study was approved by the Ethics Review Committee of Changshu Hospital of Nantong University (Changshu, China; approval number: 2022-KYW-K51) and was conducted in accordance with the principles of the Declaration of Helsinki. Informed consent was obtained from all patients. The informed consent form was signed by the patient or their agent after admission.
Study Population
This retrospective study included patients admitted with mild to severe or critical COVID-19 who received inpatient treatment at Changshu Hospital of Nantong University between December 29, 2022, and January 11, 2023 during the epidemic period in Changshu caused by the SARS-CoV-2 BA.5.2 lineage. We aimed to investigate cytokine levels (pg/mL) in the peripheral serum of COVID-19 patients in the Changshu area during this phase of the pandemic. The estimated standard deviation (σ) was 40 pg/mL, which required two-sided testing. The α was 0.05 and the acceptable error (δ) was 5 pg/mL. The sample size was calculated using the following formula,
According to the above requirements, an infinite sample size calculation method was required, and the sample size was calculated as 246 cases. Taking into account the 15% drop-out and refusal rate, a minimum of 283 COVID-19 patients was determined to be optimal for the study.
The inclusion criteria were as follows: 1) patients aged 18 years or older, 2) chest computed tomography (CT) scan performed at the time of admission that revealed a new inflammatory lesion; this method of diagnosing pneumonia was consistent with previous reports,12,13 3) positive pharyngeal swab specimen test for SARS-CoV2 infection using qPCR, and 4) first blood collection within 5 days after disease onset. Patients who did not undergo an initial CT evaluation or related testing were excluded. RF was defined as hypoxemia demonstrated by arterial blood gas or by pulse oximetry that required oxygen supplementation according to the individual trial’s criteria. After breathing indoor air at rest, patients with intracardiac anatomical shunt and primary cardiac output reduction, those for which the arterial partial pressure of oxygen (PaO2) was lower than 8k Pa (60 mmHg) or the partial pressure of carbon dioxide (PaCO2) was higher than 6.65 kPa (50 mmHg) were excluded.
Assessments
Patients were diagnosed with COVID-19 based on quantitative reverse transcription PCR (RT-qPCR) results. A pharyngeal swab was collected on admission for RT-qPCR, and CT was performed. None of the patients included in this study had received antiviral therapy at the time of laboratory examination. A minimum of 1.0 mL of serum was collected from each patient within 5 days of symptom onset and analyzed. The Seven-link detection kit (Cellgene Biotech, Jiangxi, China) was used for the detection of IL-2, IL-4, IL-6, IL-10, IL-17, IFN-γ, and TNF-α based on MBFFI. Data on patient demographics, comorbidities, clinical manifestations, and laboratory results were collected from medical records. Fasting (>8 h) blood samples were collected for analysis. Blood (cell) counts were assessed using a blood cell analyzer (ADVIA 2120i Hematology System; Siemens Healthcare Diagnostics Inc., USA). Serum biochemical variables were assessed using conventional automated blood analyses (calcium and magnesium: AU5800 Series Chemistry Analyzers; Beckman Coulter, USA; thyrocalcitonin, ferritin and C-reactive protein: IMMULITE 2000 XPi Immunoassay System Siemens Healthcare Diagnostics Inc., USA; oxygen partial pressure, oxygen saturation and lactic acid: RAPID Point 500 Systems, Siemens Healthcare Diagnostics Inc., USA; D-dimer: STA Compact Max, STAGO, France).
Statistical Analysis
For quantitative variables, histograms, box graphs, and Shapiro–Wilk tests were used to evaluate the normal distribution. Normally distributed measurement data were represented by mean ± standard deviation values. Comparisons between groups were performed using an independent-sample t-test, and skewed distribution data were represented by quartiles. The Mann–Whitney U-test was used for intergroup comparisons. Categorical variables were expressed as frequency (percentage) and compared between groups using the χ2 or Fisher’s exact test. Spearman’s rank correlation was used for correlation analysis. Statistical analyses were performed using SPSS 19.0 statistical software (IBM Corp., Armonk, NY, USA) and GraphPad Prism 8.0 (GraphPad Software Inc., USA). All tests were two-sided, and statistical significance was set at P<0.05.
Results
Baseline Characteristics
In total, 302 patients with COVID-19 participated in this study, 31 of whom experienced RF. Among them, patients with COVID-19 over 60 years old accounted for 82.1%. Compared with patients without RF, the proportion of males in patients with RF was higher (80.6%, P=0.034). After stratified analysis of age, the difference was not statistically significant. Demographic data are summarized in Table 1.
 |
Table 1 Demographic Data of Patients in the Study |
Different Laboratory Findings in Patients with COVID-19
In laboratory examination, patients with RF had a lower oxygen partial pressure (70.54±24.82 mmHg, P=0.007) compared to patients without RF. The serum levels of thyroid calcitonin [0.30 (0.10, 0.95)] and D- dimer [0.30 (0.10, 0.95)] in patients with RF were higher than those in patients without RF (P<0.0001 and P<0.0001, respectively). Lactic acid (2.49±1.15) increased in all patients with RF, and the difference was statistically significant (P=0.048), but it was not statistically significant in patients over 60 years old.
Patients with RF had a higher peripheral white blood cell count (9.34±5.80 × 109/L), neutrophil count (8.28±5.76 × 109/L), platelet volume distribution width (16.65±0.70%) than those without RF (P=0.043, P=0.024, P=0.023, respectively). In patients over 60 years old, the difference was statistically significant (P=0.004, P=0.002, and P=0.004, respectively). Patients with RF had a lower lymphocyte count (0.64±0.34 × 109/L) than those without RF (0.87±0.54 × 109/L, P=0.022), and the difference was statistically significant among patients younger than 60 years of age (P=0.022). Patient laboratory test data are summarized in Table 2.
 |
Table 2 Laboratory Findings of Study Patients |
Different Immunoinflammatory Biomarker Levels in Patients with COVID-19
Patients with RF had higher levels of serum IL-4 [0 (0, 0.54)], IL-6 [40.76 (12.33, 90.28)], IL-10 [6.65 (4.12, 11.34)], and IL-17 [9.48 (4.31, 12.13)] than those without RF (P=0.042, P<0.0001, P=0.012, and P=0.036, respectively). According to the stratified analysis of age, there was no significant difference in IL-4 between the groups with and without RF, regardless of whether the patient was over 60 years old (P=0.07, P=0.114). In the two different age groups, IL-6 was increased in patients with RF [71.07 (20.37, 271.77), 37.60 (12.33, 81.42)], and the difference was statistically significant (P=0.019, P=0.005).
Among the patients over 60 years old, IL-10 was increased in the patients with RF [6.65 (4.12, 14.01)], and the difference was statistically significant (P=0.012). In patients younger than or equal to 60 years old, IL-17 was increased in patients with RF [11.35 (10.56, 14.97)], and the difference was statistically significant (P=0.023). However, there was no significant difference in the levels of serum IL-2, IFN-γ, and TNF-α between the two groups with and without RF. Table 3 presents the levels of the immunoinflammatory biomarkers.
 |
Table 3 Immunoinflammatory Biomarker Levels of Study Patients |
Clinical Parameters and Immunoinflammatory Biomarkers Correlated with RF
Among the clinical parameters, the occurrence of RF was positively correlated with sex (R=0.122, P=0.034), lactic acid (R=0.193, P=0.007), white blood cell count (R=0.121, P=0.038), erythrocyte distribution width (R=0.131, P=0.024), thyrocalcitonin (R=0.280, P<0.0001), and D-dimer levels (R=0.214, P<0.0001) but negatively correlated with oxygen partial pressure (R=−0.208, P=0.004), oxygen saturation (R=−0.220, P=0.002), lymphocyte count (R=−0.129, P=0.026), and calcium (R=−0.152, P=0.042). No association was found between the occurrence of RF and age, C-reactive protein level, monocyte count, platelet volume distribution width, erythrocyte sedimentation rate, ferritin level, or magnesium level. Among the immunoinflammatory biomarkers, the occurrence of RF was positively correlated with IL-4 (R=−0.117, P=0.042), IL-6 (R=0.206, P<0.0001), IL-10 (R=0.145, P=0.012), and IL-17 (R=0.121, P=0.036) levels; however, there was no association between the occurrence of RF and IL-2, IFN-γ, and TNF-α (Table 4).
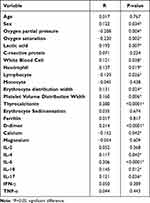 |
Table 4 Clinical Parameters and Immunoinflammatory Biomarkers Correlated with Respiratory Failure |
Prediction Value of Immunoinflammatory Biomarker Levels for RF in Patients with COVID-19
As illustrated in Figure 1, the marked total had the largest area under the curve (0.806; 95% CI, 0.718–0.895; Table 5) and a greater risk prediction value.
 |
Table 5 Predictive Value of the Serum Immunoinflammatory Biomarker Levels for the Occurrence of Respiratory Failure |
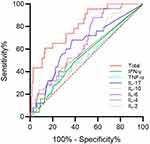 |
Figure 1 Prediction value of serum immunoinflammatory biomarker levels for respiratory failure in patients with COVID-19. |
Discussion
In this study, of 302 patients who were diagnosed with SARS-CoV-2 and required hospitalization at Changshu Hospital affiliated with Nantong University, 31 developed hypoxemic RF during the course of their disease.
The main features of COVID-19 include pulmonary and systemic inflammations. Due to the superiority of the female X chromosome and the protective effects of female sex hormones, men are considered more susceptible to COVID-19 than women.14 In our research, the proportion of males in patients with RF was higher (80.6%, P=0.034) than that among patients without RF. After stratified analysis of age, this difference was not statistically significant. This may be related to the decrease of statistical power due to the reduction of sample size in each group after stratified analysis. However, Spearman correlation analysis showed that the occurrence of RF was weak related to sex (R=0.122, P=0.034). The patients’ mean age was higher, primarily because we collected data on hospitalized patients, which also reflected that older patients were more likely to be hospitalized due to COVID-19.
It has been previously reported that approximately 90% of deaths from COVID-19 pneumonia are associated with at least one comorbidity such as hypertension, obesity, diabetes, hyperlipidemia, dementia, coronary artery disease, nephropathy, atrial fibrillation, chronic obstructive pulmonary disease, cancer, or stroke.15 In our research, the differences in the composition of diabetes, hypertension, coronary heart disease, and smoking history between the two groups were not statistically significant.
Our study revealed that patients with RF had elevated white blood cell counts at an earlier stage than those without RF, and this elevation was attributed to an increased neutrophil count. Moreover, patients with RF had lower lymphocyte counts than those without RF, which is consistent with previous studies.16,17
Elevated erythrocyte and platelet distribution widths have often been reported in patients with severe COVID-19.18 Our results showed no statistically significant difference in erythrocyte volume width between the two groups; however, erythrocyte volume width was negatively correlated with the occurrence of RF; the platelet distribution width data were consistent with those of previous studies.
The overall serum lactic acid level was above the normal range and higher in patients with COVID-19 who developed RF, suggesting that elevated lactic acid levels may presage a worse prognosis. Elevated serum lactic acid levels have been reported as a distinct feature of COVID-19, with pathway analysis showing disorders of energy production and amino acid metabolism.19 Recent findings demonstrated that elevated D-dimer levels, which are common in patients with COVID-19, can predict the clinical severity of COVID-19.20 In our study, serum D-dimer levels were significantly elevated in patients with RF and were associated with the development of RF. This discovery of high serum D-dimer levels provided evidence of a hypercoagulable state in SARS-CoV-2 infection.21 This may be a sign of increased risk and may even indicate the presence of thrombotic events. These findings support the use of prophylactic anticoagulation therapy in patients with moderate-to-severe COVID-19. This hypercoagulable state can be attributed to the activation of neutrophil extracellular traps, which trigger immune thrombosis during COVID-19.22
After acute infection with SARS-CoV-2, inflammatory cytokines, such as interleukin IL-2, IL-6, TNF-α, and IFN-γ are overproduced and secreted. The levels of anti-inflammatory cytokines such as IL-4 and IL-10 are also elevated.23,24 Simultaneously, monocytes, macrophages, and cells of the adaptive immune system such as CD8+ and CD4+T cells are mobilized to the respiratory tract to promote the secretion of IFN-γ, promote additional inflammation, and establish a sustained pro-inflammatory response.24 Inflammatory changes known as CRS are associated with different clinical features of COVID-19. Furthermore, they are associated with the progression of the acute phase of SARS-CoV-2 infection.24,25 Exacerbation of lung inflammation caused by cytokine imbalance is a fundamental cause of RF in patients with SARS-CoV-2 infection.14
In this study, we found that the serum levels of pro-inflammatory cytokines IL-6 and IL-17 and anti-inflammatory cytokines IL-4 and IL-10 in patients with RF were significantly increased; and positively correlated with the occurrence of RF, compared to patients without RF in COVID-19. The correlation coefficients were all weak R (<0.3) but still significant. Significant increases in IL-6 levels have been described as important biomarkers in critically ill patients.14 IL-6 is a major cytokine associated with various inflammatory diseases. In addition to acting as a pro-inflammatory cytokine, IL-6 promotes resistance to pathogens and tissue homeostasis.26 Previous studies have shown a significant correlation between high serum IL-6 levels and adverse clinical outcomes.27,28 In addition, several have recommended early detection of serum IL-6 levels after admission to identify patients with the highest risk of disease progression and death.27,29 These results are consistent with our findings in hospitalized patients.
IL-17 is considered a biomarker of disease severity and a potential therapeutic target for alleviating the damage caused by SARS-CoV-2, especially in the lungs.30 Our study showed that serum IL-17 levels were higher in patients with RF, and IL-17 was associated with the occurrence of RF (R=0.121).
A unique feature of the COVID-19 cytokine storm is a dramatic increase in IL-10 levels. This is considered to be a negative feedback mechanism for the inhibition of inflammation. However, multiple lines of clinical evidence suggest that a significant increase in early pro-inflammatory IL-10 may play a pathological role in COVID-19 severity.31,32
In contrast, our results showed no significant differences in IL-2, TNF-α, and IFN-γ between the two groups, and no correlation with the occurrence of RF. Our results are similar to those reported by another team of scholars from Harbin.33 However, IL-4 levels were elevated early in the course of the disease and were associated with RF. The secretion and activation of IL-4 ultimately stimulates IL-4 receptors; inhibits the secretion of other inflammatory cytokines including TNF-α, IL-1, and PGE 2; increases LDL oxidation; and ultimately reduces inflammation.34 In COVID-19, one of the pathways of inflammation in vivo is increased apoptotic activity due to increased activity of Th2 cells and the IL-4-induced JAK-STAT6 signaling pathway by the virus.35 Based on the receiver operating characteristic curves in our results, the cytokine combination assay had the largest AUC value. Therefore, combined cytokine detection at the time of admission may predict patient prognosis and guide treatment according to changes in individual cytokines.
In this study, we aimed to mitigate selection bias by clearly defining the inclusion and exclusion criteria as well as specifying the selection scope. To prevent information bias, we also developed rigorous and thorough data collection methods that include the use of blind techniques during research information gathering and the utilization of quantitative objective indicators when recording research information. The testing instruments and equipment functioned as expected and underwent regular calibration checks, and the reagents met the necessary requirements. A stratified analysis approach was used to minimize the impact of any potential mixed bias, taking into account age differences between participants.
Nonetheless, this study had a few limitations. First, it was a single-center study. Additionally, data were from clinical patients after admission; therefore, we lacked data on mild cases that did not arrive at the hospital. Moreover, although our samples were from within 5 days after onset, earlier data were lacking. As the COVID-19 pandemic evolved, our study was conducted on a seemingly milder variant of Omicron infection. Therefore, further studies are warranted to explore these key immune mechanisms to help clinicians better cope with acute complications, long-term sequelae, and possible future COVID-19 variants and to be flexible in dealing with future epidemics and similar public health threats.
Conclusion
In this study, we analyzed the clinical characteristics, laboratory parameters, and inflammatory markers of 302 patients with SARS-CoV-2. We found that sex, oxygen partial pressure, oxygen saturation, lactic acid, white blood cell, neutrophil, lymphocyte, platelet volume distribution width, thyrocalcitonin, and D-dimer were all weakly correlated with RF in patients with COVID-19. The serum levels of pro-inflammatory cytokines IL-6 and IL-17 and anti-inflammatory cytokines IL-4 and IL-10 in patients with RF were significantly increased and weakly positively correlated with the occurrence of RF compared with patients without RF in COVID-19. Our results also showed that there was no significant difference in IL-2, TNF-α, and IFN-γ levels between the two groups, and no correlation with the occurrence of RF.
Abbreviations
COVID-19, Coronavirus disease 2019; ARDS, Acute respiratory distress syndrome; IL, Serum interleukin; IFN, Interferon; TFN, Tumor necrosis factor; CT, Computed tomography; RT-qPCR, Quantitative reverse transcription PCR; MBFFI, Multiplex bead-based flow fluorescent immunoassay.
Ethics Approval and Informed Consent
This study was approved by the Ethics Review Committee of Changshu Hospital of Nantong University (Changshu, China; approval number: 2022-KYW-K51). It was conducted in accordance with the principles of the Declaration of Helsinki. Informed consent for study participation was obtained from all patients.
Consent to Publish
The authors affirm that human research participants provided informed consent for publication of the images in Figure 2.
 |
Figure 2 Study population flowchart. The final study cohort comprised 302 patients. |
Author Contributions
XX, ZZ, and TJ: conception and design. YL, WX, JG, SY and LZ: data collection and collation, data analysis and interpretation. XX: manuscript writing. TJ: final review of the manuscript. All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
This study was funded by the National Natural Science Foundation of China (82270556), the Youth Medical Talent Project of Suzhou (GSWS2020106), the Youth Project of Changshu Health Committee (CSWSQ202104), the Guiding Project of Changshu Health Committee (cswszd202213) and the Changshu Science and Technology Development Project (CS2020016).
Disclosure
The authors declare that there is no conflict of interest regarding the publication of this paper.
References
1. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi:10.1001/jama.2020.1585
2. Chan M, Vijay S, McNevin J, et al. Machine learning identifies molecular regulators and therapeutics for targeting SARS-CoV2-induced cytokine release. Mol Syst Biol. 2021;17(9):e10426. doi:10.15252/msb.202110426
3. Chan M, Holland EC, Gujral TS. Olverembatinib inhibits SARS-CoV-2-Omicron variant-mediated cytokine release in human peripheral blood mononuclear cells. EMBO Mol Med. 2022;14(6):e15919. doi:10.15252/emmm.202215919
4. Monteleone G, Sarzi-Puttini PC, Ardizzone S. Preventing COVID-19-induced pneumonia with anticytokine therapy. Lancet Rheumatol. 2020;2(5):e255–e256. doi:10.1016/S2665-9913(20)30092-8
5. Saleki K, Alijanizadeh P, Azadmehr A. Is neuropilin-1 the neuroimmune initiator of multi-system hyperinflammation in COVID-19? Biomed Pharmacother. 2023;167:115558. doi:10.1016/j.biopha.2023.115558
6. Hazrati E, Gholami M, Farahani RH, et al. The effect of IGF-1 plasma concentration on COVID-19 severity. Microb Pathog. 2022;164:105416. doi:10.1016/j.micpath.2022.105416
7. Jafarzadeh A, Chauhan P, Saha B, et al. Contribution of monocytes and macrophages to the local tissue inflammation and cytokine storm in COVID-19: lessons from SARS and MERS, and potential therapeutic interventions. Life Sci. 2020;257:118102. doi:10.1016/j.lfs.2020.118102
8. Moore JB, June CH. Cytokine release syndrome in severe COVID-19. Science. 2020;368(6490):473–474. doi:10.1126/science.abb8925
9. Wang J, Wang C. The coming Omicron waves and factors affecting its spread after China reopening borders. BMC Med Inform Decis Mak. 2023;23(1):186. doi:10.1186/s12911-023-02219-y
10. Xiao H, Wang Z, Liu F, et al. Excess all-cause mortality in China after ending the zero COVID policy. JAMA Netw Open. 2023;6(8):e2330877. doi:10.1001/jamanetworkopen.2023.30877
11. Hu W, Li X, Yan Z, et al. Impact of the first wave of COVID-19 on Crohn’s disease after the end of “zero-COVID” policy in China. Front Public Health. 2023;11:1186275. doi:10.3389/fpubh.2023.1186275
12. Machnicki S, Patel D, Singh A, et al. The usefulness of chest CT imaging in patients with suspected or diagnosed COVID-19: a review of literature. Chest. 2021;160(2):652–670. doi:10.1016/j.chest.2021.04.004
13. Ebrahimzadeh S, Islam N, Dawit H, et al. Thoracic imaging tests for the diagnosis of COVID-19. Cochrane Database Syst Rev. 2022;5(5):CD013639. doi:10.1002/14651858.CD013639.pub5
14. Ye H, Liu ZM, Zhou L, et al. Levels of peripheral IL-6 and CD4+ and CD8+ T cells and their prognostic significance in COVID-19. Eur Rev Med Pharmacol Sci. 2023;27(6):2686–2691. doi:10.26355/eurrev_202303_31806
15. Ramasamy S, Subbian S. Critical determinants of cytokine storm and Type I interferon response in COVID-19 pathogenesis. Clin Microbiol Rev. 2021;34(4):e00299–20. doi:10.1128/CMR.00299-20
16. Qi X, Kong H, Ding W, et al. Abnormal coagulation function of patients with COVID-19 is significantly related to hypocalcemia and severe inflammation. Front Med (Lausanne). 2021;8:638194. doi:10.3389/fmed.2021.638194
17. Zheng Y, Zhang Y, Chi H, et al. The hemocyte counts as a potential biomarker for predicting disease progression in COVID-19: a retrospective study. Clin Chem Lab Med. 2020;58(7):1106–1115. doi:10.1515/cclm-2020-0377
18. Shen L, Chen L, Chi H, et al. Parameters and morphological changes of erythrocytes and platelets of COVID-19 subjects: a longitudinal cohort study. Infect Drug Resist. 2023;16:1657–1668. doi:10.2147/IDR.S400735
19. Caterino M, Costanzo M, Fedele R, et al. The serum metabolome of moderate and severe COVID-19 patients reflects possible liver alterations involving carbon and nitrogen metabolism. Int J Mol Sci. 2021;22(17):9548. doi:10.3390/ijms22179548
20. Lippi G, Mullier F, Favaloro EJ. D-dimer: old dogmas, new (COVID-19) tricks. Clin Chem Lab Med. 2023;61(15):841–850. doi:10.1515/cclm-2022-0633
21. Abou-Ismail MY, Diamond A, Kapoor S, Arafah Y, Nayak L. The hypercoagulable state in COVID-19: incidence, pathophysiology, and management. Thromb Res. 2020;194:101–115. doi:10.1016/j.thromres.2020.06.029
22. Middleton EA, He XY, Denorme F, et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood. 2020;136(10):1169–1179. doi:10.1182/blood.2020007008
23. Akbari H, Tabrizi R, Lankarani KB, et al. The role of cytokine profile and lymphocyte subsets in the severity of coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. Life Sci. 2020;258:118167. doi:10.1016/j.lfs.2020.118167
24. Filgueira TO, Carvalho PRC, de Sousa Fernandes MS, et al. The impact of supervised physical exercise on chemokines and cytokines in recovered COVID-19 patients. Front Immunol. 2022;13:1051059. doi:10.3389/fimmu.2022.1051059
25. Hu B, Huang S, Yin L. The cytokine storm and COVID-19. J Med Virol. 2021;93(1):250–256. doi:10.1002/jmv.26232
26. Jones SA, Hunter CA. Is IL-6 a key cytokine target for therapy in COVID-19? Nat Rev Immunol. 2021;21(6):337–339. doi:10.1038/s41577-021-00553-8
27. Laguna-Goya R, Utrero-Rico A, Talayero P, et al. IL-6-based mortality risk model for hospitalized patients with COVID-19. J Allergy Clin Immunol. 2020;146(4):799–807.e9. doi:10.1016/j.jaci.2020.07.009
28. Han H, Ma Q, Li C, et al. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg Microbes Infect. 2020;9(1):1123–1130. doi:10.1080/22221751.2020.1770129
29. Rotundo S, Borelli M, Scaglione V, et al. Interleukin-62/lymphocyte as a proposed predictive index for COVID-19 patients treated with monoclonal antibodies. Clin Exp Med. 2023;23(1):1–7. doi:10.1007/s10238-021-00781-1
30. Pacha O, Sallman MA, Evans SE. COVID-19: a case for inhibiting IL-17? Nat Rev Immunol. 2020;20(6):345–346. doi:10.1038/s41577-020-0328-z
31. Lu L, Zhang H, Dauphars DJ, He YW. A potential role of interleukin 10 in COVID-19 pathogenesis. Trends Immunol. 2021;42(1):3–5. doi:10.1016/j.it.2020.10.012
32. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi:10.1016/S0140-6736(20)30183-5
33. Luo W, Zhang JW, Zhang W, Lin YL, Wang Q. Circulating levels of IL-2, IL-4, TNF-α, IFN-γ, and C-reactive protein are not associated with severity of COVID-19 symptoms. J Med Virol. 2021;93(1):89–91. doi:10.1002/jmv.26156
34. Bhattacharjee A, Shukla M, Yakubenko VP, Mulya A, Kundu S, Cathcart MK. IL-4 and IL-13 employ discrete signaling pathways for target gene expression in alternatively activated monocytes/macrophages. Free Radic Biol Med. 2013;54:1–16. doi:10.1016/j.freeradbiomed.2012.10.553
35. Renu K, Subramaniam MD, Chakraborty R, et al. The role of interleukin-4 in COVID-19 associated male infertility – a hypothesis. J Reprod Immunol. 2020;142:103213. doi:10.1016/j.jri.2020.103213
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.