Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 19
Circular RNA_0025843 Alleviated Cigarette Smoke Extract Induced Bronchoalveolar Epithelial Cells Ferroptosis
Authors Chen J, Deng X, Xie H, Wang C, Huang J , Lian N
Received 12 October 2023
Accepted for publication 26 January 2024
Published 3 February 2024 Volume 2024:19 Pages 363—374
DOI https://doi.org/10.2147/COPD.S444402
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jill Ohar
Jia Chen,1– 3 Xiaoyu Deng,1– 3 Hansheng Xie,1– 3 Caiyun Wang,1– 3 Jiefeng Huang,1– 3 Ningfang Lian1– 3
1Department of Respiratory and Critical Care Medicine, The First Affiliated Hospital of Fujian Medical University, Fuzhou, People’s Republic of China; 2Fujian Provincial Sleep-Disordered Breathing Clinic Center, Institute of Respiratory Disease, Fujian Medical University, Fuzhou, People’s Republic of China; 3Department of Respiratory and Critical Care Medicine, National Regional Medical Center, Binhai Campus of the First Affiliated Hospital, Fujian Medical University, Fuzhou, People’s Republic of China
Correspondence: Ningfang Lian, Department of Respiratory and Critical Care Medicine, The First Affiliated Hospital of Fujian Medical University, No. 20, Chazhong Road, Taijiang District, Fuzhou, Fujian Province, 350005, People’s Republic of China, Tel +86-591-87981698, Email [email protected]
Purpose: Circular RNA (circRNA) plays an important role in various biological processes. However, their functions in cigarette smoke extract (CSE) induced human normal lung epithelial cells (BEAS-2B) injury remain vague. The study aimed to explore circRNA expression profiles and reveal their potential roles in CSE-treated BEAS-2B cells.
Methods: 5% CSE exposure for 24 hours were used to build the BEAS-2B cells ferroptosis model. Differentially expressed circRNAs (DECs) were identified by next-generation RNA sequencing. Six randomly selected DECs were validated via quantitative reverse transcription polymerase chain reaction (qRT-PCR). Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis and Gene Ontology (GO) analysis were conducted to clarify the potential functions of the DECs. Furthermore, the role of hsa_circ_0025843 in CSE-related BEAS-2B cells ferroptosis was confirmed.
Results: 5% CSE exposure induced BEAS-2B cells ferroptosis. Fifty-one up-regulated cirRNAs and 80 down-regulated circRNAs were revealed in CSE-treated BEAS-2B cells. Hsa_circ_0003461, hsa_circ_0007548, hsa_circ_0025843, hsa_circ_0068896, hsa_circ_0005832, and hsa_circ_0053378 were selected randomly to validate the reliability of next-generation RNA sequencing by qRT-PCR. After KEGG pathway analysis, DECs were found to participate in the process of EGFR tyrosine kinase inhibitor resistance and glycerophospholipid metabolism. The knockdown of hsa_circ_0025843 significantly alleviated CSE-induced BEAS-2B cells ferroptosis.
Conclusion: The study indicated the circRNA expression profiles in CSE-treated BEAS-2B cells. Hsa_circ_0025843 alleviated CSE induced BEAS-2B cells ferroptosis, which might be a potential therapeutic target of CSE related lung injury.
Keywords: cigarette smoke extract, Circular RNA, next-generation RNA sequencing, lung injury, ferroptosis
Introduction
Nearly 1 million people died from smoking-related diseases in China each year, accounting for about 12% of all deaths. Tobacco abuse is an important risk factor for various pulmonary diseases, such as lung cancer, chronic obstructive pulmonary disease (COPD), and interstitial lung disease.1,2 Tobacco inhalation directly injured the human normal lung epithelial cells (BEAS-2B).3 However, no treatment can completely reverse tobacco-related lung injury.2,4 Therefore, studying the potential pathophysiology of cigarette smoke extract (CSE) induced BEAS-2B cells injury and revealing the exact underlying mechanisms are quite necessary.
Previous research revealed that cigarette smoke exposure induced BEAS-2B cells ferroptosis.5 Ferroptosis, a recently discovered form of cell death, involves iron-dependent lipid peroxidation, oxidative stress, mitochondrial disorder, excessive Fe2+ level, and an overabundance of reactive oxygen species (ROS). This process was first identified by Dixon in 2012.6 Glutathione peroxidase 4(GPX4), an enzyme containing both selenium and cysteine, acts to diminish the levels of peroxide, resulting in keeping cellular redox homeostasis. This action effectively prevents the chain oxidation of cell membranes.7 Additionally, Acyl-CoA synthetase long-chain family member 4 (ACSL4) plays a critical role in the biosynthesis and remodeling of cell membrane-specific polyunsaturated fatty acid-phosphatidylethanolamine (PUFA-PE). Activation of ACSL4 facilitates the conversion of free polyunsaturated fatty acid (PUFA) into PUFA-PE.8 Consequently, GPX4 and ACSL4 are recognized as indicative biomarkers of ferroptosis.
Circular RNA (circRNA) is a special class of newly discovered non-coding RNA. The closed ring structure of circRNA makes it impervious to RNA exonuclease, so the expression levels are stable. It is one of the research hotspots recently. The function of circRNA covers almost all levels of gene expression regulation, such as histone modification, transcription regulation, post-transcriptional processing, protein modification, localization, and chromatin remodeling. Accumulating evidence confirmed that circRNAs affected numerous biological processes of various diseases, such as cell apoptosis, ferroptosis, epithelial-mesenchymal transformation, etc.9–11 However, a few studies focused on the circRNA expression profiles in CSE-treated BEAS-2B cells.
Last decade, the development of second-generation sequencing technology benefited in exploring the mechanism of disease occurrence and development.12 High-throughput data have the advantage of fully understanding the alters in the expression profile of non-coding RNA. However, this technology was used to investigate the issue of CSE-related lung injury in a few studies.
The present study was designed to perform cirRNA sequencing in CSE-treated BEAS-2B cells and normal BEAS-2B cells. The potential functions of differentially expressed circRNAs were predicted by bioinformatics methods. This study was aimed to explore the function of circRNAs in CSE-related lung injury.
Materials and Methods
Cell Culture
BEAS-2B cells were purchased from Biyuntian Biological Technology Co., Ltd (Shanghai, China). BEAS-2B cells were cultured in RPMI-1640 (HyClone) with 1% Streptomycin penicillin mixture (HyClone) and 10% fetal bovine serum (Gibco). The incubator was set at 37°C with 5% CO2 (Thermo Fisher Scientific, Waltham, MA, USA).
CSE Preparation and the Cell Model Establishment
Prepare 100% CSE as following procedures: Combusted a cigarette (Septwolves, Longyan Cigarette Factory, Fujian, China) in a 500 mL glass bottle with the suction device.13 Dissolved the smoke in the 20 mL culture medium of RPMI-1640. Filtered the solution through a filter of 0.22 μm pore size and moved out the bacteria and large particles. Adjusted the pH value to 7.4. The obtained solution was 100% CSE. The 5% CSE was the mixture of RPMI-1640 complete medium and 100% CSE. The 5% CSE was used for subsequent experiments within 30 min of preparation. In the CSE group, the BEAS-2B cells were exposed in 5% CSE for 24 hours when BEAS-2B cells reached 70–80% confluency. The experimental methods of cell apoptosis rate were reviewed in the previous study.5
Isolation of RNA
TRIzol Reagent (Invitrogen, USA) was used for RNA extraction. The quantity and quality of the total RNA were detected by DNA/RNA concentration assay (Pharmathea INC., China). At least 3μg of total RNA was isolated from BEAS-2B cells.
Screening for Differentially Expressed circRNA and Bioinformatics Analysis
The differentially expressed circRNA was screened by next-generation RNA sequencing. First, removing Ribosomal RNA and digesting the linear RNAs with the manufacturer’s instructions. After that, it will amplify suitable fragments and construct the cDNA library via polymerase chain reaction (PCR). Then, assess the cDNA library’s quantity and quality via qPCR quantification methods and Agilent 2100 Bioanalyzer (Agilent, USA). Last, sequencing the libraries by NovaSeq 6000 (Illumina, USA). The trimmed data were compared to the reference transcriptome to obtain Backsplice Junction. The circRNA was annotated and quantified by analyzing the comparison results. The circRNA with CSE vs control fold change >1.50 (or <1/1.5), and p-value < 0.05 were defined as differentially expressed circRNA. The scatter diagram, volcano diagram, and cluster diagram were drawn to obtain visual data.
Gene Ontology (GO) analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis were carried out to clarify the functions and interactions among these dysregulated expressed circRNAs by GO database (http://geneontology.org) and KEGG database (https://www.genome.jp/kegg/). The enrichment GO terms and KEGG pathways of the differentially expressed circRNAs were selected and ranked by enrichment score [-log10 (p-value)].
Real-Time Quantitative PCR (qRT-PCR)
The cDNA was obtained by reverse transcription of the total RNA of BEAS-2B cells via the PrimeScript RT reagent Kit (Takara Bio Inc., Japan). Quantitative reverse transcription polymerase chain reaction (qRT-PCR) was performed to detect the expression levels of GPX4, ACSL4, hsa_circ_0003461, hsa_circ_0005832, hsa_circ_0007548, hsa_circ_0025843, hsa_circ_0053378 and hsa_circ_0068896 on an ABI 7500 thermocycler (Applied Biosystems, Foster City, CA, USA) with the SYBR Green PCR Master Mix (Takara Bio Inc., Japan). The sequences of primers were listed in Table 1. 2−ΔΔCT methods were used to calculate the Fold changes.
 |
Table 1 List of Primer Sequence |
Cell Transfection
Hsa_circ_0025843 small interfering RNA (Si-circ_0025843), and the corresponding control (Si-nc-circ_0025843) were synthesized by Hanbio Biotechnology Co. (Shanghai, China). The transfection of Si- circ_0025843 was performed according to the manufacturer’s instructions of Lipofectamine 3000 (Invitrogen, USA).
Detection of ROS Levels
The fluorescence probe DCFH-DA was used to detect intracellular ROS levels. BEAS-2B cells were seeded in 6-well plates and subjected to different treatments. After 24 h of CSE exposure, cells were collected in the 1.5 mL ep tubes and then suspended in serum-free RPMI-1640 with 10 μM DCFH-DA (Beyotime, China). After incubating in a cell incubator at 37°C for 20 minutes, the cells were washed three times with serum-free RPMI-1640. When the samples were ready, a C6 flow cytometer was used to detect the ROS levels (Becton Dickinson, USA).
Detection of Fe2+ Levels
To measure the Fe2+ levels in different groups, FerroOrange (F-374, Dojindo, China) was employed. Cells were collected into 1.5 mL ep tubes, washed with non-serum RPMI-1640, and then incubated at 37°C in an incubator with 1 µM FerroOrange for 30 minutes before being tested by a C6 flow cytometry (Becton Dickinson, USA).
Detection of Mitochondrial Membrane Potential
Mitochondrial membrane potential (MMP) was assessed using the JC-1 assay (Beyotime, C2006, China). Following the instructions, the JC-1 working solution was prepared. After the cell collection from each group, they were thoroughly mixed with 500 μL of the JC-1 working solution, and incubated at 37°C for 20 minutes. Then, the cells were washed twice with precooled 1× JC-1 Assay Buffer and then tested by a C6 flow cytometry (Becton Dickinson, USA).
Statistical Analysis
After the normality test, the data were described by the mean ± standard or the interquartile range deviation. An independent t-test or the Mann–Whitney test was used to compare the mean of continuous variables. A p-value less than 0.05 was defined as a statistically significant difference. Statistical analysis and the figures were performed by GraphPad Prism 7.0 (GraphPad Software Inc., USA).
Results
CSE Induced BEAS-2B Cells Injury
After being exposed in 5% CSE for 24 hours, the apoptosis rate of BEAS-2B cells in the CSE group increased (Figure 1a), the ROS levels and the Fe2+ levels of BEAS-2B cells in the CSE group increased (Figure 1b and d), while the MMP levels of BEAS-2B cells in the CSE group decreased (Figure 1c), all p<0.05. The mRNA and protein expression levels of GPX4 decreased (Figure 1e and f); The mRNA and protein expression levels of ACSL4 increased (both p<0.05) (Figure 1e and f). The original WB images were shown in Figure S1.
The Expressions of circRNAs
After 24-hour exposure to 5% CSE, differentially expressed circRNAs were explored by RNA sequence. Fifty-one upregulated circRNAs and 80 downregulated circRNAs were screened out when the differential multiple was more than 1.5 times and the p-value was less than 0.05. Among them, the circRNAs with the top ten differential expression multiples were shown in Tables 2 and 3.
 |
Table 2 List of Top Upregulated CircRNAs in CSE Group Compared to Control Group |
 |
Table 3 List of Top Downregulated CircRNAs in CSE Group Compared to Control Group |
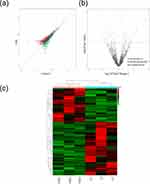
To visualize the differentially expressed genes, the scatter plots and volcano figures of differentially expressed circRNAs were shown in Figure 2. Hierarchical clustering showed the distinguishable expression profiles of differentially expressed circRNAs. The color legend of the small squares of the heat map illustrated the corresponding relationship between color and expression, with red indicating high expression and green indicating low expression.
GO Analysis and KEGG Pathway Analysis
GO analysis was performed to determine the biological process (BP), cellular component (CC), and molecular function (MF) of the differentially expressed circRNAs. GO analysis for the upregulated circRNAs were enriched in organelle organization (Figure 3a; BP), nucleoplasm, nuclear lumen (Figure 3a; CC), protein binding, and enzyme binding (Figure 3a; MF). GO analysis for the downregulated circRNAs were enriched in the regulation of the cellular process, regulation of signal transduction, (Figure 3b; BP), cytosol, cytoplasm (Figure 3b; CC), and protein binding, GTPase regulator activity, and GTPase activator activity (Figure 3b; MF). Additionally, 7 pathways for upregulated circRNAs and 20 pathways for downregulated circRNAs were involved in CSE-induced BEAS-2B cells injury via KEGG pathway analysis. We selected all upregulated pathways and the top 10 downregulated pathways based on the values of enrichment score and showed them in Figure 4. The significantly upregulated circRNAs were associated with glycerophospholipid metabolism, Phospholipase-D signaling pathway; The significantly downregulated circRNAs were associated with EGFR tyrosine kinase inhibitor resistance, Ubiquitin mediated proteolysis, Platinum drug resistance, ErbB signaling pathway.
Verification of Differentially Expressed circRNAs
To validate the results of the RNA sequence, 6 circRNAs were selected randomly and performed qRT-PCR. The results of qRT-PCR had similar trends to the results of RNA-sequence. The expression of hsa_circ_0003461, hsa_circ_0007548, hsa_circ_0025843 and hsa_circ_0068896 upregulated. The expression of hsa_circ_0005832 and hsa_circ_0053378 was downregulated, as shown in Figure 5.
Knockdown of hsa_circ_0025843 Alleviated CSE Induced BEAS-2B Cells Injury
After transfected with Si-circ_0025843 for 48 hours, the transfection efficiency was verified. The Fold change of hsa_circ_0025843 mRNA was 0.18 ± 0.11 (p < 0.01) after transfection (Figure 6e).
Compared with the CSE group, hsa_circ_0025843 knockdown alleviated CSE induced BEAS-2B cells injury. After hsa_circ_0025843 knockdown, the apoptosis rate, ROS levels, and Fe2+ levels decreased, MMP levels increased (Figure 6a–d), the mRNA and protein levels of GPX4 increased, the mRNA and protein levels of ACSL4 decreased (Figure 6f and g), all p < 0.05. The original WB images were shown in Figure S2.
Discussion
The present study used the method of next-generation sequencing to provide a comprehensive and quantitative analysis of circRNA in CSE treated BEAS-2B cells. The results revealed that the expression profiles of circRNA were significantly different in CSE-treated BEAS-2B cells. Hsa_circ_0025843 knockdown relieved CSE-related BEAS-2B cells ferroptosis.
Cigarette smoking is one of the important risk factors for COPD and various cell types of lung cancer. Tobacco-induced airway epithelial cell injury, as an important pathogenesis of COPD and lung cancer, has received widespread attention.14–18 Cigarette exposure is related to the biological process of apoptosis, ferroptosis, inflammatory response, oxidative stress and et al.5,19 In the study, after 24 h exposure to CSE, the cell apoptosis rate increased, the mRNA and protein expression levels of GPX4 and ACSL41 changed. GPX4 and ACSL4 were the typical genes in ferroptosis. These changes suggested that 5% CSE treatment for 24 hours successfully induced the BEAS-2B cells ferroptosis. Altered levels of Fe2+ and mitochondrial membrane potential also support the occurrence of Ferroptosis.
Increasing evidence revealed that circRNAs play crucial roles in various diseases. Many studies have focused on the different expression profiles of cirRNAs in COPD. CircRNA0001859 might act as a new diagnostic and prognostic biomarker for COPD and AECOPD.20 Ding Wx. found that CircTMEM30A promotes the malignant progression of COPD with primary lung cancer.21 However, the interests in these studies are the diagnostic biomarkers. Furthermore, circRNA regulated the CSE-related lung injury. In animal studies, Lei Zhou. Found knockdown circFOXO3 ameliorated cigarette-related lung injury.22 In this study, a high-throughput approach was used to have a comprehensive description of the circRNAs, which was possible to better understand the role of circRNAs in CSE-related lung injury.
A total of 131 dysregulated circRNAs were identified to reveal the potential roles of circRNAs on CSE induced BEAS-2B cells injury. qRT-PCR verification showed a consistent trend with the RNA sequencing in the randomly selected 6 differentially expressed circRNAs. The results confirmed the reliability of the RNA second-generation sequencing technology. Furthermore, the results of KEGG pathway analysis showed that dysregulated circRNAs may be involved in tobacco-induced lung injury. Energy metabolism processes, such as glycerophospholipid and phospholipase D, affected the oxidative stress, which participated in the occurrence and development of COPD;23–25 EGFR tyrosine kinase inhibitor resistance, Platinum drug resistance, and ErbB signaling pathway are closely related to the occurrence of lung cancer and tumor drug resistance.26,27 Therefore, we speculate that regulation of these differentially expressed circRNAs may provide novel clues for the prevention and treatment of tobacco-related lung diseases.
Ferroptosis, as a novel mode of programmed cell death, has been confirmed to occur in CSE-associated bronchoalveolar epithelial cell injury.5 Ferroptosis was also involved in the development of COPD.28 The role of circRNA in regulating ferroptosis has been confirmed in previous studies. CircRNA affects the occurrence and development of lung cancer and breast cancer by regulating cellular ferroptosis.29,30 In another study, circRNA relieved post-traumatic brain injury by regulating cellular ferroptosis.31 Among the differentially expressed circRNA in high-throughput sequencing screening, we selected hsa_circ_0025843 for subsequent functional experiments. The study confirmed that the knockdown of hsa_circ_0025843 alleviated the CSE-induced BEAS-2B cells ferroptosis. Hsa_circ_0025843 may be a potential therapeutic target for CSE-associated lung injury.
There are some limitations in this study. First, the sample size included in the RNA-sequencing experiments was relatively small. Second, we did not conduct an in-depth mechanism study on differentially expressed circRNAs. The treatment effects of hsa_circ_0025843 may be different between in vitro and in vivo. So, we need to further verify the role of hsa_circ_0025843 in lung injury in vivo.
In summary, the study indicated the altered circRNA expression profiles in CSE-treated BEAS-2B cells. Hsa_circ_0025843 alleviated CSE induced BEAS-2B cells ferroptosis. Hsa_circ_0025843 may serve as a potential therapeutic target for CSE-associated lung injury.
Abbreviations
BEAS-2B, human normal lung epithelial cells; CSE, cigarette smoke extract; circRNA, Circular RNA; PCR, polymerase chain reaction; GO, Gene Ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes; ceRNA, competing endogenous RNA; BP, biological process; CC, cellular component; MF, molecular function; COPD, chronic obstructive pulmonary disease; ROS, reactive oxygen species; MMP, mitochondrial membrane potential; ACSL4, Acyl-CoA synthetase long-chain family member 4; GPX4, glutathione peroxidase 4.
Ethics Approval and Consent to Participate
This article does not contain any studies with human participants or animals performed by any of the authors.
Funding
This study was funded by the National Natural Science Foundation of China (Grant number: 82170101) and the Joint Funds for the Innovation of Science and Technology, Fujian Province (Grant number: 2019Y9116).
Disclosure
The authors have no relevant financial or non-financial interests to disclose for this work.
References
1. Halpin DMG, Criner GJ, Papi A, et al. Global initiative for the diagnosis, management, and prevention of chronic obstructive lung disease. The 2020 GOLD science committee report on COVID-19 and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2021;203(1):24–36. doi:10.1164/rccm.202009-3533SO
2. Vogelmeier CF, Román-Rodríguez M, Singh D, Han MK, Rodríguez-Roisin R, Ferguson GT. Goals of COPD treatment: focus on symptoms and exacerbations. Respir Med. 2020;166:105938. doi:10.1016/j.rmed.2020.105938
3. Madan A, Turner AM. Identifying the at risk smokers: who goes on to get COPD? Eur Respir J. 2019;54(4):1901613. doi:10.1183/13993003.01613-2019
4. Barnes PJ. Oxidative stress-based therapeutics in COPD. Redox Biol. 2020;33:101544. doi:10.1016/j.redox.2020.101544
5. Lian N, Zhang Q, Chen J, Chen M, Huang J, Lin Q. The role of ferroptosis in bronchoalveolar epithelial cell injury induced by cigarette smoke extract. Front Physiol. 2021;12:751206. doi:10.3389/fphys.2021.751206
6. Dixon SJ, Lemberg KM, Lamprecht MR, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060–1072. doi:10.1016/j.cell.2012.03.042
7. Xu C, Sun S, Johnson T, et al. The glutathione peroxidase Gpx4 prevents lipid peroxidation and ferroptosis to sustain Treg cell activation and suppression of antitumor immunity. Cell Rep. 2021;35(11):109235. doi:10.1016/j.celrep.2021.109235
8. Doll S, Proneth B, Tyurina YY, et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol. 2017;13(1):91–98. doi:10.1038/nchembio.2239
9. Jiang X, Ning Q. Long noncoding RNAs as novel players in the pathogenesis of hypertension. Hypertens Res. 2020;43(7):597–608. doi:10.1038/s41440-020-0408-2
10. Shen Q, Zheng J, Wang X, Hu W, Jiang Y, Jiang Y. LncRNA SNHG5 regulates cell apoptosis and inflammation by miR-132/PTEN axis in COPD. Biomed Pharmacother. 2020;126:110016. doi:10.1016/j.biopha.2020.110016
11. Hu C, Li J, Du Y, et al. Impact of chronic intermittent hypoxia on the long non-coding RNA and mRNA expression profiles in myocardial infarction. J Cell Mol Med. 2021;25(1):421–433. doi:10.1111/jcmm.16097
12. Nazarov PV, Kreis S. Integrative approaches for analysis of mRNA and microRNA high-throughput data. Comput Struct Biotechnol J. 2021;19:1154–1162. doi:10.1016/j.csbj.2021.01.029
13. Sampilvanjil A, Karasawa T, Yamada N, et al. Cigarette smoke extract induces ferroptosis in vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2020;318(3):H508–H518. doi:10.1152/ajpheart.00559.2019
14. Ghosh B, Reyes-Caballero H, Akgün-ölmez SG, et al. Effect of sub-chronic exposure to cigarette smoke, electronic cigarette and waterpipe on human lung epithelial barrier function. BMC Pulm Med. 2020;20(1):216. doi:10.1186/s12890-020-01255-y
15. Sun J, Gu X, Wu N, Zhang P, Liu Y, Jiang S. Human antigen R enhances the epithelial-mesenchymal transition via regulation of ZEB-1 in the human airway epithelium. Respir Res. 2018;19(1):109. doi:10.1186/s12931-018-0805-0
16. Chen L, He X, Xie Y, et al. Up-regulated miR-133a orchestrates epithelial-mesenchymal transition of airway epithelial cells. Sci Rep. 2018;8(1):15543. doi:10.1038/s41598-018-33913-x
17. Sun X, Feng X, Zheng D, et al. Ergosterol attenuates cigarette smoke extract-induced COPD by modulating inflammation, oxidative stress and apoptosis in vitro and in vivo. Clin Sci. 2019;133(13):1523–1536. doi:10.1042/CS20190331
18. Zinellu E, Zinellu A, Fois AG, et al. Reliability and usefulness of different biomarkers of oxidative stress in chronic obstructive pulmonary disease. Oxid Med Cell Longev. 2020;2020:4982324. doi:10.1155/2020/4982324
19. Kono Y, Colley T, To M, et al. Cigarette smoke-induced impairment of autophagy in macrophages increases galectin-8 and inflammation. Sci Rep. 2021;11(1):335. doi:10.1038/s41598-020-79848-0
20. Chen S, Yao Y, Lu S, et al. CircRNA0001859, a new diagnostic and prognostic biomarkers for COPD and AECOPD. BMC Pulm Med. 2020;20(1):311. doi:10.1186/s12890-020-01333-1
21. Ding W, Dong Y. CircTMEM30A/hsa-miR-130a-3p regulates TNFα and promotes the malignant progression of COPD with primary lung cancer. Minerva Med. 2021;114(3):332–344.
22. Zhou L, Wu B, Yang J, et al. Knockdown of circFOXO3 ameliorates cigarette smoke-induced lung injury in mice. Respir Res. 2021;22(1):294. doi:10.1186/s12931-021-01883-w
23. Zhu T, Li S, Wang J, et al. Induced sputum metabolomic profiles and oxidative stress are associated with chronic obstructive pulmonary disease (COPD) severity: potential use for predictive, preventive, and personalized medicine. EPMA J. 2020;11(4):645–659. doi:10.1007/s13167-020-00227-w
24. Gai X, Guo C, Zhang L, et al. Serum glycerophospholipid profile in acute exacerbation of chronic obstructive pulmonary disease. Front Physiol. 2021;12:646010. doi:10.3389/fphys.2021.646010
25. Huang Q, Wu X, Gu Y, et al. Detection of the disorders of glycerophospholipids and amino acids metabolism in lung tissue from male COPD patients. Front Mol Biosci. 2022;9:839259. doi:10.3389/fmolb.2022.839259
26. da Cunha Santos G, Shepherd FA, Tsao MS. EGFR mutations and lung cancer. Annu Rev Pathol. 2011;6:49–69. doi:10.1146/annurev-pathol-011110-130206
27. Mazières J, Peters S, Lepage B, et al. Lung cancer that harbors an HER2 mutation: epidemiologic characteristics and therapeutic perspectives. J Clin Oncol. 2013;31(16):1997–2003. doi:10.1200/JCO.2012.45.6095
28. Xia H, Wu Y, Zhao J, et al. N6-methyladenosine-modified circSAV1 triggers ferroptosis in COPD through recruiting YTHDF1 to facilitate the translation of IREB2. Cell Death Differ. 2023;30(5):1293–1304. doi:10.1038/s41418-023-01138-9
29. Zhang X, Xu Y, Ma L, et al. Essential roles of exosome and circRNA_101093 on ferroptosis desensitization in lung adenocarcinoma. Cancer Commun. 2022;42(4):287–313. doi:10.1002/cac2.12275
30. Zhang H, Ge Z, Wang Z, Gao Y, Wang Y, Qu X. Circular RNA RHOT1 promotes progression and inhibits ferroptosis via mir-106a-5p/STAT3 axis in breast cancer. Aging. 2021;13(6):8115–8126. doi:10.18632/aging.202608
31. Wu C, Du M, Yu R, et al. A novel mechanism linking ferroptosis and endoplasmic reticulum stress via the circPtpn14/miR-351-5p/5-LOX signaling in melatonin-mediated treatment of traumatic brain injury. Free Radic Biol Med. 2022;178:271–294. doi:10.1016/j.freeradbiomed.2021.12.007
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.