Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 19
Circular RNA Expression of Peripheral Blood Mononuclear Cells Associated with Risk of Acute Exacerbation in Smoking Chronic Obstructive Pulmonary Disease
Authors Shen XR , Liu YY, Qian RQ, Zhang WY, Huang JA, Zhang XQ, Zeng DX
Received 30 November 2023
Accepted for publication 13 March 2024
Published 20 March 2024 Volume 2024:19 Pages 789—797
DOI https://doi.org/10.2147/COPD.S448759
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Min Zhang
Xu-Rui Shen,1,* Ying-Ying Liu,2,* Rui-Qi Qian,1,* Wei-Yun Zhang,2 Jian-An Huang,1 Xiu-Qin Zhang,1 Da-Xiong Zeng2
1Department of Pulmonary and Critical Care Medicine, The First Affiliated Hospital of Soochow University, Jiangsu, People’s Republic of China; 2Department of Pulmonary and Critical Care Medicine, Suzhou Dushu Lake Hospital, Suzhou, Jiangsu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiu-Qin Zhang, Department of Pulmonary and Critical Care Medicine, The First Affiliated Hospital of Soochow University, No. 188, Shizi Street, Suzhou, Jiangsu, 215006, People’s Republic of China, Tel +86-18912616972, Email [email protected] Da-Xiong Zeng, Department of Pulmonary and Critical Care Medicine, Suzhou Dushu Lake Hospital, No. 9 Chongwen Road, Suzhou, Jiangsu, 215006, People’s Republic of China, Tel +86-15895567963, Email [email protected]
Purpose: Circular RNAs (circRNAs) are newly identified endogenous non-coding RNAs that function as crucial gene modulators in the development of several diseases. By assessing the expression levels of circRNAs in peripheral blood mononuclear cells (PBMCs) from patients with chronic obstructive pulmonary disease (COPD), this study attempted to find new biomarkers for COPD screening.
Patients and Methods: We confirmed altered circRNA expression in PBMCs of COPD (n=41) vs controls (n=29). Further analysis focused on the highest and lowest circRNA expression levels. The T-test is used to assess the statistical variances in circRNAs among COPD patients in the smoking and non-smoking cohorts. Additionally, among smokers, the Spearman correlation test assesses the association between circRNAs and clinical indicators.
Results: Two circRNAs, hsa_circ_0042590 and hsa_circ_0049875, that were highly upregulated and downregulated in PBMCs from COPD patients were identified and verified. Smokers with COPD had lower hsa_circ_0042590 and higher hsa_circ_0049875, in comparison to non-smokers. There was a significant correlation (r=0.52, P< 0.01) between the number of acute exacerbations (AEs) that smokers with COPD experienced in the previous year and the following year (r=0.67, P< 0.001). Moreover, hsa_circ_0049875 was connected to the quantity of AEs in the year prior (r=0.68, P< 0.0001) as well as the year after (r=0.72, P< 0.0001). AUC: 0.79, 95% CI: 0.1210– 0.3209, P< 0.0001) for hsa_circ_0049875 showed a strong diagnostic value for COPD, according to ROC curve analysis. Hsa_circ_0042590 showed a close second with an AUC of 0.83 and 95% CI: − 0.1972--0.0739 (P < 0.0001).
Conclusion: This research identified a strong correlation between smoking and hsa_circ_0049875 and hsa_circ_0042590 in COPD PBMCs. The number of AEs in the preceding and succeeding years was substantially linked with the existence of hsa_circ_0042590 and hsa_circ_0049875 in COPD patients who smoke. Additionally, according to our research, hsa_circ_0049875 and hsa_circ_0042590 may be valuable biomarkers for COPD diagnosis.
Keywords: chronic obstructive pulmonary disease, circular RNAs, smoking, acute exacerbation, biomarker
Introduction
Chronic obstructive pulmonary disease (COPD) is mainly manifested as incompletely reversible airflow limitation in the lungs, and the airflow limitation is usually gradual, moreover, its degree is often closely related to the lung’s inflammatory response to harmful particles or gases.1 At present, COPD is a global public health problem.2 According to a systematic analysis, 391.9 million people worldwide had COPD in 2019 at a prevalence of 10.3% according to the GOLD criteria.3 COPD-related fatalities accounted for 5.7% of all deaths in 2017. The bulk of COPD cases worldwide are concentrated in China, the nation with the largest population. According to estimates, the prevalence of COPD in China was 13.6%, and in 2016,4 COPD was the country’s fifth most prevalent cause of death. The percentage of COPD patients who have at least one AECOPD episode per year varies from 9.16% to 22.40%.5 However, most current COPD treatments focus on relieving symptoms and preventing acute exacerbations. Elucidating the pathophysiology of COPD is crucial in developing targeted interventions for respiratory diseases.
In recent years, the research of non-coding ribonucleic acid (ncRNA) in the field of disease pathogenesis has been heating up, and the research content has become more and more in-depth. Among the various types of non-coding RNA, circRNA has emerged as a particularly noteworthy focus in current research into the molecular mechanisms of diseases due to its distinctive properties and diverse functions.6 CircRNAs have emerged as important players in gene regulation by absorbing miRNAs or proteins,7 They possess several noteworthy properties that contribute to their vital role in this process. Firstly, circRNAs are widely expressed and produced by a significant proportion of actively transcribed human genes (ranging from 5.8% to 23%).8 Secondly, Circular RNAs have a closed-loop structure, which enhances their stability and reduces their susceptibility to exonucleases such as ribonuclease R (RNase R). Thirdly, most circRNAs are evolutionarily conserved, meaning they have been maintained throughout evolution across different species. Fourthly, a high concentration of circRNAs is found in the cytoplasm, with many derived from introns present in the nucleus.9 Fifthly, circRNAs exhibit tissue- and cancer-specific expression patterns, rendering them potential biomarkers with high sensitivity and specificity for different tissues and cancer types.6 Furthermore, recent research has revealed that certain circular RNAs (circRNAs) can produce functional proteins.6 Overall, due to their unique structural conformation, stability, and diverse functional properties, circRNAs, covalently closed transcripts, can serve as biomarkers and regulate genes and biological processes.10
Multiple studies have demonstrated the importance of circRNAs in PBMCs as pivotal biomolecules for elucidating the pathogenesis of associated diseases. For systemic diseases, many studies on circRNAs in PBMC have focused on diseases such as rheumatoid arthritis,11 cancer,12 systemic lupus erythematosus,13 and coronary artery disease.14 For respiratory system diseases, emphasis has been placed on lung cancer,15 pneumonia,16 and pulmonary tuberculosis.17 However, no reports have been on the expression of circRNAs in COPD patients’ PBMCs and their potential for COPD diagnosis and treatment. The project aims to explore the possible application of circRNAs in PBMCs as a diagnostic tool for COPD.
Materials and Methods
Patients Characteristics
This research was centered on COPD patients at the First Affiliated Hospital of Soochow University from January to December 2022. To be eligible for the study, patients had to meet the following criteria: (1) an FEV1/FVC ratio of less than 70% after 20 minutes of albuterol administration; (2) aged between 45 and 75 years; and (3) no acute exacerbation within the last 6 months. The control group comprised individuals who received a physical examination at the hospital within the same time frame and met the following criteria: (1) a post-albuterol inhalation spirometry test showing a FEV1/FVC score of at least 0.7; (2) no history of chronic cough, wheezing, or expectoration; and (3) no COPD or other chronic respiratory diseases. Both the COPD group and control group were ineligible if they exhibited any of the following: (1) cardiovascular diseases, cerebrovascular disease, hepatic insufficiency, kidney insufficiency, diabetes mellitus, or rheumatic disease; (2) malignant tumor; (3) acute infectious disease; or (4) pregnancy. The participants provided informed consent and the study was approved by the Medical Ethics Committee of the First Affiliated Hospital of Soochow University. The study protocols were conducted in accordance with the principles outlined in the Declaration of Helsinki of the World Medical Association.
PBMC Preparation and RNA Extraction
PBMCs were separated from 5 mL of blood using Ficoll-Paque PLUS (1.077 gmL, GE Healthcare, Uppsala, Sweden) through density centrifugation (400 g, 35 min, 25C). The cells were then preserved in Trizol (5–7 106mL concentration, Invitrogen, Carlsbad, CA, USA) and stored at −80C. Total RNA was isolated from PMBCs using Trizol reagent following the manufacturer’s protocol. The amount of RNA was determined using a NanoDrop ND-1000 (Agilent, Santa Clara, CA, USA).
Reverse Transcription and Quantitative Real-Time PCR
1 μg of RNA was reverse-transcribed using the ReverTra Ace® qPCR RT Kit (616,700, TOYOBO, Japan). Each sample was repeated 3 times using cDNA as a template to amplify the target gene and used customized qRT-PCR primer sequences (Aksomics, China) for reaction, with GAPDH as an internal reference. Finally, we used the Quant Studio 6 Flex system (442,800, TOYOBO, Japan) and followed the react instructions. The 2–ΔΔCt method was utilized to determine the level of gene expression.
Statistical Analysis
All statistical analyses were performed using GraphPad Prism 8.0, a software developed by GraphPad Software, based in San Diego, California, USA. The numerical data were presented as mean ± SD. To identify differences in circRNA expression between groups, Student’s t-tests were employed. A P-value less than 0.05 and fold change (FC) greater than 1.5 were considered indicative of significant gene expression. The Spearman rank correlation was used to evaluate the relationship between circRNA levels and clinical data. ROC curves were used to evaluate the diagnostic potential of dysregulated circRNAs in PBMCs of COPD patients compared to healthy controls.
Results
All research participants’ baseline characteristics are shown in Table 1. There were 70 people in all, 41 in the COPD group and 29 in the control group. Age, gender, and BMI did not significantly differ between the two groups. In contrast to the control group, the FEV1/FVC (%), FEV1%, and FEV1 predicted values were considerably lower in the COPD group (P < 0.01). Additionally, CAT and MMRC scores were considerably higher (P < 0.01) in COPD patients. Patients with COPD showed differential expression of circRNAs. The most upregulated circRNA (hsa_circ_0049875) and the most downregulated circRNA (hsa_circ_0042590) were chosen for additional examination to determine the most clinically relevant biomarker.
 |
Table 1 Clinical Characters and Laboratory Measures of the Participants |
Comparison of Different Expressions of Hsa_circ_0042590 and Hsa_circ_0049875 in PBMCs in the COPD and Control Group as Well as in Smoking COPD and Non-Smoking COPD Groups
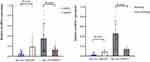
The COPD group exhibited aberrant levels of hsa_circ_0042590 and hsa_circ_0049875 in PBMCs. In the COPD group, The expressions of hsa_circ_0049875 were upregulated by (0.3470±0.2625) in comparison to the control group. (0.1261±0.0714). Conversely, the expressions of hsa_circ_0042590 were downregulated by (0.04634±0.04436) compared with the control group (0.1820±0.1911). These differences were found to be statistically significant (P <0. 05). We conducted a T-test to evaluate the levels of hsa_circ_0042590 and hsa_circ_0049875 in the PBMCs of patients with COPD, with and without a history of smoking. In the COPD group with a smoking history, the levels of hsa_circ_0042590 showed a significant decrease (P<0.01), while hsa_circ_0049875 levels were found to be significantly increased (P<0.01) compared to the non-smoking COPD group (Figure 1).
The levels of expression for two circRNAs were confirmed using qRT-PCR in PBMCs obtained from all the enrolled patients. Student’s t-test was used to analyze the data obtained from hsa_circ_0042590 and hsa_circ_0049875. Within the COPD group, participants were further divided into smoking and non-smoking subgroups based on their smoking history, and the differences in the levels of expression for hsa_circ_0042590, and hsa_circ_0049875 were compared between these two groups using the T-test. The results are reported as means ± SD.
Spearman Correlation Analysis Was Performed to Assess the Relationship Between Clinical Variables and Confirmed circRNAs in PBMCs Obtained from Both Smoking and Non-Smoking COPD Patients
The aim was to determine whether hsa_circ_0042590 and hsa_circ_0049875 in PBMCs could be biomarkers for COPD severity. According to the findings of the Spearman correlation tests, a significant correlation between the levels of has_circ_0042590 in PBMCs from smoking COPD patients and the number of acute exacerbations (AEs) in the previous year (r=0.52, P<0.01) and the following year (r=0.67, P<0.001) were found. Additionally, Furthermore, it was shown that hsa_circ_0049875 was connected to the quantity of AEs in the year prior (r=0.68, P<0.0001) and the year after (r=0.72, P<0.0001), as demonstrated in Table 2 and Figure 2.
 |
Table 2 Spearman Rank Correlation Coefficients of Clinical Variables and Quantitative RT-PCR-Confirmed circRNAs in PBMCs from Smoking and Non-Smoking COPD Patients |
 |
Figure 2 Correlation between the expression levels of confirmed circRNAs and the number of AEs in the previous and following year in smoking COPD patients. |
ROC Curve Analysis of PMBC circRNAs That Have Been Verified in COPD Patients
We conducted an ROC curve analysis to evaluate the potential use of substantially and differentially expressed circRNAs in COPD diagnosis. The levels of hsa_circ_0049875 and has_circ_0042590 in PBMCs were able to identify COPD patients from healthy controls, as shown by the ROC curves for validated circRNAs. AUC values for has_circ_0042590 (AUC: 0.83, 95% CI 0.1972--0.0739, P<0.0001) and hsa_circ_0049875 (AUC: 0.79, 95% CI 0.1210–0.3209, P<0.0001) were the highest, respectively, according to Table 3, Figure 3. As a result, has_circ_0042590 and hsa_circ_0049875 may be useful biomarkers for COPD diagnosis.
 |
Table 3 Sensitivity and Specificity of the Candidate Biomarkers in Controls and COPD Patients |
 |
Figure 3 Receiver operating characteristic (ROC) curve analysis of confirmed circRNAs in PBMCs from COPD patients. The AUC values are given on the graphs. |
Discussion
COPD is the result of prolonged exposure to harmful inhaled particles, specifically from tobacco smoke and pollutants.18 It is distinguished by a progressive and irreversible decline in airflow and deterioration of lung tissue.19 AECOPD is the main cause of hospitalization and death in people with COPD.20 It is a medical condition marked by an abrupt decline in airway function and respiratory symptoms. CircRNAs are distinct, very conserved molecules that are expressed in several human tissues, including the skin, lung, breast, digestive tract, urinary system, and nervous system.21,22 Numerous investigations have shown that circRNAs control many diseases via various pathways. The mononuclear cells in the blood called peripheral blood mononuclear cells (PBMCs) are essential to the immune system of the body.23 It has been demonstrated that the gene expression signatures found in PBMCs, which represent the body’s general immunological function, are important in the development of COPD.24
This paper used circRNA microarray to determine the circRNA expression patterns in PBMCs from COPD patients and healthy controls. Because of their potential role in the development of COPD, circRNAs’ unique expression patterns may serve as biomarkers for the identification of the disease. We identified two differentially expressed circRNAs with significant potential as clinically applicable biomarkers. In COPD patients, hsa_circ_0049875 was overexpressed whereas hsa_circ_0042590 demonstrated a substantial decrease when compared to healthy controls. There were statistically significant differences in the expression levels of hsa_circ_0049875 and hsa_circ_0042590 between COPD patients who smoked and those who did not. The expression of hsa_circ_0042590 was correlated with the number of acute exacerbations (AEs) in the prior year (r=0.52, P<0.01) and the subsequent year (r=0.67, P<0.001) in COPD patients with a history of smoking, whereas hsa_circ_0049875 was correlated with the number of AEs in the prior year (r=0.68, P<0.0001) and the subsequent year (r=0.72, P<0.0001). Furthermore, hsa_circ_0049875 (AUC=0.79) and hsa_circ_0042590 (AUC=0.83) have substantial diagnostic values for COPD, according to ROC curve analysis.
Previous reports of circRNA dysregulation in COPD patients are inconsistent due to varying samples and techniques. For example, Circ_0061041 has been linked to airway remodeling and the CSE-triggered epithelial-mesenchymal switch in COPD.25,26 Meanwhile, hsa_circRNA_0003060 has been shown to significantly down-regulate the primary human small-airway epithelial cell (HSAECs) model of COPD that is induced by.27 Additionally, CircFOXO3 may be a promising target for the treatment of inflammatory illnesses as it has been found to play a critical role in the pathological remodeling of inflammatory processes brought on by exposure to cigarette smoke.28 Moreover, emphysema development later on has been seen to benefit from the protective effects of CD69. Xue et al discovered that in human pulmonary microvascular endothelial cells, circ0006872 induces apoptosis, inflammation, and oxidative stress.29 Furthermore, significant differences in the downregulation of hsa_circ_0022342 in PBMC were observed in Chronic thromboembolic pulmonary hypertension.30 Research on the expression of circRNAs in patient-derived peripheral blood mononuclear cells suggests that circRNA expression may impact immunological homeostasis and contribute to the pathogenesis of COPD.
According to earlier research, RNAs are crucial in the remodeling and inflammation of the airways brought on by smoking.31–33 These results imply that circRNAs’ role in the pathophysiology of COPD may be connected to their dysregulated expression. Additionally, we found that hsa_circ_0049875 and hsa_circ_0042590 levels in PBMCs had potential as COPD diagnostic indicators. The aberrant expression of hsa_circ_0048775 and hsa_circ_0042590 in smokers with COPD can forecast the frequency of exacerbations in the preceding and following years. This is especially the case with hsa_circ_0048775 and hsa_circ_0042590, which stood out among the rest in terms of ROC AUC value and so have the potential to be used as diagnostic biomarkers. Furthermore, our extensive review of the literature confirmed that our study was the first to identify abnormalities in hsa_circ_0049875 and hsa_circ_0042590. These results point to a potential function for circRNAs in the chronic inflammatory immune response associated with COPD and imply that the dysregulation of these two circRNAs, as emphasized in our investigation, is implicated in the development of COPD.34–36
In conclusion, we have deduced that hsa_circ_0049875 and hsa_circ_0042590 may be important factors in the onset of COPD. A crucial first step in understanding COPD better is the identification of novel circRNAs with differential expression. Future investigations into the pathogenesis of COPD may benefit from these results, which may assist in determining if circRNAs in PBMCs might function as innovative, non-invasive biomarkers for the diagnosis and treatment of COPD. However, further research is required to corroborate our findings because of our inadequate understanding of circRNAs. The tiny sample size in this study is one of its limitations. To support the results of our investigation, we thus plan to collect additional samples.
Conclusion
This study found a strong correlation between smoking and hsa_circ_0049875 and hsa_circ_0042590 in COPD PBMCs. The frequency of AEs in the prior and subsequent years was shown to be substantially linked with hsa_circ_0049875 and hsa_circ_0042590 in COPD patients with a previous history of smoking. Our findings also suggest that hsa_circ_0049875 and hsa_circ_0042590 may also be used as COPD diagnostic biomarkers.
Acknowledgments
Xiu-Qin Zhang and Da-Xiong Zeng are equal corresponding authors.
Funding
There is no funding to report.
Disclosure
The authors had no conflicts of interest.
References
1. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease; 2023. Available from: https://goldcopd.org/2023-gold-report-2.
2. Soriano JB, Kendrick PJ, Paulson KR, et al. Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir Med. 2020;8(6):585–596. doi:10.1016/S2213-2600(20)30105-3
3. Adeloye D, Song P, Zhu Y, et al. Global, regional, and national prevalence of, and risk factors for, chronic obstructive pulmonary disease (COPD) in 2019: a systematic review and modeling analysis. Lancet Respir Med. 2022;10(5):447–458. doi:10.1016/S2213-2600(21)00511-7
4. Fang L, Gao P, Bao H, et al. Chronic obstructive pulmonary disease in China: a nationwide prevalence study. Lancet Respir Med. 2018;6(6):421–430. doi:10.1016/S2213-2600(18)30103-6
5. Zinellu A. Clinical significance of the neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in acute exacerbations of COPD: present and future. Eur Respir Rev. 2022;31(166):220095. doi:10.1183/16000617.0095-2022
6. Han B, Chao J, Yao H. Circular RNA and its mechanisms in disease: from the bench to the clinic. Pharmacol Ther. 2018;187:31–44. doi:10.1016/j.pharmthera.2018.01.010
7. Kong P, Yu Y, Wang L, et al. circ-Sirt1 controls NF-kB activation via sequence-specific interaction and enhancement of SIRT1 expression by binding to miR-132/212 in vascular smooth muscle cells. Nucleic Acids Res. 2019;4(7):3580–3593. doi:10.1093/nar/gkz141
8. Conn SJ, Pillman K, Toubia J, et al. The RNA binding protein quaking regulates formation of circRNAs. Cell. 2015;160(6):1125–1134. doi:10.1016/j.cell.2015.02.014
9. Ebbesen KK, Kjems J, Hansen TB. Circular RNAs: identification, biogenesis and function. BBA. 2016;1859(1):163–168. doi:10.1016/j.bbagrm.2015.07.007
10. Wang N, Wang Q, Du T, et al. The potential roles of exosomes in chronic obstructive pulmonary disease. Front Med. 2021;7:618506. doi:10.3389/fmed.2020.618506
11. Lu H, Yang Y, Kuang D, Liu P, Yang J. Expression profile of circRNA in peripheral blood mononuclear cells of patients with rheumatoid arthritis. BMC Med Genomics. 2022;15(1):77. doi:10.1186/s12920-022-01225-9
12. Chen X The functional roles of the circRNA/Wnt axis in cancer. Mol Cancer. 2022;2:1.
13. Guo G, Wang H, Ye L, Lin K, Li B, Xue X, Zhang H. Hsa_circ_0000479 as a Novel Diagnostic Biomarker of Systemic Lupus Erythematosus. Front Immunol. 2019;10:464046.
14. Zhou H, Gan X, He S, et al. Identification of circular RNA BTBD7_hsa_circ_0000563 as a novel biomarker for coronary artery disease and the functional discovery of BTBD7_hsa_circ_0000563 based on peripheral blood mononuclear cells: a case-control study. Clin Proteomics. 2022;19:37.
15. Zhao Y, Zheng R, Chen J, Ning D. CircRNA CDR1as/Mir-641/HOXA9 Pathway-Regulated Stemness Contributes to Cisplatin Resistance in Non-Small Cell Lung Cancer (NSCLC). Cancer Cell International; 2020.
16. Yin YD. Differential circRNA expression profiles in peripheral blood mononuclear cells among mild and severe influenza-associated pneumonia patients. Nat Med J China. 2021;2:1.
17. Qian Z. Potential diagnostic power of blood circular RNA expression in active pulmonary tuberculosis. EBio Med. 2018;27:18–26.
18. Christenson SA, Smith BM, Bafadhel M, Putcha N. Chronic obstructive pulmonary disease. Lancet. 2022;399(10342):2227–2242. doi:10.1016/S0140-6736(22)00470-6
19. Mathyssen C, Aelbrecht C, Serré J, et al. Local expression profiles of vitamin D-related genes in airways of COPD patients. Respir Res. 2020;21(1):137. doi:10.1186/s12931-020-01405-0
20. Ko FW, Chan KP, Hui DS, et al. Acute exacerbation of COPD. Respirology. 2016;21(7):1152–1165. doi:10.1111/resp.12780
21. Lei M, Zheng G, Ning Q, Zheng J, Dong D. Translation and functional roles of circular RNAs in human cancer. Mol Cancer. 2020;19(1):30. doi:10.1186/s12943-020-1135-7
22. Meng Q, Wang J, Cui J, et al. Prediction of COPD acute exacerbation in response to air pollution using exosomal circRNA profile and Machine learning. Environ Int. 2022;168:107469. doi:10.1016/j.envint.2022.107469
23. Khan S, Kaihara KA. Single-cell RNA-sequencing of peripheral blood mononuclear cells with ddSEQ. Single Cell Meth. 2019;1979:155–176.
24. Bahr TM, Hughes GJ, Armstrong M, et al. Peripheral blood mononuclear cell gene expression in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2013;49(2):316–323. doi:10.1165/rcmb.2012-0230OC
25. Liu Q. For COPD, regulation of miR-515-5p by hsa_circ_0061052, acting via the FoxC1/Snail pathway, is involved in cigarette smoke-induced airway remodeling; 2020. Available from: https://www.researchsquare.com/article/rs-15783/v1.
26. Chen -L-L. The expanding regulatory mechanisms and cellular functions of circular RNAs. Nat Rev Mol Cell Biol. 2020;21(8):475–490. doi:10.1038/s41580-020-0243-y
27. Duan R, Niu H, Yu T, et al. Identification and bioinformatic analysis of circular RNA expression in peripheral blood mononuclear cells from patients with chronic obstructive pulmonary disease. Int J Chronic Obstr. 2020;15:1391–1401. doi:10.2147/COPD.S252896
28. Zhou L, Wu B, Yang J, et al. Knockdown of circFOXO3 ameliorates cigarette smoke-induced lung injury in mice. Respir Res. 2021;22(1):294. doi:10.1186/s12931-021-01883-w
29. Xue M, Peng N, Zhu X, Zhang H. Hsa_circ_0006872 promotes cigarette smoke-induced apoptosis, inflammation, and oxidative stress in HPMECs and BEAS-2B cells through the miR-145-5p/NF-κB axis. Biochem Biophys Res Commun. 2021;534:553–560. doi:10.1016/j.bbrc.2020.11.044
30. Miao R, Wang Y, Wan J, et al. Microarray expression profile of circular RNAs in chronic thromboembolic pulmonary hypertension. Medicine. 2017;96(27):e7354. doi:10.1097/MD.0000000000007354
31. Lee H. Targeting insulin-like growth factor-I and insulin-like growth factor–binding protein-3 signaling pathways. A novel therapeutic approach for asthma. Am J Respir Cell Mol Biol. 2014;50(4):667–677. doi:10.1165/rcmb.2013-0397TR
32. Tsuyusaki J, Kuroda F, Kasuya Y, et al. Cigarette smoke-induced pulmonary inflammation is attenuated in CD69-deficient mice. J Recep Sig Transd. 2011;31(6):434–439. doi:10.3109/10799893.2011.631929
33. Zeng N, Wang T, Chen M, et al. Cigarette smoke extract alters genome‐wide profiles of circular RNAs and mRNAs in primary human small airway epithelial cells. J Cell Mol Med. 2019;23(8):5532–5541. doi:10.1111/jcmm.14436
34. Gaffey K, Reynolds S, Plumb J, Kaur M, Singh D. Increased phosphorylated p38 mitogen-activated protein kinase in COPD lungs. Eur Respir J. 2013;42(1):28–41. doi:10.1183/09031936.00170711
35. Chung KF. p38 mitogen-activated protein kinase pathways in asthma and COPD. Chest. 2011;139(6):1470–1479. doi:10.1378/chest.10-1914
36. Wu D, Yuan Y, Lin Z, et al. Cigarette smoke extract induces placental growth factor release from human bronchial epithelial cells via ROS/MAPK(ERK-1/2)/Egr-1 axis. COPD. 2016;11:3031–3042. doi:10.2147/COPD.S120849
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.