Back to Journals » OncoTargets and Therapy » Volume 15
Circular RNA Controls Tumor Occurrence and Development via Cell Cycle Regulation
Authors Liu F, Qu R, Yang L, Shi G , Hao S, Hu C
Received 23 April 2022
Accepted for publication 25 August 2022
Published 15 September 2022 Volume 2022:15 Pages 993—1009
DOI https://doi.org/10.2147/OTT.S371629
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Sanjay Singh
Fang Liu,* Rongfeng Qu,* Limin Yang, Guang Shi, Shuhong Hao, Chunmei Hu
Department of Hematology and Oncology, The Second Hospital of Jilin University, Changchun, Jilin, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Chunmei Hu, Department of Hematology and Oncology, The Second Hospital of Jilin University, Ziqiang Street No. 265, Changchun, Jilin, 130041, People’s Republic of China, Tel/Fax +86043181136427, Email [email protected]
Abstract: Circular RNAs (circRNAs) participate in the occurrence and development of various diseases through different mechanisms, such as by acting as a microRNA (miRNA) sponge, interacting with RNA-binding proteins, and regulating gene transcription and protein translation. For example, the abnormal expression of specific circRNAs in tumor cells can alter key regulatory factors and the cell cycle network, resulting in cell cycle disorders and the development and metastasis of tumors. Here, we summarize the mechanisms involved in the circRNA-mediated processes that lead to uncontrolled cell cycle and tumor cell proliferation. Extensive studies investigating the abnormal expression of circRNAs in different cancer types have been conducted. The unique characteristics of circRNAs and their ability to regulate the cell cycle through diverse mechanisms is extremely valuable in tumor diagnosis, treatment, and prognosis. Our review may assist in further understanding the circRNA-mediated regulation of the cell cycle in tumors and provide insights for research on circRNA-based therapeutic strategies and biological diagnosis for cancer.
Keywords: circRNA, cancer, cyclins, CDKs, CKIs, p53
Introduction
With the development of advanced molecular technologies, such as circular RNA (circRNA) microarray screening and high-throughput sequencing, extensive research on circRNAs has been performed, revealing their biological function and potential therapeutic and diagnostic value.1
Unlike linear RNAs, circRNAs possess special covalent bond ring structures, which ensures high stability. They can be differentially expressed in tissues and cells, especially in accessible body fluids such as saliva, plasma, and blood. These characteristics make this class of RNA molecules promising cancer biomarkers.2 Li et al found that hsa_circ_0001649 is expressed at low levels in gastric cancer (GC) tissues, plotted its associated receiver operating characteristic (ROC) curve, and evaluated its diagnostic value in gastric cancer.3 Circular RNAs are differentially expressed in a variety of cancers, and their clinical significance as potential diagnostic markers has been shown for different malignancies, such as liver cancer,4 colorectal cancer,5 glioblastoma,6 and head and neck squamous cell carcinoma.7 In addition, many studies have used survival analysis to evaluate the relationship between the expression level of circRNAs in different tumors and the survival rates of patients, reflecting the value of circRNAs in judging tumor prognosis.8–10 Circular RNAs primarily play a role in tumors by acting as competing endogenous RNA (ceRNA) sponge miRNAs to attenuate the miRNA repression of target genes.11 In addition, circRNAs can also participate in tumorigenesis and development by interacting with proteins, acting as transcriptional regulators, and participating in protein translation.11–13 The regulation of circRNA expression can have an impact on many diseases, including cancer, in which abnormally expressed circRNAs play a critical function in activating carcinogenic or tumor suppressor effects during tumor development and progression.13,14
The cell cycle involves a strict regulatory process facilitating DNA replication and cellular reproduction.15 Hence, cell cycle disorders play a vital role in tumor proliferation, metastasis, and prognosis. In eukaryotic cells, the cell cycle consists of gap 1 (G1, pre-DNA synthesis), synthesis (S, DNA synthesis), gap 2 (G2, pre-division), and mitosis (M, cell division) phases.15 Cell cycle progression is mainly driven by the complexes formed by cyclins (CCN) and cyclin-dependent kinases (CDKs).16 The four main types of CCNs in mammalian cells, CCNA, CCNB, CCND, and CCNE, form complexes with various CDKs, such as CDK1, CDK2, CDK4, and CDK6.16 Simultaneously, cell cycle progression is regulated by the CCN-dependent kinase inhibitors (CKIs), including the INK4 and Cip/Kip families.17 These inhibitors can block specific CCN/CDK complexes, thereby terminating the cell cycle at certain points.16,17 Normal cell cycle operation is also inseparable from the regulatory network involving the retinoblastoma protein (pRb) and p53 pathways.17,18 Hence, cell cycle dysregulation is often a marker of the transformation of normal cells into tumor cells.15 Aberrantly expressed circRNAs in tumors can affect tumor cell proliferation by regulating key cell cycle regulators, including p53, pRB, CDKs, CDK inhibitors (CKIs), and cyclins.19 In addition, circRNAs can play a role in tumor invasion, metastasis, the tumor lymph node metastasis (TNM) stage, the histological grade, malignant phenotypes, and chemotherapy drug sensitivity by affecting the aforementioned cell cycle factors.20–24 Table 1 lists the functions of cyclin/CDK complexes in various phases of the cell cycle. Figure 1 depicts cell cycle regulation in cancer involving circRNA targets.
 |
Table 1 Functions of the Cyclin/CDK Complexes in Cell Cycle Phases |
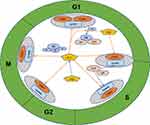 |
Figure 1 Schematic diagram of cell cycle regulation in cancer involving circular RNA targets. Orange arrow – inhibition, blue arrow – promotion. |
Circular RNAs can regulate the aforementioned key proteins in the cell cycle in a variety of ways. For example, circRNAs can form a circRNA/miRNA/messenger RNA (mRNA) axis through the adsorption of miRNA and regulate the expression of downstream cell cycle-associated target genes. CircRNAs can also modulate cell cycle processes by regulating the key regulatory factors of phosphatidylinositol-3 kinase (PI3K)/AKT/FOXO, Wnt/β-catenin, and other signaling pathways, altering cell proliferation by directly forming complexes with cell cycle-related factors, and binding to RNA-binding proteins to regulate CCNs.25–31 Interestingly, circRNAs can also be controlled by cell cycle regulators, forming a regulatory feedback loop directing cell cycle progression.32,33 Figure 2 shows the regulatory mechanism of the cancer-associated circRNA-mediated cell cycle.
Here, we summarize the mechanisms involved in the circRNA-mediated processes that lead to uncontrolled cell cycle and tumor proliferation through an extensive review of the abnormal expression of circRNAs in different cancer types. We found that circRNAs are extremely valuable for the diagnosis, treatment, and prognosis of tumors.
CircRNA-Mediated Regulation of Cell Cycle via CDKs
The main driving force of cell cycle progression is the sequential activation of CDKs, a group of serine/threonine kinases that form complexes with CCNs, which stably activate and phosphorylate CDKs at specific stages.15 CCN/CDK formation also can regulate cell cycle progression through the phosphorylation of pRB. The dysregulation of CDKs primarily leads to the development of many diseases, including cancer.17,34
Cdk1
Generally, CDK1 facilitates the G2/M transition and M phase progression by forming a complex with CCNA and CCNB.15 In tumor cells, the abnormal expression of circRNAs can affect CDK1 expression through miRNA sponging. For example, the estrogen-induced circPGR, which is highly expressed in estrogen receptor (ER)-positive breast cancer (BrC) cell lines and clinical BrC tissue samples, regulates the cell cycle-associated proteins CDK1 and CDK6 by sponging miR-301a-5p and promotes the growth and tumorigenesis of ER-positive BrC cells.35 In addition, this study also found that an anti-sense oligonucleotide targeting circPGR could effectively inhibit the growth of breast cancer cells, providing a new approach for the treatment of ER-positive breast cancer. Li et al discovered that circ-METTL3, which was also highly expressed in BrC, acted as a ceRNA of miR-31-5p and upregulated its target CDK1, facilitating cell proliferation, migration, and invasion in BrC.36 Huang et al found that circSLC7A11 is significantly overexpressed in tissue and cells of hepatocellular carcinoma (HCC) and that its expression level is positively correlated with the tumor size, microvascular invasion, and TNM stage.37 Survival analysis showed that HCC patients with high expression of circSLC7A11 usually have worse prognoses, and the results indicated that circSLC7A11 has the potential to become a prognostic marker for HCC. Huang et al further investigated the mechanism of action of circSLC7A11 in HCC cells and found that it accelerates HCC progression and metastasis by dampening the inhibitory effect of miR-330-3p on CDK1.37 Aside from sponging miRNAs, circRNAs can also directly bind to CKD1. For example, another study found that HBV_circ_1, produced by hepatitis B virus (HBV), is significantly more abundant in HBV-related HCC tissues than in adjacent tissues.38 In addition, the survival rates of HBV_circ_1-positive patients is significantly lower than that of HBV_circ_1-negative patients. The role of HBV_circ_1 in HBV-related HCC was further discovered via RNA pull-down and cell function assays; specifically, HBV_circ_1 was found to directly interact with CDK1 and upregulate CDK1 expression in HBV-related HCC tissues, thereby stimulating tumor proliferation, migration, and invasion.38
Cdk2
CDK2 normally facilitates G1/S transition and S phase progression by forming a complex with CCNE and CCNA.15 Similar with CDK1, circRNAs mainly regulate the level of CDK2 via miRNA sponging. For instance, Zheng et al assessed the expression of hsa_circ_0000520 in 52 pairs of cervical cancer (CC) tissues and their corresponding adjacent normal tissues using RT-qPCR. The expression of hsa_circ_0000520 in CC was found to be higher than that in normal tissues.39 Furthermore, the overexpression of hsa_circ_0000520 was determined to promote the proliferation of CC cells. Zheng et al further investigated the downstream mechanism of hsa_circ_0000520. That study found that hsa_circ_0000520 promotes cell proliferation and cell cycle progression by inhibiting miR-1296 and upregulating CDK2 expression, and in vivo experiments were performed to verify this conclusion.39 This study suggests potential strategies for the treatment of CC. Similarly, circ_0084927, which is upregulated in CC tissues and cells, sponges miR-1179 to promote tumor cell proliferation and adhesion by regulating CDK2 expression.23 Xie et al found that has_circ_0078710, which was upregulated in HCC, enhanced cell proliferation, migration, and invasion and promoted tumor growth by sponging miR-31 and activating CDK2 expression.40
Cdk3
In mammals, CDK3 can participate in cell cycle progression by promoting the transition of cells from G1 to S phase, and even play a role in tumor progression.20,41 CDK3 reportedly also promotes the G0/G1 transition by interacting with the non-CDK8-associated pool of CCNC for pRB phosphorylation, which is required for cells to efficiently exit from the G0 phase.42 CDK3 is overexpressed in several cancer cell lines and may play an important role in cell proliferation and malignant transformation.43 Liu et reported that circRNA_141539 was significantly upregulated in patients with esophageal squamous cell carcinoma (ESCC), which in turn upregulated the CDK3 expression level by sponging miR-4469, which is significantly associated with TNM stage, differentiation, and poor prognosis.20
Cdk4
CDK4 and its homolog CDK6 are classic cell cycle kinases that facilitate the progression of cells through the early G1 phase by forming complexes with D‐type CCNs, specifically D1, D2, and D3.44 The CDK4/6/CCND complexes enable the phosphorylation of the RB protein family members, resulting in the release of E2F transcription factors from RB‐mediated inhibition. Subsequently, E2F‐dependent gene activation facilitates the G1/S transition and DNA synthesis.44 Previous studies have reported that miR-198 is abnormally expressed in various cancers, including HCC, and that it is associated with the initiation and progression of various cancers.45–48 Li et al used bioinformatics analysis to predict that circSP3 might sponge miR-198 and the miR-198-target gene CDK4 and further verified this prediction.49 A series of in vitro and in vivo experiments was further conducted to study the expression and function of circSP3 in HCC. The results showed that high expression of circSP3 in HCC could promote the growth of HCC cells by inducing the expression of CDK4 by sponging miR-198.49
Cdk6
CDK6 commonly interacts with CDK4 and CCND to form a complex during the G1 phase of the cell cycle.15 A recent study found that the expression level of circ-ZEB1.33 in HCC tissues and serum is higher than that in normal tissue.50 Gong et al further analyzed the clinical value of circ-ZEB1.33 in HCC by generating Kaplan–Meier curves, and the survival rates of patients with high expression were found to be significantly reduced. This result indicated that circ-ZEB1.33 could be used as a biomarker for HCC diagnosis and prognosis. Furthermore, Gong et al explored the biological function of circ-ZEB1.33 in HCC cells and found that it promotes HCC cell proliferation by attenuating the downregulation of CDK6 expression mediated by the sponge miR-200a-3p.50 A previous report revealed that the expression levels of circ_ASAP2 and CDK6 were increased in gastric cancer (GC) tissues and cells, whereas the expression of miR-770-5p was decreased. Specifically, circ_ASAP2 sponged miR-770-5p and consequently altered CDK6 expression, thereby boosting cell cycle progression in GC cells.51 In addition, circ_0058063 is significantly overexpressed in bladder cancer (BCa) tissues and promotes tumor progression by sponging miR-145-5p and regulating CDK6 expression.52
CircRNA-Mediated Regulation of Cell Cycle via Cyclins
The normal progression of the cell cycle greatly depends on the CCN family proteins and the subsequent activation of CDKS.53 In turn, the kinase activity of CDKs is positively regulated by the binding of specific CCNs.15
CCNDs
CircRNAs indirectly regulate the expression of CCNDs by sponging miRNAs. For example, Zhu et al found that hsa_circ_0013958 expression is upregulated in lung adenocarcinoma (LAC) tissues, cells, and plasma.54 Furthermore, hsa_circ_0013958 levels were found to be associated with the TNM stage and lymphatic metastasis. The diagnostic value of circ-SHPRH for LAC was evaluated by generating an ROC curve, and the area under the curve was 0.815, the sensitivity was 0.755, and the specificity was 0.796, indicating that hsa_circ_0013958 has high accuracy, specificity, and sensitivity and can be used as a new diagnostic biomarker for LAC. Through in vitro experiments, hsa_circ_0013958 was found to play a tumor-promoting role in LAC cells by upregulating CCND1 levels via the sponging of miR-134.54 Similarly, a previous study reported that circ-PITX1, which was highly expressed in NSCLC, formed the circ-PITX1/miR-1248/CCND2 regulatory axis that promoted tumor proliferation, migration, and invasion.21 Yang et al also showed that the overexpression of circDPP4 induced the expression of CCND1 by acting as a ceRNA for miR-195 and enhanced the proliferation, migration, invasion, and cell cycle progression of prostate cancer cells.55 In addition, hsa_circ_0000467 expression is higher in GC tissues than in controls and may play a carcinogenic role in tumor progression by regulating the associated miR-326-3p/CCND1 axis.56 In BrC, the upregulation of circ-UBR1 expression increases the expression of CCND1 by sponging miR-1299 and promotes tumor cell proliferation.57 Interestingly, the interaction of circRNAs with RNA-binding proteins can prevent the inhibition of CCND expression. A circRNA derived from CCND1, circ-CCND1, is significantly upregulated in laryngeal squamous cell carcinoma and positively correlates with aggressive clinical features, resulting in poor prognosis and enhanced tumorigenesis.31 Zang et al analyzed the survival curve and found that patients with high circ-CCND1 expression had a shorter survival time and that it could be a biomarker for the prognosis of laryngeal squamous cell carcinoma. The mechanism underlying the effects of circ-CCND1 in laryngeal squamous cell carcinoma was further explored.Circ-CCND1 can directly interact directly interacts with the human antigen R protein to increase the stability of CCND1 mRNA. In addition, as an effective sponge for miR-646, circ-CCND1 reduces the miR‐646-mediated inhibition of CCND1 and further elevates its expression.31
CircRNAs can also regulate the expression of CCND1 by targeting the PI3K/AKT pathway. Akt is a serine-threonine kinase that can be regulated following activation of phosphatidylinositol 3-kinase (PI3K),58 which inhibits FOXO transcription factors via phosphorylation and contributes to cell survival, growth, and proliferation.58 FOXOs, which regulate numerous cell cycle-related proteins, can induce cell cycle arrest by suppressing CCND1 and CCND2 expression.58,59 In addition, AKT promotes cell cycle progression by inactivating GSK-3β and promoting the accumulation of β-catenin, activating the Wnt/β-catenin signaling pathway, and increasing the expression of its target, CCND1.60–63 Yang et al demonstrated that circZFR expression was elevated in HCC tissues and cells and promoted tumor progression by downregulating miR-511 expression, activating AKT1 signaling, and upregulating CCND1 expression.60 The circPSMA1 overexpression observed in triple-negative BrC (TNBC) cells and their exosomes attenuated the miR-637-mediated inhibition of AKT1, elevated the expression of CCND1, and enhanced the tumorigenesis, metastasis, and immunosuppression of tumor cells.64 Yang et al performed Kaplan–Meier analysis to further explore the relationship between the circPSMA1/miR-637/Akt1 axis and the clinical outcomes of TNBC patients; the results showed that high Akt1 expression and low miR-637 expression were closely associated with poor outcomes for TNBC patients with lymphatic metastases.64 Taken together, these findings suggest that circPSMA1 might serve as a potential prognostic biomarker for TNBC and provide a new research direction for TNBC immunotherapy. circRNAs regulate CCND1-mediated cell proliferation by modulating the Wnt-β/catenin signaling pathway.25 In cancer, aberrant Wnt/β-catenin signaling facilitates stem cell renewal, cell proliferation, and cell differentiation, thus playing crucial roles in tumorigenesis and showing potential for therapy.61 In particular, the abnormal regulation of the transcription factor β-catenin, a pivotal component of the Wnt signaling pathway, leads to early events in carcinogenesis.61 In the canonical Wnt cascade, β-catenin is the key effector responsible for signal transduction to the nucleus that triggers the transcription of Wnt-specific genes determining cell fate.62 Without a Wnt signal, cytoplasmic β-catenin is ubiquitinated and degraded by the APC complex and cannot enter the nucleus, consequently blocking the transcription of Wnt/β-catenin target genes.62 Normally, Wnt signaling blocks the activity of the destruction complex, resulting in increased levels of cytoplasmic β-catenin that are translocated to the nucleus.62 In the nucleus, β-catenin interacts with T cell-specific factor (TCF)/lymphoid enhancer-binding factor (LEF) and other co-activators and induces the transcription of Wnt target genes, such as c-Myc, CCND1, and CDKN1A, which in turn upregulate the expression of TCF/LEF target genes.61,62 Zhang et al revealed that the expression of circRNA_069718 in TNBC tissues and cell lines was significantly correlated with advanced TNM stage, lymph node metastasis, and poor overall survival in cancer patients.63 Notably, circRNA_069718 upregulated the expression of Wnt/β-catenin pathway-related genes (eg β-catenin, c-Myc, and CCND1), subsequently promoting TNBC cell proliferation and invasion in vitro.63 These findings provide a theoretical basis for developing new diagnostic markers and treatment strategies for TNBC. By contrast, circRNA-ITCH expression was downregulated in HCC tissues. CircRNA-ITCH can reduce the expression of CCND1 by inhibiting the Wnt/β-catenin pathway, affecting the occurrence and development of tumors.65 In addition, hsa_circ_0005615 (circ5615) is significantly upregulated in colorectal cancer (CRC) tissues,25 and exhibits oncogenic function as a ceRNA of miR-149-5p. In addition, CRC patients with high levels of circ5615 have shorter overall survival, and multivariable analysis confirmed that circ5615 could serve as an independent prognostic biomarker for CRC patients. Tankyrase (TNKS), a regulator of β-catenin stabilization, was identified as the potential miR-149-5p target by a series of experiments. Circ5615 can serve as a miR-149-5p sponge to upregulate TNKS levels, activate the Wnt/β-catenin pathway, and upregulate the expression of β-catenin and cyclin D1 to promote CRC.25
CCNBs
The Cyclin B-CDK1 complex formed by CCNB and CDK1 is the key to triggering and promoting mitosis.53 In normal cells, the CCNB1/CDK1 complex rapidly accumulates in the nucleus after activation in the cytoplasm, thereby inducing the cell to enter mitosis.28,53 CircRNAs can regulate cell cycle progression by altering the CCNB level in the nucleus. In HCC tissues, the low hsa_circ_0079929 expression influences cell proliferation by regulating the expression of nuclear CCNB1.28
CCNEs
CircRNAs can also alter the expression of CCNE via sponging miRNA. One study discovered that circDENND2A was abnormally expressed in NSCLC samples, which induced the expression of CCNE1 after miR-34a sponging and promoted the proliferation, migration, and invasion of tumor cells.27
CircRNA-Mediated Regulation of Cell Cycle via CDK/Cyclin Interactions
Cell cycle regulators are considered to be promising targets in cancer therapy since cell proliferation depends on the progression of the cell cycle phases regulated by several CDKs acting in complex with their CCN partners.67 CircRNAs also can regulate cell cycle progression by altering the expression of CCN/CDK complexes. At the end of G1 phase, the accumulation of CCNE activates CDK2 to form a CCNE/CDK2 complex, which can promote G1/S transition.66
In addition, the activation of the CDK2/CCNE1 complexes can induce pRB phosphorylation, thus releasing the transcription factor E2F1, which induces the expression of its downstream target genes (eg CCNA2, CCNB1), thereby promoting cell cycle progression.66,68 CircRNAs can enhance the formation of the CDK2/CCNE complex by interacting with single-stranded DNA binding proteins (SSBPs). In cervical cancer, the significantly upregulated hsa_circ_0072088 (circZFR) recruits and activates the CDK2/CCNE1 complex by interacting with SSBP1 to promote cervical cancer proliferation.68 In addition, an evaluation of circZFR by ROC analysis indicated that it has good predictive value for the diagnosis of cervical cancer.68 CCNA replaces CCNE as the partner of CDK2 during the cell cycle to control DNA replication in the S phase and is subsequently associated with CDK1 to promote entry into the M phase.15 CircRNAs can also directly form a complex with CDKs to hinder cell cycle progression. Interestingly, the high ectopic expression of circ-Foxo3 in non-cancer cells results in the formation of a ternary complex with CDK2 and p21.30 The formation of the circ-Foxo3/p21/CDK2 complex hijacks CDK2 and p21 together and blocks the formation of the CCNE/CDK2 and CCNA/CDK2 complexes, thereby inhibiting cell cycle progression.30 Important tumor suppressors involved in cell cycle regulation (eg pRB and p53), which are mainly regulated by post-translational modifications (eg phosphorylation, sumoylation, and acetylation), are functionally related and can form a complex regulatory network.69 The abnormal regulation of these tumor suppressors alters the expression of cell cycle regulators and leads to tumor occurrence and development.
CircRNA-Mediated Regulation of Cell Cycle via pRb-E2Fs
As a tumor suppressor, RB loses its function in various cancers; thus, RB has an inseparable relationship with tumor formation.70 As a product of a tumor suppressor gene found in hereditary retinoblastoma, pRB plays a central role in G1/S transition.69,70 The dynamic regulation of pRB phosphorylation by the CCN/CDK complex triggers the cell to exit the G0 phase, cross the G1 phase, and enter the S phase.68 In the early G1 phase, pRB is phosphorylated by the CCND/CDK4/6 complex to release the transcription factor E2F, which positively regulates the transcription of certain genes, such as CCNA and CCNE, that are necessary for cells to enter the S phase.17,68 CircRNAs directly influence the expression of RB via sponging its related miRNAs. For instance, circRNA_100782, which exhibits low expression in GC cells, regulates the proliferation and metastasis of GC cells by regulating RB expression via miR-574-3p sponging.71 The transcription factor E2F binds to the promoter regions of genes that are required for S phase entry, including DNA polymerase subunits, CCNA, and CCNE.17,68 Lin et al used quantitative real-time PCR to evaluate the expression of circCYFIP2 in GC tissues and adjacent normal tissues, and the results showed that its expression was significantly increased in GC tissues.72 Furthermore, high levels of circCYFIP2 were found to be positively correlated with International Union for International Cancer Control (UICC) stages, pathological T stages, lymphatic metastasis, distant metastasis, and grades.72 Lin et al identified the diagnostic and prognostic value of circCYFIP2 in GC by generating ROC curves and by Kaplan–Meier analysis. Subsequently, through a series of in vitro and in vivo experiments, it was found that circCYFIP2 increases E2F1 expression via miR-1205 sponging and accelerates tumor cell proliferation and invasion.72 These findings provide potential biomarkers and prognostic indicators to reflect the deadly disease status of and develop more therapeutic targets for GC. Likewise, in GC tissues and cell lines, the significantly upregulated circ_PGPEP1 elevates E2F3 expression via miR-1297 sponging and enhanced the proliferation of tumor cells.73 In addition, circ_0016760 is upregulated in NSCLC and acts as a sponge for miRNA-4295, consequently enhancing the expression of E2F3 and promoting cell proliferation.74 In malignant melanoma, circ_0119872 encourages cell proliferation by relieving the inhibitory effect of miR-582-3p on E2F3 via sponging.75 Another study reported that circAGAP1 (circ0058792) expression was significantly upregulated in clear cell renal cell carcinoma (ccRCC) tissues compared to that in adjacent non-tumor tissues. In particular, circAGAP1 played a carcinogenic role in ccRCC progression by regulating the miR-15-5p/E2F3 axis.76 CircFAM13B, which is highly expressed in HCC, serves as a sponge of miR-212 and enhances tumor progression by upregulating E2F5.77 Furthermore, circFOXM1, which is highly expressed in glioblastoma tumor tissues and cells, alleviates targeted inhibition of E2F5 by miR-577 by competing with miR-577 and upregulates its expression to promote tumor cell proliferation.78 Similarly, in CRC tissues, the upregulated circDUSP16 elevates the expression of E2F6 by competitively binding with miR-432-5p and promotes tumor progression.79 In CC tissues and cell lines, the increased expression of circ-ZNF609 significantly promotes the viability, migration, and invasion of tumor cells by acting as a ceRNA of miRNA-197-3p and reducing its inhibitory effect on E2F6.80 In lung cancer, the upregulated expression of circ-CCS can be used to predict poor prognosis. Circ-CCS indirectly increases the expression of E2F7 by sponging miR-383 and promotes cancer progression.81
CircRNA-Mediated Regulation of Cell Cycle via CKIs
CircRNA can regulate the cell cycle at multiple levels by targeting different molecules. Aside from RB family proteins, CKIs can also negatively regulate CDK activity by binding to either free CDKs or CDK/CCN complexes.15,17 There are two distinct families of CKIs: the INK4 family, composed of p16 (INK4a), p15 (INK4b), p18 (INK4c), and p19 (INK4d), which specifically inactivate CDK4 and CDK6, thereby preventing their interaction with CCND, and the Cip/Kip family, comprising p21 (Cip1), p27 (Kip1), and p57 (Kip2), which form heterotrimeric complexes with CCND-, CCNE-, and CCNA-dependent kinase complexes, thus inhibiting their activities.15 CKI dysregulation can cause a variety of diseases, including cancers.15,82 CircRNAs can also alter the levels of CKIs in tumors and interfere with cell cycle progression.
p21
P21, a potent universal CKI that belongs to the CIP/Kip family, possesses high binding affinity for CCN/CDK2, CCN/CDK1, and CCN/CDK4/6 complexes, which subsequently prevents the phosphorylation of pRB and blocks cell cycle progression.82–84 In addition, p21 directly interacts with the E2F complexes and inhibits E2F activity to induce cell cycle arrest.83 As a tumor suppressor, p21 exerts oncogenic effects and is usually downregulated in several cancer types, contributing to poor prognosis and decreased overall survival in patients.83 As a critical effector of various intra- and extracellular stress signals, p21 is highly regulated by multiple transcriptional factors, distinct post-transcriptional regulators (eg miRNAs, RNA-binding proteins), and different post-translational modifications.82 In addition, the p21 gene can be activated by p53.85,86 Notably, a previous study reported that circMTO1 was significantly downregulated in HCC.CircMTO1 could elevate the expression of p21 by sponging miR-9,thus reducing tumor cell proliferation.87 In addition, HCC patients with low levels of circMTO1 have shorter survival, suggesting that it could be used to evaluate the survival rate of HCC patients as a predictive prognostic indicator. In GC tissues and cell lines, the downregulated expression of the circGRAMD1B alters the expression of p21 by sponging miR-130a-3p and influences the proliferation, migration, and invasion of tumor cells.88 In addition, the circRHOBTB3 expression is downregulated in GC.CircRHOBTB3 can act as a tumor suppressor to inhibit the proliferation of tumor cells by acting as a sponge for miR-654-3p to elevate the expression of p21.89 Circ-ITCH, which is downregulated in BCa tissues and cell lines, regulates the expression levels of p21 by sponging miR-17 or miR-224 and influences the aggressive biological behaviors of BCa.90 Yang et al included and analyzed the clinicopathological parameters and survival data of BCa patients and found that the level of circ-ITCH expression was positively correlated with the histological grade and that BCa patients with lower cyclic ITCH expression have a shorter overall survival and worse prognosis.90 Similarly, the circCdr1as, which acts as a tumor suppressor in BCa, has significantly reduced expression in BCa.CircCdr1as can partly upregulate p21 expression by sponging miR-135a and exerts anti-cancer effects.91 In BrC, the circDDX17 reduces CDK1 and increases p21 expression by sponging miR-605, thereby inhibiting tumor cell proliferation.92 Circ_0021977, which acts as a tumor suppressor in CRC, has significantly reduced expression in tumor tissues, plasma, and cell lines. Circ_0021977 indirectly regulates p21/p53 by acting as a sponge of miR-10b-5p, thus inhibiting the proliferation, migration, and invasion of CRC cells.93 Previous reports have found that circFAM114A2 levels were decreased in urothelial carcinoma (UC) cell lines and tissues. Furthermore, circFAM114A2 inhibited the proliferation of UC cells in the G1 phase and heightened the sensitivity of UC to cisplatin chemotherapy. Mechanistically, circFAM114A2 directly sponges miR-222-3p and miR-146a-5p, altering the expression of their downstream target genes p27 and p21, respectively, and thus inhibiting the progression of UC and enhancing the sensitivity of cancer cells to cisplatin chemotherapy.22 In addition, this study also found that the expression level of circFAM114A2 is positively correlated with the survival rate of patients with UC. Therefore, circFAM1142 has great potential as a prognostic biomarker and therapeutic target for UC. CircRNAs can also regulate the expression of p21 by targeting the AKT/FOXO signaling axis. For example, FOXO is an important target in the PI3K/AKT pathway regulated by AKT phosphorylation.58 One of its functions is to activate p21 gene transcription, which plays a key role in G1 arrest.58,94 Jie et al revealed that circRNAs could directly affect acetylation at the regulatory region of target genes and “unlock” their expression, suggesting that noncoding RNAs play a significant role in the histone modification of gene promoters.95 Lysine acetyltransferase 7 (KAT7) is a member of the histone acetyltransferase family that regulates histone acetylation and participates in histone modification.96 Jie et al found that circMRPS35 expression is downregulated in GC.95 In addition, this study found that the area under the ROC curve of circMRPS35 was 0.6976 and its expression level was closely related to the survival time of patients. This study showed that circMRPS35 has important clinical significance for GC diagnosis and prognosis. A further investigation of the mechanism underling the effects of circMRPS35 in GC revealed that circMRPS35 increased the H4K5 acetylation associated with the promoter regions of FOXO1 and FOXO3a via recruitment of KAT7, which resulted in the upregulation of FOXO1 and FOXO3a.95 Notably, FOXO1 and FOXO3a can inhibit cell proliferation and invasion by upregulating the expression of their target genes p21 and p27.58
p27
The CKI p27 (or KIP1), one of the members of the Cip/Kip family, can block cell cycle progression via CDK inhibition, with CDK2 as the main target.97 In addition, p27 can inhibit the CCN-mediated phosphorylation of RB and prevent the separation of E2F from RB, subsequently inhibiting the transcription of genes required for G1/S transition.97 As the most important pathway involved in the p27 function, PI3K/AKT signaling promotes the G1/S transition by phosphorylating FOXO and downregulating the expression of cell cycle inhibitory genes.58,97 CircRNAs can regulate cell cycle progression by altering p27 expression via miRNA sponging. For instance, circRNA BCRC-3, which exhibits low expression in BCa tissues and cell lines, acts as a tumor suppressor to inhibit the proliferation of tumor cells through the miR-182-5p/p27 axis. Specifically, circRNA BCRC-3 directly interacts with miR-182-5p, weakening its inhibitory effect on p27 at the 3′-UTR and resulting in the significant upregulation of p27, preventing tumor cell proliferation.98 In GC tissues, the expression level of circYAP1 is significantly lower than that in adjacent normal tissues. Furthermore, circYAP1 acts as a tumor suppressor to inhibit the proliferation of GC cells by blocking the miR-367-5p/p27 axis.99
CircRNA-Mediated Regulation of Cell Cycle via p53
The tumor suppressor p53 encoded by the TP53 gene is also an important part of the cell cycle control mechanism as a transcription factor that specifically binds to DNA and activates the transcription of key genes that induce cell cycle arrest (eg CKI p21 for G1 arrest).16 Many human tumors carry inactivating missense mutations in TP53 that disrupt p53 activity, resulting in cell cycle dysregulation and promoting tumor proliferation.16,100 CircRNAs can directly affect the expression of p53 via sponging its associated miRNAs. For instance, Li et al discovered that circ_100395 was lowly expressed in ovarian cancer tissues and functioned as a miR-1228 sponge, which altered the p53 expression and influenced tumor progression.101 CircRNAs can also indirectly modulate p53 expression by changing the levels of p53 antagonists through miRNA sponging. One p53 inhibitor, MDM, creates an autoregulatory feedback loop with p53.102 In cells containing wild-type p53 and subjected to various stimuli, p53 normally transcribes the MDM2 gene to produce MDM2 protein, and in turn, the binding of MDM2 to p53 directly inhibits p53 function.102 Notably, MDM2 expression is abnormally upregulated in different cancer types.103 In BCa, circNUDT21 is overexpressed in tumor tissues and cell lines. Additionally, MDM2 is a potential downstream target of miR-16-1-3p, and circNUDT21 promotes BCa progression by sponging miR-16-1-3p and activating the miR-16-1-3p/MDM2/p53 axis.104 Similarly, Zhou et al found that circ_0080229 expression was upregulated in both glioma tissues and cell lines. Circ_0080229 relieved the inhibitory effect of miR-1827 on MDM2 via sponging and promoted tumorigenesis and metastasis.105 The MDM2 homolog MDM4 can also inhibit p53 function through multiple pathways.103 In CC, MDM4 expression is regulated by circ-0000263 via sponging miR-150-5p, which indirectly downregulates the expression of p53, and consequently promotes cell proliferation and migration and inhibits cell apoptosis.106
CircRNAs can also be regulated by p53, forming a regulation feedback loop between the two. For instance, MDM2 is both the direct transcription target and primary negative regulator of p53.103 Circ-MDM2 was confirmed to be a circRNA derived from MDM2 pre-mRNA, which could promote tumor growth. Interestingly, circ-MDM2 was resistant to RNase R digestion, and its transcription was induced in a p53-dependent manner. Simultaneously, although the specific mechanism underlying the regulation of p53 level remains to be determined, experiments have confirmed that circ-MDM2 can negatively regulate the expression of p53, thus forming a self-regulating negative feedback loop that facilitates tumor cell proliferation.107 The expression of another circRNA derived from PGAP3, circ-PGAP3, is significantly downregulated in CC tissues, and the low expression of circ-PGAP3 is closely related to a larger tumor size, advanced FIGO stage, and lymph node metastasis.32 In addition, the survival times of CC patients with low levels of circ-PGAP3 were verified by analyzing survival curves, and they were found to be shorter than those of patients with high levels of circ-PGAP3, indicating that circ-PGAP3 has the potential to be used to judge the prognosis of CC. Jun et al further investigated the biological function and mechanism of circ-PGAP3 in CC cells and found that its overexpression could significantly reduce tumor cell proliferation. Circ-PGAP3 attenuates the miR-769-5p-mediated inhibition of wild-type p53 via sponging and indirectly promotes the expression of p53. In addition, p53 promotes the transcription of circ-PGAP3 by combining with its promoter and subsequently forming a regulatory feedback loop that inhibits CC cell proliferation and invasion.32 Wang et al reported significantly downregulated circCNTNAP3 expression in ESCC tissues compared to paired normal tissues.33 CircCNTNAP3 also inhibited the proliferation and increased apoptosis of ESCC cells with wild-type p53 both in vitro and in vivo. On one hand, circCNTNAP3 promoted the expression of p53 by sponging miR-513a-5p. However, p53 could also regulate the expression of circCNTNAP3 by interacting with RNA-binding-motif protein 25 (RBM25), which is a direct transcriptional target of p53 that can bind to circCNTNAP3 and positively regulate its expression.33 Overall, circCNTNAP3 can inhibit the malignant progression of ESCC through the circCNTNAP3/TP53 positive feedback loop. The roles and specific targets of cancer-associated circular RNAs involved in the cell cycle are summarized in Table 2.
 |  |  |  |
Table 2 Roles and Specific Targets of Cancer-Associated Circular RNAs Involved in the Cell Cycle |
Conclusion
In recent years, cancer incidence and mortality have been increasing to critical levels.108 Therefore, it is particularly important to discover effective tumor markers and therapeutic targets. Disorders of the cell cycle are known to play an important role in tumor proliferation, metastasis, and prognosis.15 Several studies have confirmed that cell cycle progression can be blocked by targeting specific regulators, thereby preventing tumor growth and survival.70,109 For example, in the treatment of patients with HR+, HER2- breast cancer, the CDK4/6 inhibitor palbociclib can play an important therapeutic role in inhibiting tumor cell proliferation by inhibiting cell cycle progression.109 In recent years, the dysregulation of circRNA expression has opened a new chapter in the etiology of various human diseases, especially cancer.110 This review summarizes the mechanism of circRNAs involved in tumor cell cycle regulation and finds that their abnormal expression in tumors not only plays a role in tumor proliferation but also in tumor invasion and metastasis, TNM staging, histological grade, malignant phenotype, sensitivity to chemotherapy drugs and patient survival.3,5,8,9,25–33,111–114 These findings highlight potential biomarkers for tumor diagnosis and provide an effective reference for developing future tumor treatments involving the regulation of the cell cycle based on circRNA.
Abbreviations
circRNAs, Circular RNAs; miRNA, microRNA; CCN, cyclins; CDKs, cyclin-dependent kinases; CKI, cyclin-dependent kinase inhibitor; pRb, retinoblastoma protein; BrC, breast cancer; HCC, hepatocellular carcinoma; HCCT, hepatitis B virus (HBV)-related HCC tissues; CC, cervical cancer; GC, gastric cancer; BCa, bladder cancer; NSCLC, non-small cell lung cancer; TNBC, triple-negative breast cancer; CRC, colorectal cancer; ccRCC, clear cell renal cell carcinoma.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
This work was funded by the Science and Technology Department of Jilin Province, P. R. C. [grant number 20200201543JC] and the Development and Reform Commission of Jilin Province, P. R. C. [grant number 2016C053].
Disclosure
The authors declare that they have no competing interests.
References
1. Liu J, Li D, Luo H, Zhu X. Circular RNAs: the star molecules in cancer. Mol Aspects Med. 2019;70:141–152. doi:10.1016/j.mam.2019.10.006
2. Verduci L, Strano S, Yarden Y, Blandino G. The circRNA-microRNA code: emerging implications for cancer diagnosis and treatment. Mol Oncol. 2019;13(4):669–680. doi:10.1002/1878-0261.12468
3. Li WH, Song YC, Zhang H, et al. Decreased expression of Hsa_circ_00001649 in gastric cancer and its clinical significance. Dis Markers. 2017;2017:4587698. doi:10.1155/2017/4587698
4. Qin M, Liu G, Huo X, et al. Hsa_circ_0001649: a circular RNA and potential novel biomarker for hepatocellular carcinoma. Cancer Biomark. 2016;16(1):161–169. doi:10.3233/CBM-150552
5. Ji W, Qiu C, Wang M, Mao N, Wu S, Dai Y. Hsa_circ_0001649: a circular RNA and potential novel biomarker for colorectal cancer. Biochem Biophys Res Commun. 2018;497(1):122–126. doi:10.1016/j.bbrc.2018.02.036
6. Chen A, Zhong L, Ju K, Lu T, Lv J, Cao H. Plasmatic circRNA predicting the occurrence of human glioblastoma. Cancer Manag Res. 2020;12:2917–2923. doi:10.2147/CMAR.S248621
7. Shen Z, Wang L, Ye D. The expression profile and clinical application value of hsa_circ_0016148 in head and neck squamous cell carcinoma. J Clin Lab Anal. 2021;35(11):e23997. doi:10.1002/jcla.23997
8. Jiang Y, Wang T, Yan L, Qu L. A novel prognostic biomarker for pancreatic ductal adenocarcinoma: hsa_circ_0001649. Gene. 2018;675:88–93. doi:10.1016/j.gene.2018.06.099
9. Xing L, Zhang L, Feng Y, Cui Z, Ding L. Downregulation of circular RNA hsa_circ_0001649 indicates poor prognosis for retinoblastoma and regulates cell proliferation and apoptosis via AKT/mTOR signaling pathway. Biomed Pharmacother. 2018;105:326–333. doi:10.1016/j.biopha.2018.05.141
10. Wang Y, Sui X, Zhao H, et al. Decreased circular RNA hsa_circ_0001649 predicts unfavorable prognosis in glioma and exerts oncogenic properties in vitro and in vivo. Gene. 2018;676:117–122. doi:10.1016/j.gene.2018.07.037
11. Geng Y, Jiang J, Wu C. Function and clinical significance of circRNAs in solid tumors. J Hematol Oncol. 2018;11(1):98. doi:10.1186/s13045-018-0643-z
12. Meng S, Zhou H, Feng Z, et al. CircRNA: functions and properties of a novel potential biomarker for cancer. Mol Cancer. 2017;16(1):94. doi:10.1186/s12943-017-0663-2
13. Li J, Sun D, Pu W, Wang J, Peng Y. Circular RNAs in cancer: biogenesis, function, and clinical significance. Trends Cancer. 2020;6(4):319–336. doi:10.1016/j.trecan.2020.01.012
14. Xiao MS, Ai Y, Wilusz JE. Biogenesis and functions of circular RNAs come into focus. Trends Cell Biol. 2020;30(3):226–240. doi:10.1016/j.tcb.2019.12.004
15. Bai J, Li Y, Zhang G. Cell cycle regulation and anticancer drug discovery. Cancer Biol Med. 2017;14(4):348–362. doi:10.20892/j.issn.2095-3941.2017.0033
16. Wiman KG, Zhivotovsky B. Understanding cell cycle and cell death regulation provides novel weapons against human diseases. J Intern Med. 2017;281(5):483–495. doi:10.1111/joim.12609
17. Sun A, Bagella L, Tutton S, Romano G, Giordano A. From G0 to S phase: a view of the roles played by the retinoblastoma (Rb) family members in the Rb-E2F pathway. J Cell Biochem. 2007;102(6):1400–1404. doi:10.1002/jcb.21609
18. Aubrey BJ, Kelly GL, Janic A, Herold MJ, Strasser A. How does p53 induce apoptosis and how does this relate to p53-mediated tumour suppression? Cell Death Differ. 2018;25(1):104–113. doi:10.1038/cdd.2017.169
19. Xiao W, Li J, Hu JE, et al. Circular RNAs in cell cycle regulation: mechanisms to clinical significance. Cell Proliferat. 2021;54(12). doi:10.1111/cpr.13143
20. Liu ZH, Yang SZ, Li WY, Dong SY, Zhou SY, Xu S. CircRNA_141539 can serve as an oncogenic factor in esophageal squamous cell carcinoma by sponging miR-4469 and activating CDK3 gene. Aging-Us. 2021;13(8):12179–12193. doi:10.18632/aging.103071
21. Yue Q, Xu Y, Deng X, et al. Circ-PITX1 promotes the progression of non-small cell lung cancer through regulating the miR-1248/CCND2 axis. Onco Targets Ther. 2021;14:1807–1819. doi:10.2147/OTT.S286820
22. Lv J, Zhou Z, Wang J, et al. CircFAM114A2 promotes cisplatin sensitivity via miR-222-3p/P27 and miR-146a-5p/P21 cascades in urothelial carcinoma. Front Oncol. 2021;11:659166. doi:10.3389/fonc.2021.659166
23. Qu X, Zhu L, Song L, Liu S. circ_0084927 promotes cervical carcinogenesis by sponging miR-1179 that suppresses CDK2, a cell cycle-related gene. Cancer Cell Int. 2020;20:333. doi:10.1186/s12935-020-01417-2
24. Xu JH, Ni LW, Zhao FL, et al. Overexpression of hsa_circ_0002874 promotes resistance of non-small cell lung cancer to paclitaxel by modulating miR-1273f/MDM2/p53 pathway. Aging-Us. 2021;13(4):5986–6009. doi:10.18632/aging.202521
25. Ma Z, Han C, Xia W, et al. circ5615 functions as a ceRNA to promote colorectal cancer progression by upregulating TNKS. Cell Death Dis. 2020;11(5):356. doi:10.1038/s41419-020-2514-0
26. Bian L, Zhi X, Ma L, et al. Hsa_circRNA_103809 regulated the cell proliferation and migration in colorectal cancer via miR-532-3p/FOXO4 axis. Biochem Biophys Res Commun. 2018;505(2):346–352. doi:10.1016/j.bbrc.2018.09.073
27. Zhang Y, Shan C, Chen Y, et al. CircDENND2A promotes non-small cell lung cancer progression via regulating MiR-34a/CCNE1 signaling. Front Genet. 2020;11:987. doi:10.3389/fgene.2020.00987
28. Zheng H, Chen T, Li C, et al. A circular RNA hsa_circ_0079929 inhibits tumor growth in hepatocellular carcinoma. Cancer Manag Res. 2019;11:443–454. doi:10.2147/CMAR.S189338
29. Wang GJ, Yu TY, Li YR, Liu YJ, Deng BB. Circ_0000190 suppresses gastric cancer progression potentially via inhibiting miR-1252/PAK3 pathway. Cancer Cell Int. 2020;20:351. doi:10.1186/s12935-020-01422-5
30. Du WW, Yang W, Liu E, Yang Z, Dhaliwal P, Yang BB. Foxo3 circular RNA retards cell cycle progression via forming ternary complexes with p21 and CDK2. Nucleic Acids Res. 2016;44(6):2846–2858. doi:10.1093/nar/gkw027
31. Zang Y, Li J, Wan B, Tai Y. circRNA circ-CCND1 promotes the proliferation of laryngeal squamous cell carcinoma through elevating CCND1 expression via interacting with HuR and miR-646. J Cell Mol Med. 2020;24(4):2423–2433. doi:10.1111/jcmm.14925
32. Jun T, Chen W, Hailing C, Ning W, Qinxue C. The novel circular RNA circ-PGAP3 retards cervical cancer growth by regulating the miR-769-5p/p53 axis. Hum Cell. 2021;34(3):878–888. doi:10.1007/s13577-021-00493-4
33. Wang H, Song X, Wang Y, et al. CircCNTNAP3-TP53-positive feedback loop suppresses malignant progression of esophageal squamous cell carcinoma. Cell Death Dis. 2020;11(11):1010. doi:10.1038/s41419-020-03217-y
34. Gao SW, Liu F. Novel insights into cell cycle regulation of cell fate determination. J Zhejiang Univ Sci B. 2019;20(6):467–475. doi:10.1631/jzus.B1900197
35. Wang L, Yi J, Lu LY, et al. Estrogen-induced circRNA, circPGR, functions as a ceRNA to promote estrogen receptor-positive breast cancer cell growth by regulating cell cycle-related genes. Theranostics. 2021;11(4):1732–1752. doi:10.7150/thno.45302
36. Li Z, Yang HY, Dai XY, et al. CircMETTL3, upregulated in a m6A-dependent manner, promotes breast cancer progression. Int J Biol Sci. 2021;17(5):1178–1190. doi:10.7150/ijbs.57783
37. Huang Y, Ge W, Ding Y, et al. The circular RNA circSLC7A11 functions as a mir-330-3p sponge to accelerate hepatocellular carcinoma progression by regulating cyclin-dependent kinase 1 expression. Cancer Cell Int. 2021;21(1):636. doi:10.1186/s12935-021-02351-7
38. Zhu M, Liang Z, Pan J, et al. Hepatocellular carcinoma progression mediated by hepatitis B virus-encoded circRNA HBV_circ_1 through interaction with CDK1. Mol Ther Nucleic Acids. 2021;25:668–682. doi:10.1016/j.omtn.2021.08.011
39. Zheng Q, Zhang J, Zhang T, et al. Hsa_circ_0000520 overexpression increases CDK2 expression via miR-1296 to facilitate cervical cancer cell proliferation. J Transl Med. 2021;19(1):314. doi:10.1186/s12967-021-02953-9
40. Xie B, Zhao Z, Liu Q, Wang X, Ma Z, Li H. CircRNA has_circ_0078710 acts as the sponge of microRNA-31 involved in hepatocellular carcinoma progression. Gene. 2019;683:253–261. doi:10.1016/j.gene.2018.10.043
41. Lu JP, Zhang ZL, Huang DM, et al. Cdk3-promoted epithelial-mesenchymal transition through activating AP-1 is involved in colorectal cancer metastasis. Oncotarget. 2016;7(6):7012–7028. doi:10.18632/oncotarget.6875
42. Ren S, Rollins BJ. Cyclin C/cdk3 promotes Rb-dependent G0 exit. Cell. 2004;117(2):239–251. doi:10.1016/S0092-8674(04)00300-9
43. Wang L, Hu HY, Lin YL, et al. CDK3 expression and its clinical significance in human nasopharyngeal carcinoma. Mol Med Rep. 2014;9(6):2582–2586. doi:10.3892/mmr.2014.2095
44. Nebenfuehr S, Kollmann K, Sexl V. The role of CDK6 in cancer. Int J Cancer. 2020;147(11):2988–2995. doi:10.1002/ijc.33054
45. Qi B, Yao WJ, Zhao BS, et al. Involvement of microRNA-198 overexpression in the poor prognosis of esophageal cancer. Asian Pac J Cancer Prev. 2013;14(9):5073–5076. doi:10.7314/APJCP.2013.14.9.5073
46. Wang M, Wang J, Kong X, et al. MiR-198 represses tumor growth and metastasis in colorectal cancer by targeting fucosyl transferase 8. Sci Rep. 2014;4:6145. doi:10.1038/srep06145
47. Han HS, Yun J, Lim SN, et al. Downregulation of cell-free miR-198 as a diagnostic biomarker for lung adenocarcinoma-associated malignant pleural effusion. Int J Cancer. 2013;133(3):645–652. doi:10.1002/ijc.28054
48. Huang WT, Wang HL, Yang H, et al. Lower expressed miR-198 and its potential targets in hepatocellular carcinoma: a clinicopathological and in silico study. Onco Targets Ther. 2016;9:5163–5180. doi:10.2147/OTT.S108828
49. Li M, Chen H, Xia L, Huang P. Circular RNA circSP3 promotes hepatocellular carcinoma growth by sponging microRNA-198 and upregulating cyclin-dependent kinase 4. Aging. 2021;13(14):18586–18605. doi:10.18632/aging.203303
50. Gong Y, Mao J, Wu D, et al. Circ-ZEB1.33 promotes the proliferation of human HCC by sponging miR-200a-3p and upregulating CDK6. Cancer Cell Int. 2018;18:116. doi:10.1186/s12935-018-0602-3
51. Chen B, Ji F, Wen XN, Jin Z. Circular RNA circ_ASAP2 promotes cell viability, migration, and invasion of gastric cancer cells by regulating the miR-770-5p/CDK6 axis. Int J Clin Exp Patho. 2020;13(11):2806–2819.
52. Sun M, Zhao W, Chen Z, et al. Circ_0058063 regulates CDK6 to promote bladder cancer progression by sponging miR-145-5p. J Cell Physiol. 2019;234(4):4812–4824. doi:10.1002/jcp.27280
53. Martinez-Alonso D, Malumbres M. Mammalian cell cycle cyclins. Semin Cell Dev Biol. 2020;107:28–35. doi:10.1016/j.semcdb.2020.03.009
54. Zhu X, Wang X, Wei S, et al. hsa_circ_0013958: a circular RNA and potential novel biomarker for lung adenocarcinoma. FEBS J. 2017;284(14):2170–2182. doi:10.1111/febs.14132
55. Yang D, Yang B, Zhu Y, et al. Circular RNA-DPP4 serves an oncogenic role in prostate cancer progression through regulating miR-195/cyclin D1 axis. Cancer Cell Int. 2021;21(1):379. doi:10.1186/s12935-021-02062-z
56. Mo WL, Jiang JT, Zhang L, et al. Circular RNA hsa_circ_0000467 promotes the development of gastric cancer by competitively binding to MicroRNA miR-326-3p. Biomed Res Int. 2020;2020:4030826. doi:10.1155/2020/4030826
57. Zhang L, Sun D, Zhang J, Tian Y. Circ-UBR1 facilitates proliferation, metastasis, and inhibits apoptosis in breast cancer by regulating the miR-1299/CCND1 axis. Life Sci. 2021;266:118829. doi:10.1016/j.lfs.2020.118829
58. Zhang X, Tang N, Hadden TJ, Rishi AK. Akt, FoxO and regulation of apoptosis. Biochim Biophys Acta. 2011;1813(11):1978–1986. doi:10.1016/j.bbamcr.2011.03.010
59. Yan F, Liao R, Farhan M, et al. Elucidating the role of the FoxO3a transcription factor in the IGF-1-induced migration and invasion of uveal melanoma cancer cells. Biomed Pharmacother. 2016;84:1538–1550. doi:10.1016/j.biopha.2016.11.027
60. Yang X, Liu L, Zou H, Zheng YW, Wang KP. circZFR promotes cell proliferation and migration by regulating miR-511/AKT1 axis in hepatocellular carcinoma. Dig Liver Dis. 2019;51(10):1446–1455. doi:10.1016/j.dld.2019.04.012
61. Zhang Y, Wang X. Targeting the Wnt/beta-catenin signaling pathway in cancer. J Hematol Oncol. 2020;13(1):165. doi:10.1186/s13045-020-00990-3
62. MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17(1):9–26. doi:10.1016/j.devcel.2009.06.016
63. Zhang J, Xu HD, Xing XJ, Liang ZT, Xia ZH, Zhao Y. CircRNA_069718 promotes cell proliferation and invasion in triple-negative breast cancer by activating Wnt/beta-catenin pathway. Eur Rev Med Pharmacol Sci. 2019;23(12):5315–5322. doi:10.26355/eurrev_201906_18198
64. Yang SJ, Wang DD, Zhong SL, et al. Tumor-derived exosomal circPSMA1 facilitates the tumorigenesis, metastasis, and migration in triple-negative breast cancer (TNBC) through miR-637/Akt1/beta-catenin (cyclin D1) axis. Cell Death Dis. 2021;12(5):420. doi:10.1038/s41419-021-03680-1
65. Yang BM, Zhao JR, Huo TT, Zhang ML, Wu XH. Effects of CircRNA-ITCH on proliferation and apoptosis of hepatocellular carcinoma cells through inhibiting Wnt/beta-catenin signaling pathway. J Buon. 2020;25(3):1368–1374.
66. Dang F, Nie L, Wei W. Ubiquitin signaling in cell cycle control and tumorigenesis. Cell Death Differ. 2021;28(2):427–438. doi:10.1038/s41418-020-00648-0
67. Otto T, Sicinski P. Cell cycle proteins as promising targets in cancer therapy. Nat Rev Cancer. 2017;17(2):93–115. doi:10.1038/nrc.2016.138
68. Zhou M, Yang Z, Wang D, Chen P, Zhang Y. The circular RNA circZFR phosphorylates Rb promoting cervical cancer progression by regulating the SSBP1/CDK2/cyclin E1 complex. J Exp Clin Cancer Res. 2021;40(1):48. doi:10.1186/s13046-021-01849-2
69. Chen L, Liu S, Tao Y. Regulating tumor suppressor genes: post-translational modifications. Signal Transduct Target Ther. 2020;5(1):90. doi:10.1038/s41392-020-0196-9
70. Sherr CJ, McCormick F. The RB and p53 pathways in cancer. Cancer Cell. 2002;2(2):103–112. doi:10.1016/S1535-6108(02)00102-2
71. Xin D, Xin Z. CircRNA_100782 promotes proliferation and metastasis of gastric cancer by downregulating tumor suppressor gene Rb by adsorbing miR-574-3p in a sponge form. Eur Rev Med Pharmaco. 2020;24(17):8845–8854.
72. Lin J, Liao S, Li E, et al. circCYFIP2 acts as a sponge of miR-1205 and affects the expression of its target gene E2F1 to regulate gastric cancer metastasis. Mol Ther Nucleic Acids. 2020;21:121–132. doi:10.1016/j.omtn.2020.05.007
73. Wang Y, Liu X, Wang L, Zhang Z, Li Z, Li M. Circ_PGPEP1 serves as a sponge of miR-1297 to promote gastric cancer progression via regulating E2F3. Dig Dis Sci. 2021;66(12):4302–4313. doi:10.1007/s10620-020-06783-5
74. Yan X, Wang T, Wang J. Circ_0016760 acts as a sponge of MicroRNA-4295 to enhance E2F transcription factor 3 expression and facilitates cell proliferation and glycolysis in nonsmall cell lung cancer. Cancer Biother Radiopharm. 2020;37(2):147–158. doi:10.1089/cbr.2020.3621
75. Qu J, Yuan C, Jia Q, Sun M, Jiang M, Zuo F. CircularRNA_0119872 regulates the microRNA-582-3p/E2F transcription factor 3 pathway to promote the progression of malignant melanoma. Clinics. 2021;76:e3036. doi:10.6061/clinics/2021/e3036
76. Lv Q, Wang G, Zhang Y, et al. CircAGAP1 promotes tumor progression by sponging miR-15-5p in clear cell renal cell carcinoma. J Exp Clin Cancer Res. 2021;40(1):76. doi:10.1186/s13046-021-01864-3
77. Xie Y, Hang X, Xu W, et al. CircFAM13B promotes the proliferation of hepatocellular carcinoma by sponging miR-212, upregulating E2F5 expression and activating the P53 pathway. Cancer Cell Int. 2021;21(1):410. doi:10.1186/s12935-021-02120-6
78. Fan X, Liu M, Fei L, Huang Z, Yan Y. CircFOXM1 promotes the proliferation, migration, invasion, and glutaminolysis of glioblastoma by regulating the miR-577/E2F5 axis. Bosn J Basic Med Sci. 2021. doi:10.17305/bjbms.2021.6028
79. Wang G, Yang H. CircRNA DUSP16 knockdown suppresses colorectal cancer progression by regulating the miR-432-5p/E2F6 axis. Cancer Manag Res. 2021;13:6599–6609. doi:10.2147/CMAR.S323437
80. Gu Q, Hou W, Shi L, Liu H, Zhu Z, Ye W. Circular RNA ZNF609 functions as a competing endogenous RNA in regulating E2F transcription factor 6 through competitively binding to microRNA-197-3p to promote the progression of cervical cancer progression. Bioengineered. 2021;12(1):927–936. doi:10.1080/21655979.2021.1896116
81. Yuan Y, Zhou X, Kang Y, et al. Circ-CCS is identified as a cancer-promoting circRNA in lung cancer partly by regulating the miR-383/E2F7 axis. Life Sci. 2021;267:118955. doi:10.1016/j.lfs.2020.118955
82. Kreis NN, Louwen F, Yuan J. The multifaceted p21 (Cip1/Waf1/CDKN1A) in cell differentiation, migration and cancer therapy. Cancers. 2019;11(9):1220.
83. Al Bitar S, Gali-Muhtasib H. The role of the cyclin dependent kinase inhibitor p21(cip1/waf1) in targeting cancer: molecular mechanisms and novel therapeutics. Cancers. 2019;11(10):1475. doi:10.3390/cancers11101475
84. Karimian A, Ahmadi Y, Yousefi B. Multiple functions of p21 in cell cycle, apoptosis and transcriptional regulation after DNA damage. DNA Repair. 2016;42:63–71. doi:10.1016/j.dnarep.2016.04.008
85. Fischer M, Quaas M, Steiner L, Engeland K. The p53-p21-DREAM-CDE/CHR pathway regulates G2/M cell cycle genes. Nucleic Acids Res. 2016;44(1):164–174. doi:10.1093/nar/gkv927
86. Mansilla SF, de la Vega MB, Calzetta NL, Siri SO, Gottifredi V. CDK-independent and PCNA-dependent functions of p21 in DNA replication. Genes. 2020;11(6):593. doi:10.3390/genes11060593
87. Han D, Li J, Wang H, et al. Circular RNA circMTO1 acts as the sponge of microRNA-9 to suppress hepatocellular carcinoma progression. Hepatology. 2017;66(4):1151–1164. doi:10.1002/hep.29270
88. Dai XL, Guo X, Liu JJ, et al. Circular RNA circGRAMD1B inhibits gastric cancer progression by sponging miR-130a-3p and regulating PTEN and p21 expression. Aging-Us. 2019;11(21):9689–9708. doi:10.18632/aging.102414
89. Deng G, Mou T, He J, et al. Circular RNA circRHOBTB3 acts as a sponge for miR-654-3p inhibiting gastric cancer growth. J Exp Clin Cancer Res. 2020;39(1):1. doi:10.1186/s13046-019-1487-2
90. Yang C, Yuan W, Yang X, et al. Circular RNA circ-ITCH inhibits bladder cancer progression by sponging miR-17/miR-224 and regulating p21, PTEN expression. Mol Cancer. 2018;17(1):19. doi:10.1186/s12943-018-0771-7
91. Li P, Yang X, Yuan W, et al. CircRNA-Cdr1as exerts anti-oncogenic functions in bladder cancer by sponging MicroRNA-135a. Cell Physiol Biochem. 2018;46(4):1606–1616. doi:10.1159/000489208
92. Peng HH, Wen YG. CircDDX17 acts as a competing endogenous RNA for miR-605 in breast cancer progression. Eur Rev Med Pharmaco. 2020;24(12):6794–6801.
93. Lu C, Jiang W, Hui B, et al. The circ_0021977/miR-10b-5p/P21 and P53 regulatory axis suppresses proliferation, migration, and invasion in colorectal cancer. J Cell Physiol. 2020;235(3):2273–2285. doi:10.1002/jcp.29135
94. Farhan M, Wang H, Gaur U, Little PJ, Xu J, Zheng W. FOXO signaling pathways as therapeutic targets in cancer. Int J Biol Sci. 2017;13(7):815–827. doi:10.7150/ijbs.20052
95. Jie M, Wu Y, Gao M, et al. CircMRPS35 suppresses gastric cancer progression via recruiting KAT7 to govern histone modification. Mol Cancer. 2020;19(1):56. doi:10.1186/s12943-020-01160-2
96. Miotto B, Struhl K. HBO1 histone acetylase activity is essential for DNA replication licensing and inhibited by Geminin. Mol Cell. 2010;37(1):57–66. doi:10.1016/j.molcel.2009.12.012
97. Abbastabar M, Kheyrollah M, Azizian K, et al. Multiple functions of p27 in cell cycle, apoptosis, epigenetic modification and transcriptional regulation for the control of cell growth: a double-edged sword protein. DNA Repair. 2018;69:63–72. doi:10.1016/j.dnarep.2018.07.008
98. Xie F, Li Y, Wang M, et al. Circular RNA BCRC-3 suppresses bladder cancer proliferation through miR-182-5p/p27 axis. Mol Cancer. 2018;17(1):144. doi:10.1186/s12943-018-0892-z
99. Liu H, Liu Y, Bian Z, et al. Circular RNA YAP1 inhibits the proliferation and invasion of gastric cancer cells by regulating the miR-367-5p/p27 (Kip1) axis. Mol Cancer. 2018;17(1):151. doi:10.1186/s12943-018-0902-1
100. Soussi T, Wiman KG. TP53: an oncogene in disguise. Cell Death Differ. 2015;22(8):1239–1249. doi:10.1038/cdd.2015.53
101. Li X, Lin S, Mo Z, et al. CircRNA_100395 inhibits cell proliferation and metastasis in ovarian cancer via regulating miR-1228/p53/epithelial-mesenchymal transition (EMT) axis. J Cancer. 2020;11(3):599–609. doi:10.7150/jca.35041
102. Wang S, Zhao Y, Aguilar A, Bernard D, Yang CY. Targeting the MDM2-p53 protein-protein interaction for new cancer therapy: progress and challenges. Cold Spring Harb Perspect Med. 2017;7(5):a026245. doi:10.1101/cshperspect.a026245
103. Mendoza M, Mandani G, Momand J. The MDM2 gene family. Biomol Concepts. 2014;5(1):9–19. doi:10.1515/bmc-2013-0027
104. Chen L, Li W, Li Z, et al. circNUDT21 promotes bladder cancer progression by modulating the miR-16-1-3p/MDM2/p53 axis. Mol Ther Nucleic Acids. 2021;26:625–636. doi:10.1016/j.omtn.2021.08.032
105. Zhou Z, Zheng X, Mei X, et al. Hsa_circ_0080229 upregulates the expression of murine double minute-2 (MDM2) and promotes glioma tumorigenesis and invasion via the miR-1827 sponging mechanism. Ann Transl Med. 2021;9(9):762. doi:10.21037/atm-20-7123
106. Cai H, Zhang P, Xu M, Yan L, Liu N, Wu X. Circular RNA hsa_circ_0000263 participates in cervical cancer development by regulating target gene of miR-150-5p. J Cell Physiol. 2019;234(7):11391–11400. doi:10.1002/jcp.27796
107. Chaudhary R, Muys BR, Grammatikakis I, et al. A circular RNA from the MDM2 locus controls cell cycle progression by suppressing p53 levels. Mol Cell Biol. 2020;40(9). doi:10.1128/MCB.00473-19
108. Li YF, Zhang J, Yu L. Circular RNAs regulate cancer onset and progression via wnt/beta-catenin signaling pathway. Yonsei Med J. 2019;60(12):1117–1128. doi:10.3349/ymj.2019.60.12.1117
109. Braal CL, Jongbloed EM, Wilting SM, Mathijssen RHJ, Koolen SLW, Jager A. Inhibiting CDK4/6 in breast cancer with palbociclib, ribociclib, and abemaciclib: similarities and differences. Drugs. 2021;81(3):317–331. doi:10.1007/s40265-020-01461-2
110. Zhang C, Hu J, Yu Y. CircRNA is a rising star in researches of ocular diseases. Front Cell Dev Biol. 2020;8:850. doi:10.3389/fcell.2020.00850
111. Zhang Y, Tan X, Lu Y. Exosomal transfer of circ_0006174 contributes to the chemoresistance of doxorubicin in colorectal cancer by depending on the miR-1205/CCND2 axis. J Physiol Biochem. 2022;78(1):39–50. doi:10.1007/s13105-021-00831-y
112. Lin D, Lin X, He T, Xie G. Gambogic acid inhibits the progression of gastric cancer via circRNA_ASAP2/miR-33a-5p/CDK7 axis. Cancer Manag Res. 2020;12:9221–9233. doi:10.2147/CMAR.S269768
113. Sava GP, Fan H, Coombes RC, Buluwela L, Ali S. CDK7 inhibitors as anticancer drugs. Cancer Metastasis Rev. 2020;39(3):805–823. doi:10.1007/s10555-020-09885-8
114. Wang Q, Li M, Zhang X, et al. Upregulation of CDK7 in gastric cancer cell promotes tumor cell proliferation and predicts poor prognosis. Exp Mol Pathol. 2016;100(3):514–521. doi:10.1016/j.yexmp.2016.05.001
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.