Back to Journals » Journal of Inflammation Research » Volume 16
circHECTD1 Promotes the Proliferation and Migration of Human Brain Vascular Smooth Muscle Cells via Interacting with KHDRBS3 to Stabilize EZH2 mRNA Expression
Authors Feng M, Tu W, Zhou Q, Du Y, Xu K, Wang Y
Received 28 November 2022
Accepted for publication 15 March 2023
Published 24 March 2023 Volume 2023:16 Pages 1311—1323
DOI https://doi.org/10.2147/JIR.S398199
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Meina Feng,* Wenxian Tu,* Qin Zhou, Yuanmin Du, Kang Xu, Yunfeng Wang
Department of Neurology, Wuhan Brain Hospital, General Hospital of the YANGTZE River Shipping, Wuhan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yunfeng Wang, Email [email protected]
Purpose: The objective of this paper is to explore the role of circHECTD1 in vascular smooth muscle cells (VSMCs) and atherosclerosis (AS).
Methods: VSMCs were treated with platelet-derived growth factor-BB (PDGF-BB) in vitro, and the level of circHECTD1 was determined using qRT-PCR. Cell proliferation, migration, and invasion were analyzed using CCK8 and transwell assays. Cell apoptosis and cell cycle were analyzed using flow cytometry. The binding interaction between circHECTD1 and KHDRBS3 or EZH2 was investigated using the RIP, RNA pull-down.
Results: CircHECTD1 was upregulated in PDGF-BB-induced VSMCs with a dose-dependent and time-dependent manner. Knockdown of circHECTD1 suppressed VSMCsproliferation and migration and enhanced cell apoptosis in VSMCs, while circHECTD1 overexpression yielded opposite effects. Mechanistically, circHECTD1 could interact with KHDRBS3, thus enhanced the stability of EZH2 mRNA and increased EZH2 protein level. In addition, silencing EZH2 in VSMCs reversed the proliferation-enhancing effect of circHECTD1 overexpression.
Conclusion: Our findings provided providing a potential prognostic and therapy biomarker for AS.
Keywords: circHECTD1, KHDRBS3, EZH2, VSMCs, atherosclerosis
Introduction
Atherosclerosis (AS) is considered to be a major underlying cause of cardiovascular disease resulting in high rate of mortality globally.1 Atherosclerotic lesion formation is a complex process characterized by a formation of atherosclerotic plaques. Vascular smooth muscle cells (VSMCs) are the main cells in the arterial middle layer and play an important role in maintaining the complete structure of the vascular wall.2 However, proliferative phenotype of VSMCs is a critical contributor to the AS, in which defective VSMC autophagy accelerated the progression of stress-induced premature senescence as well as AS.3–5 Hence, understanding the regulatory mechanisms governing SMC gene expression may provide new sights in AS pathophysiology and therapeutic management.6
Circular RNAs (circRNAs) are a new type of endogenous RNAs that exist in closed loop RNA forms and lack 5′ cap and 3′ polyadenylation tail in contrast to linear RNAs.7,8 CircRNAs can tolerate digestion by RNA enzymes due to their lack of 5′ and 3′ ends.9–11 Accumulating evidence shows that CircRNAs play essential roles in regulating cellular processes and contribute to numerous diseases progression.12–14 Recent studies have also shown that circRNA also plays an important role in the cardiovascular system.15,16 For example, circCHFR participates in atherosclerosis by promoting the proliferative phenotype of VSMCs via the miR-370/FOXO1/Cyclin D1 axis.17 Silencing circHIPK3 inhibits cardiomyocyte proliferation and angiogenesis.18 CircSirt1 was found to sponge miR-132/212 that disturbed SIRT1 mRNA thereby preventing the inflammatory phenotype in VSMCs.19 Therefore, it is necessary to explore the exact mechanism of circRNA in AS formation.
Studies on the function of circRNAs remain challenging. Previous studies have shown that circular RNAs (circRNAs) can competitively sponge miRNAs and diminished their repression of target mRNAs.20,21 Recently, more and more studies indicate that circRNAs can “sponge” other factors, such as RBPs and regulate their functions. For instance, Li et al found that downregulated circNDUFB2 inhibits NSCLC progression via destabilizing IGF2BPs.22 Garikipati et al reported that overexpression of circFNDC3B in post myocardial infarction hearts reduces cardiomyocyte apoptosis via FUS/VEGF-A axis.23 Meanwhile, the tumor-promoting effect of circHECTD1 has been demonstrated in a variety of tumors.24–26 However, the function and mechanism of circHECTD1 in AS need to be further explored.
In this study, we found that circHECTD1 could promote the proliferation of human brain VSMCs (HBVSMCs) and mouse VSMCs (mVSMCs). Moreover, circHECTD1 was proved to interact with KHDRBS3, thus stabilized EZH2 (Enhancer of zeste homolog 2) mRNA. The influence of circHECTD1 on the proliferation and migration of VSMCs was abrogated by silencing EZH2. This study discovered that the circHECTD1/KHDRBS3/EZH2 axis played a key role in the progressive of VSMCs and could be a potential therapeutic target for cerebro-cardiovascular AS.
Methods
Cell Culture
HBVSMCs were purchased from ATCC, (Manassas, VA, USA) and were cultured in DMEM; (Gibco, Grand Island, NY, USA) with 10% fetal bovine serum (FBS, Gibco, USA), 1% penicillin and 1% streptomycin (Gibco) supplement. HBVSMCs were cultured in an incubator of 5% CO2 at 37 °C.
The isolation and culture of primary mVSMCs were performed according to previous protocol.27,28 Briefly, mVSMCs were extracted from the aorta of 6 weeks old C57BL/6J and plated in DMEM supplemented with 20% FBS, 1% penicillin and 1% streptomycin at 37℃ with 5% CO2. Cells passed through 3–5 generations were used for follow-up experiments.
Cell Transfection
CircHECTD1 overexpression vector was constructed by GenePharma (Shanghai, China), and the pLC5-ciR vector was regarded as a negative control (vector). To knockdown circHECTD1 or EZH2, short hairpin RNAs targeting the splicing junction sites of circHECTD1 (sh-circHECTD1-1 and sh-circHECTD1-2) or EZH2 (sh-EZH2-1 and sh-EZH2-2) were designed and introduced into the pLKO.1 plasmid. The VSMCs were plated in six-well plates with a concentration of 3×104 cells per well. Lipofectamine 3000 (Invitrogen, USA) was utilized for the plasmids transfection. Briefly, plasmids were first mixed with Opti-MEM (Invitrogen, Carlsbad, CA, USA), incubated for 5 min, then co-incubated with Lipofectamine 3000 for 20 min and then transfected into cells for 72h, cells were harvested for the later study.
Cell Counting Kit‑8 (CCK‑8) Assay
Cells viability was assessed using a CCK-8 colorimetric assay (Dojindo, Japan). Briefly, the cells were plated in a 96-well plate (6000/well) and the tr ansfection was performed. After 72 h, 10μL CCK-8 solution was added into each well with cell culture medium following 24, 48, 72, and 96 h, and then Placed the plate in the dark at 37°C incubator for 1h. After the cells were completely dissolved, the relative cell viability was measured by detecting the absorbance at 570 nm using an ELISA reader (Thermo Fisher Scientific).
Colony Formation Assay
Different groups of transfected VSMCs were seeded into the 6-well plates with a density of 5×102 cells each well. After 2 weeks incubation, these plates were fixed by 10% formaldehyde and stained with 0.5% crystal violet solution. The colonies were defined as > 50 cells/colony and photographed using an inverted microscope (Olympus IX71, Japan).
Quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR)
Trizol (Invitrogen) was utilized to isolate total RNA. Reverse transcriptions were performed by PrimeScriptTM RT reagent Kit (Takara, Dalian, China) and the first strand cDNA synthesis kit for miRNA (Sangon Biotech, Shanghai, China) based on provided directions. SYBR®Premix Ex TaqTM (TaKaRa) was used for RT-qPCR on Applied Biosystems StepOne-Plus Real-time PCR System based on provided directions. β-actin was served as a control for cellular RNA and mRNA. The level of miRNA was normalized against the endogenous reference U6 and was quantified by the 2−ΔΔCt Method. The sequences of primers were positioned in Table S1.
RNA Fluorescence in situ Hybridization (FISH)
RNA-FISH was performed using the circRNA FISH Kit (RiboBio, Guangzhou, China) according to the manufacturer’s instructions. Biotin-labeled probes targeting circHECTD1 were synthesized by RiboBio Technology Co. The Nuclei were counterstained with 4′,6-diamidino-2- phenylindole (DAPI). The images were analyzed via Nikon A1Si Laser Scanning Confocal Microscope (Nikon Instruments Inc., Japan).
Transwell Assay
Control and specific plasmid transfected VSMCs (5 × 104) were harvested from monolayer culture, resuspended in serum-free medium with 0.1% bovine serum albumin (Sigma-Aldrich), and then plated onto a Transwell Boyden chamber (NeuroProbe, Gaithersburg, MD, USA), with or without a Matrigel coating on the upper chamber, for invasion and migration assays respectively. The chambers were incubated in DMEM for 24h with the addition of 10% FBS to the lower chamber. Cells that did not move were removed using cotton swabs, and the chambers were stained with crystal violet. Photomicrographs of three random regions were captured from duplicated assay chambers. The cell numbers were counted and normalized to the control. Each experiment was conducted in triplicate and repeated three times.
Flow Cytometry
Harvested cells were fixed in 75% ethanol for 8h at 4 °C. Then, the cells were washed twice with cold PBS buffer, resuspended in propidium iodide (PI; 50 μg/mL)/RNaseA(50 μg/mL)-mixed staining solution or/and annexin V-fluorescein isothiocyanate for 30min. Finally, the results were analyzed by FlowJo software (TreeStar, OR, USA).
RNA Immunoprecipitation (RIP) Assay
An RNA-Binding Protein Immunoprecipitation Kit (Magna RIP™, Millipore) was used for RIP assay based on provided directions. Briefly, a lysis cocktail containing protease and RNase inhibitors was used to lyse cells, after which lysates were mixed overnight with anti-Antibody (anti Flag, 1:30, ab205606) or anti-IgG that had been mixed ahead of time with magnetic beads (Magna RIP™, Millipore). The magnetic beads were subsequently immobilized, and antibody-protein complex was collected, with RNA then being purified from the RNA-protein complex prior to RT-qPCR analysis.
RNA Pull-Down
With Biotin RNA Labeling Mix kit (Roche) and T7 RNA polymerase, the biotin-labeled RNA probe for targeting junction sites of circHECTD1 was in vitro transcribed. RNA pull-down assay was performed with the PierceTM RNA 3′ end Desthiobiotinylation Kits (Thermo Scientific, USA) and PierceTM magnetic RNA-protein pull-down kits (Thermo Scientific, USA) in accordance with the manufacturer’s instructions.
Western Blot
Cell extract was thermotropy and separated via 10% SDS-PAGE, transferred to PVDF membranes (Millipore), and probed with the following primary antibodies, including anti-MCM2 antibody (1:3000, 10513-1-AP, proteintech), anti-PCNA antibody (1: 5000, 10205-2-AP, proteintech), anti-KHDRBS3 antibody (1: 2500, ab68515, abcam), anti-EZH2 antibody (1:1000, #5246, CST) and anti-GAPDH antibody (1: 50,000, 60004-1-Ig, proteintech), mouse and rabbit secondary antibodies were brought from Cell Signaling Technology, USA. ECL (Pierce, USA) was implemented to visualize the band signals, which were collected by the ChemiDoc XRS molecular imager system (Bio-Rad, USA).
Statistical Analysis
All the data were represented as mean value ± standard deviation (SD). Unless otherwise noted, all experiments were performed at least three times. Non-paired t-test was used to estimate the statistical differences between two groups. One-way analysis of variance (ANOVA) was used to determine the differences between three or more groups. The data meet the assumptions of the tests. An estimate of variation was performed within each group of data. The variance is similar between the groups. All the experiments were repeated at least three times. *P<0.05, **P<0.01 and ***P<0.001. NS means the difference is not significant.
Results
circHECTD1 Was Elevated in PDGF-BB-Induced VSMCs
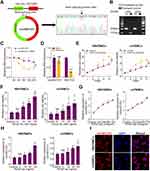
According to circRNA database (http://circbase.org/), hsa_circ_0031485, named as circHECTD1, is 344nt from exon 23 and exon 24 processing body assembly factor and validated by sanger sequence (Figure 1A). The circular structure of circHECTD1 was also confirmed by divergent primers (Figure 1B). After treatment with actinomycin D in HBVSMCs, RT-qPCR results showed that the half-life of circHECTD1 was significantly increased compared with that of linear transcripts (Figure 1C). Moreover, the tolerance of circHECTD1 to RNA R exonuclease further confirmed the circRNA ring structure (Figure 1D). The proliferation of both HBVSMCs and mVSMCs was significantly enhanced after treatment with PDGF-bb (20 ng/mL) in a time- and dose-dependent manner (Figure 1E and F). Expectedly, the expression of circHECTD1 could be promoted by PDGF-bb in a dose- and time-dependent manner in HBVSMCs and mVSMCs (Figure 1G and H). In addition, RNA-FISH experiment suggested mainly endogenous cytoplasm enrichment of circHECTD1 in HBVSMCs and mVSMCs (Figure 1I). Together, these results demonstrate that the upregulation of circHECTD1 is correlated with the proliferation of VSMCs.
circHECTD1 Facilitates Proliferation and Migration Phenotype of VSMCs
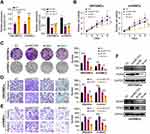
Next, we evaluated the effect of circHECTD1 on HBVSMCs and mVSMCs using gain- and loss-of-function assays, which yield a satisfied expression efficiency (Figure 2A). As expected, the proliferation, invasion, and migration abilities of VSMCs were significantly enhanced after circHECTD1 overexpression. Comparatively, circHECTD1 knockdown produced the opposite effect in HBVSMCs and mVSMCs (Figure 2B–E). Moreover, we found that circHECTD1 overexpression increased the expression levels of MCM2 and PCNA in HBVSMCs and mVSMCs, which was obviously reversed by circHECTD1 knockdown (Figure 2F). On the other hand, high circHECTD1 expression significantly enhanced VSMCs proliferation, migration and suppressed apoptosis, whereas circHECTD1 knockdown had a reverse effect (Figure 3A and B). Additionally, these data imply that circHECTD1 had a facilitating effect on cell proliferation and growth of HBVSMCs and mVSMCs.
circHECTD1 Interacts with RNA‑binding Protein KHDRBS3
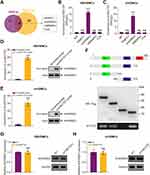
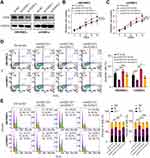
Growing evidence shows that circRNA can bind to RBPs and act as a scaffold for the binding of RBPs to other molecules.29 Firstly, we predicted the possible RBPs that interacted with circHECTD1 by intersecting targets from RBPdb and RBPmap databases. As a result, four underlying circHECTD1-interacting molecules were identified (Figure 4A). RNA immunoprecipitation (RIP) assay in HBVSMCs and mVSMCs suggested that circHECTD1 could be only pulled down by KHDRBS3 compared with IgG or other RBPs (Figure 4B and C). On the contrary, RNA pull-down assay indicated that circHECTD1 and KHDRBS3 was enriched by circHECTD1 specific probe compared with the scramble control (Figure 4D and E). KHDRBS3 has previously been shown to play a role as an RBP in ovarian cancer paclitaxel resistance,30 which is similar to the role played in present study. Additionally, in vitro binding tests indicated that the D2 domain [180–310 amino acids] of KHDRBS3 could specifically bind with circHECTD1 (Figure 4F). However, overexpression of circHECTD1 showed no apparently influence on protein or mRNA level of KHDRBS3 in HBVSMCs and mVSMCs (Figure 4G and H). Collectively, these results suggested that circHECTD1 could interact with KHDRBS3 in VSMCs.
circHECTD1/KHDRBS3 Interaction Stabilizes EZH2 mRNA
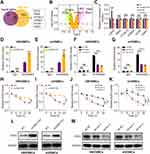
As KHDRBS3 is essential for mRNA stability,31 We inferred that circHECTD1 affects the regulation of KHDRBS3 on the stability of targeted mRNAs. Thus, RNA-sequencing of HBVSMCs transfected with circHECTD1 or empty vector was conducted to search for differentially expressed mRNAs/genes (DEMs/DEGs). As a result, a total of 6 mRNAs were identified as candidate targets of KHDRBS3 by overlapping DEGs with predicted targets of KHDRBS3 from ENCORI database and a volcano plot was generated (Figure 5A and B). qRT-PCR results showed that only EZH2 mRNAs expression levels were regulated by both overexpression of circHECTD1 and knockdown of circHECTD1 in HBVSMCs (Figure 5C). RIP assay showed that the mRNA of EZH2 could be enriched by KHDRBS3 in HBVSMCs and mVSMCs, and this could be enhanced by circHECTD1 overexpression (Figure 5D and E). Moreover, knockdown of circHECTD1 decreased the levels of EZH2 mRNA that enriched by KHDRBS3 in HBVSMCs and mVSMCs (Figure 5F and G). Besides, the half-life of EZH2 mRNA was significantly improved or suppressed by overexpression or knockdown or circHECTD1 (Figure 5H–K). In addition, the protein levels of EZH2 were also promoted by overexpression of circHECTD1 in HBVSMCs and mVSMCs, and circHECTD1 knockdown reduced EZH2 protein levels in HBVSMCs and mVSMCs (Figure 5L and M). Together, these results suggest that CircHECTD1/KHDRBS3 interaction increased the stability of EZH2 mRNA in VSMCs.
circHECTD1 Facilitates VSMCs Proliferation by Regulating EZH2 Expression
Previous studies have shown that EZH2 as a transcription factor promotes the progression of atherosclerosis.32 To explore if circHECTD1 exerted a promoting role in VSMCs through EZH2, we transfected sh-EZH2 alone or together with circHECTD1-overexpressed vector into VSMCs. Firstly, the EZH2 protein level was effectively decreased in HBVSMCs and mVSMCs through transfection with sh-EZH2 (Figure 6A). Next, cell vitality assay revealed that circHECTD1 overexpression enhanced the proliferation abilities of VSMCs, but this was completely abolished by EZH2 knockdown (Figure 6B and C). In flow cytometry, circHECTD1 overexpression promoted cell cycle and reduced the percentage of apoptotic cells in HBVSMCs and mVSMCs, however this was abrogated by EZH2 knockdown (Figure 6D and E). Therefore, these result demonstrated that circHECTD1 promotes the proliferation and migration of VSMCs through regulating the expression of KHDRBS3/EZH2 axis.
Discussion
VSMCs are the main cells in the intima of atherosclerosis and play an important role in all stages of atherosclerosis formation.33,34 In order to better understand the etiology of AS well, it is necessary to explore the mechanism VSMCs in AS. In this study, we found that circHECTD1 expression was significantly upregulated in both HBVSMCs and mVSMCs after treating with PDGF-bb compared to the control group. Moreover, circHECTD1 overexpression enhanced the proliferative phenotype of the VSMCs, while this enhancement was reversed by circHECTD1 knockdown. CircHECTD1 was verified to bind with KHDRBS3 and increase the EZH2 mRNA stability. Importantly, the influence of circHECTD1 on the proliferation and migration of VSMCs was abrogated by silencing EZH2. Therefore, the circHECTD1/KHDRBS3/EZH2 axis could be considered a novel treatment pathway for AS.
CircRNAs were covalently closed RNA molecules with non-coding ability, many of which play crucial regulatory roles in the occurrence and proliferation and migration of various disease through diverse mechanisms of action. Currently, with the development of high-throughput techniques, more and more circRNAs have been identified and revealed to play an important role in the pathogenesis of various diseases. Growing studies have shown that circRNAs are involved in VSMC dysfunction and atherogenesis. For example, Ding et al found that has_circ_0010283 regulated theproliferation and migration of ox-LDL-induced VSMCs via miR-370/HMGB1 axis.35 Dong et al showed that CTRP6 inhibits VSMCsproliferation and migration through the suppression of PI3K/Akt/mTOR pathway.36 In present study, we found that circHECTD1 was upregulated in PDGF-BB-induced VSMCs with a dose-dependent and time-dependent manner and circHECTD1 could promote the proliferative phenotype of VSMCs.
CircRNAs can act as a sponge by scavenging miRNAs or as a scaffold that binds RBPs to regulate target gene expression.29,37 Previous studies have indicated that circRNAs could interact with RBPs and plays crucial roles in human diseases and biological processes.38 KHDRBS3 was initially discovered as a new RBPs that can bind to several molecules that play an important role in the proliferation and migration of tumors.31,39 Moreover, we proved that circHECTD1 could interact with KHDRBS3, and enhancing the half-life of EZH2 mRNA. Moreover, we found that knockdown of EZH2 reversed the effects of circHECTD1 on the proliferative phenotype of VSMCs and promotes its contractile phenotype. Moreover, there are many studies indicating that non-coding RNAs (ncRNAs) modulating EZH2 signaling exert vital functions in the pathological progressions of many diseases.40 In colorectal cancer, hsa_circ_0071589 was able to promote tumor growth, invasion and migration through enhance the expression of EZH2. In laryngeal squamous cell cancer (LSCC), hsa_circ_0006232 promotes progression of LSCC cells through FUS-mediated EZH2 stabilization.32 In AS, circARHGAP12 promote the proliferation and migration of VSMC by regulating EZH2 signal axis.32
In addition, various studies indicated that VSMCs are critical to other vascular pathologies. For instance, Smith et al revealed the ending restenosis: suppression of VSMC proliferation via cAMP and they suggested that elevated VSMC proliferation promoted restenosis after angioplasty, atherogenesis and vein graft intimal thickening.41 We hypothesized that inhibitor of circHECTD1 may serve as a drug therapeutic target for restenosis after angioplasty, atherogenesis and vein graft intimal thickening. Guan et al indicated that nobiletin suppressed PDGF-BB-induced VSMC proliferation, migration and inhibited neointimal hyperplasia by a rat carotid artery injury model.42 Furthermore, Petsophonsakul et al reported the role of VSMC phenotypic switching in aortic aneurysm formation.43 We assumed that inhibitor of circHECTD1 may inhibit aortic aneurysm formation. The above hypothesis need to be tested in mice model or in clinic. Taken together, our study shows that circHECTD1 could promote VSMCs proliferation and reduce VSMCs apoptosis. Further research found that circHECTD1 could bind with KHDRBS3, thus enhanced the stability of EZH2 mRNA and protein level. Therefore, circHECTD1 potentially influences vascular disease. Our investigations revealed that circHECTD1 plays a meaningful role in VSMC response to injury, and may thus be a potential therapeutic target in various vascular diseases.
Abbreviations
VSMCs, vascular smooth muscle cells; AS, atherosclerosis; PDGF-BB, platelet-derived growth factor-BB; circRNAs, Circular RNAs; HBVSMCs, human brain VSMCs; qRT-PCR, Quantitative reverse transcription-polymerase chain reaction; FISH, fluorescence in situ hybridization; RIP, RNA immunoprecipitation; RBPs, RNA binding proteins.
Data Sharing Statement
The datasets presented during the current study are available from the corresponding author on reasonable request.
Acknowledgments
The authors sincerely appreciate all lab members. Meina Feng and Wenxian Tu are co-first authors for this study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Herrington W, Lacey B, Sherliker P, et al. Epidemiology of atherosclerosis and the potential to reduce the global burden of atherothrombotic disease. Circ Res. 2016;118(4):535–546. doi:10.1161/CIRCRESAHA.115.307611
2. Grootaert MOJ, Bennett MR. Vascular smooth muscle cells in atherosclerosis: time for a re-assessment. Cardiovasc Res. 2021;117(11):2326–2339. doi:10.1093/cvr/cvab046
3. Grootaert MOJ, Moulis M, Roth L, et al. Vascular smooth muscle cell death, autophagy and senescence in atherosclerosis. Cardiovasc Res. 2018;114(4):622–634. doi:10.1093/cvr/cvy007
4. Wu M-Y, Li C-J, Hou M-F, et al. New insights into the role of inflammation in the pathogenesis of atherosclerosis. Int J Mol Sci. 2017;18(10):2034. doi:10.3390/ijms18102034
5. Wang Z, Liu B, Zhu J, et al. Nicotine-mediated autophagy of vascular smooth muscle cell accelerates atherosclerosis via nAChRs/ROS/NF-kappaB signaling pathway. Atherosclerosis. 2019;284:1–10. doi:10.1016/j.atherosclerosis.2019.02.008
6. Rader DJ, Daugherty A. Translating molecular discoveries into new therapies for atherosclerosis. Nature. 2008;451(7181):904–913. doi:10.1038/nature06796
7. Kristensen LS, Andersen MS, Stagsted LVW, et al. The biogenesis, biology and characterization of circular RNAs. Nat Rev Genet. 2019;20(11):675–691. doi:10.1038/s41576-019-0158-7
8. Chen G, Tang W, Wang S, et al. Promising diagnostic and therapeutic circRNAs for skeletal and chondral disorders. Int J Biol Sci. 2021;17(5):1428–1439. doi:10.7150/ijbs.57887
9. Abi A, Farahani N, Molavi G, et al. Circular RNAs: epigenetic regulators in cancerous and noncancerous skin diseases. Cancer Gene Ther. 2020;27(5):280–293. doi:10.1038/s41417-019-0130-x
10. Wang L, Xu GE, Spanos M, et al. Circular RNAs in cardiovascular diseases. Adv Exp Med Biol. 2018;1087:191–204.
11. Huang A, Zheng H, Wu Z, et al. Circular RNA-protein interactions: functions, mechanisms, and identification. Theranostics. 2020;10(8):3503–3517. doi:10.7150/thno.42174
12. van Zonneveld AJ, Kölling M, Bijkerk R, et al. Circular RNAs in kidney disease and cancer. Nat Rev Nephrol. 2021;17(12):814–826. doi:10.1038/s41581-021-00465-9
13. Tao M, Zheng M, Xu Y, et al. CircRNAs and their regulatory roles in cancers. Mol Med. 2021;27(1):94. doi:10.1186/s10020-021-00359-3
14. Chen J, Yang J, Fei X, et al. CircRNA ciRS-7: a novel oncogene in multiple cancers. Int J Biol Sci. 2021;17(1):379–389. doi:10.7150/ijbs.54292
15. Huang X, Zhao Y, Zhou H, et al. Circular RNAs in atherosclerosis. Clin Chim Acta. 2022;531:71–80. doi:10.1016/j.cca.2022.03.016
16. Ding Y, Lu C, Zhang W, et al. The emerging role of circular RNAs in cardiovascular diseases. J Physiol Biochem. 2021;77(3):343–353. doi:10.1007/s13105-021-00807-y
17. Yang L, Yang F, Zhao H, et al. Circular RNA circCHFR facilitates the proliferation and migration of vascular smooth muscle via miR-370/FOXO1/Cyclin D1 pathway. Mol Ther Nucleic Acids. 2019;16:434–441. doi:10.1016/j.omtn.2019.02.028
18. Si X, Zheng H, Wei G, et al. circRNA Hipk3 induces cardiac regeneration after myocardial infarction in mice by binding to Notch1 and miR-133a. Mol Ther Nucleic Acids. 2020;21:636–655. doi:10.1016/j.omtn.2020.06.024
19. Kong P, Yu Y, Wang L, et al. circ-Sirt1 controls NF-κB activation via sequence-specific interaction and enhancement of SIRT1 expression by binding to miR-132/212 in vascular smooth muscle cells. Nucleic Acids Res. 2019;47(7):3580–3593. doi:10.1093/nar/gkz141
20. Qi X, Zhang D-H, Wu N, et al. ceRNA in cancer: possible functions and clinical implications. J Med Genet. 2015;52(10):710–718. doi:10.1136/jmedgenet-2015-103334
21. Zhang M, Bai X, Zeng X, et al. circRNA-miRNA-mRNA in breast cancer. Clin Chim Acta. 2021;523:120–130. doi:10.1016/j.cca.2021.09.013
22. Li B, Zhu L, Lu C, et al. circNDUFB2 inhibits non-small cell lung cancer progression via destabilizing IGF2BPs and activating anti-tumor immunity. Nat Commun. 2021;12(1):295. doi:10.1038/s41467-020-20527-z
23. Garikipati VNS, Verma SK, Cheng Z, et al. Circular RNA CircFndc3b modulates cardiac repair after myocardial infarction via FUS/VEGF-A axis. Nat Commun. 2019;10(1):4317. doi:10.1038/s41467-019-11777-7
24. Fang S, Guo H, Cheng Y, et al. circHECTD1 promotes the silica-induced pulmonary endothelial-mesenchymal transition via HECTD1. Cell Death Dis. 2018;9(3):396. doi:10.1038/s41419-018-0432-1
25. Li C, Liu Y, Lv Z, et al. Circular RNA circHECTD1 facilitates glioma progression by regulating the miR-296-3p/SLC10A7 axis. J Cell Physiol. 2021;236(8):5953–5965. doi:10.1002/jcp.30277
26. Cai J, Chen Z, Wang J, et al. circHECTD1 facilitates glutaminolysis to promote gastric cancer progression by targeting miR-1256 and activating beta-catenin/c-Myc signaling. Cell Death Dis. 2019;10(8):576. doi:10.1038/s41419-019-1814-8
27. Bouguerra SA, Bourdillon MC, Dahmani Y, et al. Non insulin dependent diabetes in sand rat (Psammomys obesus) and production of collagen in cultured aortic smooth muscle Cells. Influence of insulin. Int J Exp Diabetes Res. 2001;2(1):37–46. doi:10.1155/EDR.2001.37
28. Ruan C, Lu J, Wang H, et al. miR-26b-5p regulates hypoxia-induced phenotypic switching of vascular smooth muscle cells via the TGF-beta/Smad4 signaling pathway. Mol Med Rep. 2017;15(6):4185–4190. doi:10.3892/mmr.2017.6509
29. Zang J, Lu D, Xu A. The interaction of circRNAs and RNA binding proteins: an important part of circRNA maintenance and function. J Neurosci Res. 2020;98(1):87–97. doi:10.1002/jnr.24356
30. Wu X, Qiu L, Feng H, et al. KHDRBS3 promotes paclitaxel resistance and induces glycolysis through modulated MIR17HG/CLDN6 signaling in epithelial ovarian cancer. Life Sci. 2022;293:120328. doi:10.1016/j.lfs.2022.120328
31. Wu P, Gao Y, Shen S, et al. KHDRBS3 regulates the permeability of blood-tumor barrier via cDENND4C/miR-577 axis. Cell Death Dis. 2019;10(7):536. doi:10.1038/s41419-019-1771-2
32. Miao R, Qi C, Fu Y, et al. Silencing of circARHGAP12 inhibits the progression of atherosclerosis via miR-630/EZH2/TIMP2 signal axis. J Cell Physiol. 2022;237(1):1057–1069. doi:10.1002/jcp.30598
33. Bennett MR, Sinha S, Owens GK. Vascular smooth muscle cells in atherosclerosis. Circ Res. 2016;118(4):692–702. doi:10.1161/CIRCRESAHA.115.306361
34. Miano JM, Fisher EA, Majesky MW. Fate and state of vascular smooth muscle cells in atherosclerosis. Circulation. 2021;143(21):2110–2116. doi:10.1161/CIRCULATIONAHA.120.049922
35. Ding P, Ding Y, Tian Y, Lei X. Circular RNA circ_0010283 regulates the viability and migration of oxidized lowdensity lipoprotein induced vascular smooth muscle cells via an miR3703p/HMGB1 axis in atherosclerosis. Int J Mol Med. 2020;46(4):1399–1408. doi:10.3892/ijmm.2020.4703
36. Dong X, Hu H, Fang Z, et al. CTRP6 inhibits PDGF-BB-induced vascular smooth muscle cell proliferation and migration. Biomed Pharmacother. 2018;103:844–850. doi:10.1016/j.biopha.2018.04.112
37. Zheng S, Zhang X, Odame E, et al. CircRNA-protein interactions in muscle development and diseases. Int J Mol Sci. 2021;22(6):3262. doi:10.3390/ijms22063262
38. Wang Z, Lei X. Matrix factorization with neural network for predicting circRNA-RBP interactions. BMC Bioinform. 2020;21(1):229. doi:10.1186/s12859-020-3514-x
39. Ukai S, Honma R, Sakamoto N, et al. Molecular biological analysis of 5-FU-resistant gastric cancer organoids; KHDRBS3 contributes to the attainment of features of cancer stem cell. Oncogene. 2020;39(50):7265–7278. doi:10.1038/s41388-020-01492-9
40. Mirzaei S, Gholami MH, Hushmandi K, et al. The long and short non-coding RNAs modulating EZH2 signaling in cancer. J Hematol Oncol. 2022;15(1):18. doi:10.1186/s13045-022-01235-1
41. Smith SA, Newby N, Bond B. Ending restenosis: inhibition of vascular smooth muscle cell proliferation by cAMP. Cells. 2019;8(11):1447. doi:10.3390/cells8111447
42. Guan S, Tang Q, Liu W, et al. Nobiletin inhibits PDGF-BB-induced vascular smooth muscle cell proliferation and migration and attenuates neointimal hyperplasia in a rat carotid artery injury model. Drug Dev Res. 2014;75(8):489–496. doi:10.1002/ddr.21230
43. Petsophonsakul P, Furmanik M, Forsythe R, et al. Role of vascular smooth muscle cell phenotypic switching and calcification in aortic aneurysm formation. Arterioscler Thromb Vasc Biol. 2019;39(7):1351–1368. doi:10.1161/ATVBAHA.119.312787
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.