Back to Journals » OncoTargets and Therapy » Volume 13
Circ_0110805 Knockdown Enhances Cisplatin Sensitivity and Inhibits Gastric Cancer Progression by miR-299-3p/ENDOPDI Axis
Authors Yang X, Zhang Q, Guan B
Received 30 August 2020
Accepted for publication 22 October 2020
Published 6 November 2020 Volume 2020:13 Pages 11445—11457
DOI https://doi.org/10.2147/OTT.S279563
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr XuYu Yang
Xi Yang,1 Qunxiong Zhang,1 Bugao Guan2
1Digestive Department, The Affiliated Jiangsu Shengze Hospital of Nanjing Medical University, Suzhou, Jiangsu, People’s Republic of China; 2Department of General Surgery, People’s Hospital of Jinhu, Huaian, Jiangsu, People’s Republic of China
Correspondence: Bugao Guan
Department of General Surgery, People’s Hospital of Jinhu, 160 Shenhua Avenue, Jinhu, Huaian, Jiangsu, People’s Republic of China
Email [email protected]
Background: Gastric cancer is a prevalent primary stomach tumor. Cisplatin is frequently used to treat gastric cancer. However, the resistance of cisplatin in gastric cancer often occurs, which brings a heavy burden to gastric cancer treatment.
Methods: In this study, we revealed a novel underlying mechanism about cisplatin-resistant effect in gastric cancer. A Cell Counting Kit-8 (CCK-8) cell viability assay and a xenograft model were performed to evaluate the function of circRNA in the cisplatin resistance of gastric cancer.
Results: Compared with control groups, we observed that circ_0110805 was highly expressed, the mRNA and protein expression levels of ENDOPDI were dramatically upregulated, and the expression of miR-299-3p was significantly downregulated in gastric cancer cells, cisplatin-resistant gastric cancer tissues or cells. Functionally, circ_0110805 knockdown improved cisplatin sensitivity, induced cell apoptosis, whereas repressed cell viability, migration and invasion in AGS/DDP and HGC-27/DDP cells, which was reversed by miR-299-3p inhibitor. Additionally, ENDOPDI overexpression hindered the effects of miR-299-3p on cisplatin sensitivity and gastric cancer progression. Circ_0110805 knockdown enhanced cisplatin sensitivity in vivo. Mechanistically, circ_0110805 acted as a sponge of miR-299-3p and its targeted ENDOPDI.
Conclusion: We showed that circ_0110805 knockdown increased the sensitivity of gastric cancer to cisplatin, which also repressed gastric cancer progression by sponging miR-299-3p to downregulate ENDOPDI expression. It might provide a new insight for future studying cisplatin-resistant gastric cancer.
Keywords: cisplatin, sensitivity, circ_0110805, miR-299-3p, ENDOPDI, gastric cancer
Introduction
Gastric cancer is one of the most common and aggressive malignancies in the world. With high metastasis and strong proliferative ability, gastric cancer brings a heavy barrier for its therapy. At present, cisplatin is a primary first-line drug in gastric cancer therapy.1 However, cisplatin treatment achieves very modest results, and patients often have a somber prognosis with a median survival time of about 1 year. One of the leading causes of such poor responses to this treatment is cisplatin resistance. Based on the reasons mentioned above, understanding the mechanism of gastric cancer resistance to cisplatin is necessary for gastric cancer therapy.
Circular RNA (circRNA) is a long non-coding RNA with a closed structure2,3 when compared with corresponding linear RNA,4 circRNA is more stable and can be expressed in exosomes. Accumulating evidence has revealed that circRNA was related to drug resistance in cancers.5,6 In ovarian cancer, circ-Cdr1 was reported to enhance cisplatin sensitivity by downregulating microRNA-1270 (miR-1270).7 Besides, circCELSR1 was also explained to repress paclitaxel sensitivity by modulating miR-1245.8 However, the underlying effects and mechanism of circ_0110805 about cisplatin resistance in human gastric cancer remain unclear.
MiRNA is a non-coding RNA with about 22 nucleotides in size9–11 and can participate in the regulation of cell progression in various cancer.9,12–14 Besides, research unclosed that miRNA was closely linked to the drug resistance of cancer. For instance, in gastric cancer, miR-4290 regulated PDK1-mediated glycolysis, which can result in cisplatin resistance.15 miRNA-765 enhanced gastric cancer multidrug resistance with BATF2 deletion.16 Zhang et al also indicated that miR-522 contributed to gastric cancer sensitivity to cisplatin and ferroptosis.17 However, there were few studies on the role of miR-299-3p in regulating cisplatin resistance in human gastric cancer.
CircRNA phosphatidylinositol-4-phosphate 5-kinase alpha is an known oncogene in gastric cancer by affecting miR-376c-3p/ZNF146 axis18 and in non-small cell lung cancer by miR-600/HIF-1α axis.19 Moreover, microRNA-299-3p (miR-299-3p) played a suppressive role in HCC progression by targeting SIRT5.20 Endothelial protein-disulfide isomerase (ENDOPDI) acted as a downstream target of miR-218-5p to be related to the regulation of circRNA-104718 in HCC.21 In this study, we focused on the relation of circ_0110805 with miR-299-3p and ENDOPDI as a key point in regulating cisplatin-resistance gastric cancer.
In our experiment, the expression of circ_0110805, miR-299-3p and ENDOPDI was determined. We also explored the effects of circ_0110805 silencing on cisplatin sensitivity and gastric cancer progression both in vivo and in vitro. Additionally, the regulatory mechanism of circ_0110805 in cisplatin resistance and cell viability, apoptosis, migration and invasion in gastric cancer was demonstrated.
Materials and Methods
Sample Collection
People’s Hospital of Jinhu provided cisplatin-resistant and cisplatin-sensitive gastric cancer tissues derived from gastric cancer patients (N=35, respectively). The criteria for judging cisplatin-resistant and sensitive patients were followed by standard CDDP response definitions published elsewhere.22 Gastric cancer tissues were stored in liquid nitrogen. The Ethics Committee of People’s Hospital of Jinhu consented this experiment. Gastric cancer patients related to this experiment agreed with the written informed consents.
The Construction of Cisplatin-Resistant AGS and HGC-27 Cells (AGS/DDP and HGC-27/DDP Cells) and Cell Culture
Human gastric cancer cells (AGS and HGC-27) and immortalized cell lines GES-1 were purchased from Otwobiotech (Shenzhen, China). Dulbecco’s modified Eagle’s medium (DMEM; Thermo Fisher, Waltham, MA, USA) with 10% fetal bovine serum (FBS) and 1% streptomycin/penicillin (Thermo Fisher) was used to maintain the growth of cells. Cells were incubated under a humidified atmosphere containing 5% CO2 at 37°C. AGS/DDP and HGC-27/DDP cells were established by gradually treating cisplatin (Solarbio, Beijing, China) into AGS and HGC-27 cells from 1/50 IC50 concentration for more than 6 months. AGS/DDP and HGC-27/DDP cells were cultivated in a 10% DMEM medium, which maintained their resistance to cisplatin.
Cell Transfection
Small interfering RNA against circ_0110805 (si-circ_0110805), small hairpin RNA targeting circ_0110805 (sh-circ_0110805), miR-299-3p mimic (miR-299-3p), miR-299-3p inhibitor (anti-miR-299-3p), the overexpression plasmid of ENDOPDI and their control groups (si-NC, sh-NC, miR-NC, anti-miR-NC and pcDNA) were synthesized by Ribobio Co., Ltd. (Guangzhou, China). Different cells were seeded in different plates and transfected with Lipofectamine 2000 (Thermo Fisher) according to the manufacturer’s instructions.
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Cells mentioned above were lysed using TRIzol reagent (TaKaRa, Dalian, China). RNA was extracted and cDNA was amplified by prime Script™RT reagent Kit (TaKaRa). The expression of circ_0110805, miR-299-3p and ENDOPDI was detected using QRT-PCR quantity kit (TaKaRa). Data were analyzed with the 2−∆∆Ct method. U6 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were used as controls. The sense and antisense of primers were circ_0110805, 5ʹ-GAAGTTGGAGCACTCTTGGA-3ʹ (F) and 5ʹ-AAAGTCCGAGGGTTCTGGTT-3ʹ (R); miR-299-3p, 5ʹ-ACACTCCAGCTGGGTATGTGGGATGGTAAAC-3ʹ (F) and 5ʹ-GTGCAGGGTCCGAGGT-3ʹ (R); ENDOPDI, 5ʹ-TCACTGAGGGAGTACGTGGA-3ʹ (F) and 5ʹ-GCAGTGCAGTCTACTTCGG-3ʹ (R); 18s rRNA, 5ʹ-TCGAGGCCCTGTAATTGGAA-3ʹ (F) and 5ʹ-CCCAAGATCCAACTACGAGCT-3ʹ (R); U6, 5ʹ-GGAACGCTTCACGAATTTG-3ʹ (F) and 5ʹ-ATTGGAACGATACAGAGAAGATT-3ʹ (R).
Cell Counting Kit-8 (CCK-8) Assay
Cisplatin sensitivity and cell viability were tested with the CCK-8 assay. For determining cisplatin sensitivity, AGS/DDP and HGC-27/DDP cells were seeded in a 96-well plate for 16 h, followed by transfection with various treatments and cells were cultured for 24 h. After treatment, 10 μL CCK-8 solution (Beyotime, Shanghai, China) was added to each well, and cells were subsequently incubated for another 4 h. Cell sensitivity was explained by assessing the half-maximal inhibitory concentration (IC50) with detecting the absorbance at 450 nm using a microplate reader. Cells were treated as the above description to determine the cell viability. Results were assessed by detecting absorbance at 450 nm with a microplate reader.
Flow Cytometry Analysis
Cell apoptosis rate was analyzed using an Annexin V-FITC detection kit (Beyotime). In short, cells were collected at 48 h after various transfections. The cells were washed with phosphate buffer solution (PBS), harvested through centrifuging and suspended with binding buffer. Subsequently, cells were incubated with Annexin V-FITC and propidium iodide (PI). Apoptotic cells were quantified with flow cytometry (BD Biosciences, San Diego, CA, USA).
Transwell Assay
Cells migratory and invasive abilities were determined by transwell chambers without or with Matrigel (Corning, New York, Madison, USA). Cells were seeded in upper chambers with FBS-free DMEM medium, while DMEM with 10% FBS was added to lower chambers. Following cells were cultivated for 24 h. Then, the medium was discarded and cells were fixed with methanol and stained via crystal violet (Beyotime). Results were analyzed by a microscope (100×).
Western Blot Assay
Tissues and cell lysate were extracted using RIPA buffer (Beyotime). Lysate protein was resolved by 12% sodium dodecyl sulfonate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred onto polyvinylidene fluoride (Membrane Solutions, Shanghai, China). Then, membranes were blocked in 5% skim milk for 4 h. Proteins were incubated with anti-ENDOPDI (1:20,000, Abcam) and anti-GAPDH (1:10,000, Abcam). Then, bands were incubated with secondary antibody labeled horseradish peroxidase (1:20,000, Abcam). Proteins were displayed by ECL system (Pierce, Rockford, IL, USA). GAPDH was chosen as a control.
In vivo Tumor Formation Assay
Charles River (Beijing, China) provided BALB/c nude mice (5-week old). Mice were divided into four groups (sh-NC group, sh-circ_0110805 group, sh-NC+cisplatin group and shcirc_0110805+cisplatin group, N=7 per group). 4×106 cells transfected with sh-NC and sh-circ_0110805 were injected into the flank of mice, then mice were treated with cisplatin (20 mg/kg) after 8 days. Meanwhile, tissues were measured every 3 days. At 18 days after cisplatin treatment, mice were euthanized. Weights of the tumors were measured and circ_0110805 expression was assessed by qRT-PCR. The protocol was approved by People’s Hospital of Jinhu Animal Care and Use Committee, the study has followed the guidelines of the Nanjing Medical University Animal Care and Use Committee for the welfare of the laboratory animals.
Dual-Luciferase Reporter Assay
The wide-type (WT) fragment of circ_0110805 containing the targeting sequence of miR-299-3p and mutant (MUT) circ_0110805 were sub-cloned into pmirGLO vector (Miaoling Bio, Wuhan, China), and named as WT-circ_0110805 and MUT-circ_0110805. The wide-type 3ʹ-untranslated regions (3ʹUTR) of ENDOPDI containing the binding sequence of miR-299-3p and mutant ENDOPDI 3ʹUTR were inserted into pmirGLO vector, and named as ENDOPDI 3ʹUTR-WT and ENDOPDI 3ʹUTR-MUT. The constructed plasmids were transfected into AGS/DDP and HGC-27/DDP cells with miR-299-3p mimic or miR-NC, respectively. Relative luciferase activity was assayed using the Promega dual-luciferase reporter assay system. Firefly activity was normalized to internal Renilla luciferase levels.
Statistical Analysis
Figures in this experiment were made by GraphPad Prism 8.0.1 and image J software. All experiments were performed at least in triplicate unless otherwise stated. Data were expressed as means ± standard deviations. Spearman correlation analysis was performed to assess the relationship between miR-299-3p and circ_0110805 or ENDOPDI. Two-tailed Student’s t-test or one-way analysis of variance was performed for comparisons between two groups. P value < 0.05 was statistically significant.
Results
Circ_0110805 Expression is Upregulated in Cisplatin-Resistant Gastric Cancer Tissues and Cells
The expression level of circ_0110805 was detected in cisplatin-resistant gastric cancer tissues and cells. We found that circ_0110805 expression was dramatically upregulated in cisplatin-resistant gastric cancer tissues by qRT-PCR, compared with cisplatin-sensitive gastric cancer tissues (Figure 1A). Besides, qRT-PCR analysis also investigated that circ_0110805 expression was significantly upregulated in AGS and HGC-27 cells as compared to GES-1 cells, and was greatly increased in AGS/DDP and HGC-27/DDP cells relative to AGS and HGC-27 cells (Figure 1B). These results suggested that circ_0110805 might play an important role in gastric cancer resistance to cisplatin.
Circ_0110805 Knockdown Improves Cisplatin Sensitivity and Induces Cell Apoptosis, Whereas Inhibits Cell Proliferation, Migration and Invasion in AGS/DDP and HGC-27/DDP Cells
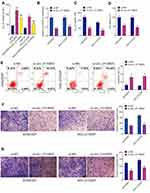
To explore the functional effects of circ_0110805 knockdown on cisplatin sensitivity and gastric cancer progression, we first detected the IC50 value of cisplatin in AGS/DDP and HGC-27/DDP cells. Figure 2A shows that, by CCK-8 assay, IC50 value of cisplatin was increased in AGS/DDP and HGC-27/DDP cells compared with that in AGS and HGC-27 cells, which indicated that cisplatin-resistant AGS and HGC-27 cells were successfully established. To detect the interfering efficiency of si-circ_0110805, circ_0110805 expression was examined by qRT-PCR and was found to be greatly downregulated after si-circ_0110805 transfection (Figure 2B). After that, the result of CCK-8 assay demonstrated that circ_0110805 knockdown decreased the IC50 value of cisplatin in AGS/DDP and HGC-27/DDP cells (Figure 2C), indicating that circ_0110805 silencing enhanced cisplatin sensitivity of cisplatin-resistant gastric cancer cells. Moreover, it also illustrated that circ_0110805 knockdown repressed cell viability in AGS/DDP and HGC-27/DDP cells (Figure 2D). Flow cytometry analysis unveiled that circ_0110805 silencing induced cell apoptosis in AGS/DDP and HGC-27/DDP cells (Figure 2E). Meanwhile, transwell assay disclosed that circ_0110805 knockdown suppressed cell migration and invasion in AGS/DDP and HGC-27/DDP cells (Figure 2F and G).
Circ_0110805 Knockdown Improves Gastric Cancer Resistance to Cisplatin in vivo
The effects of circ_0110805 knockdown on tumor growth in vivo were revealed in this part. As shown in Figures 3A, B and S1A, cisplatin treatment repressed tumor volume and weight, and circ_0110805 silencing enhanced these effects. To examine the impacts between circ_0110805 knockdown and cisplatin treatment on circ_0110805 expression, qRT-PCR was performed to illustrate that circ_0110805 deletion repressed circ_0110805 expression, and the expression of circ_0110805 was also inhibited by circ_0110805 knockdown after cisplatin exposure (Figure 3C). Taken together, these data showed that the inhibitory effects of cisplatin treatment on tumor growth in vivo were enhanced by circ_0110805 knockdown.
Circ_0110805 Binds to miR-299-3p in AGS/DDP and HGC-27/DDP Cells
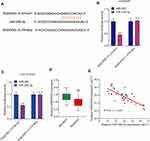
In order to reveal the regulatory mechanism of circ_0110805 in cisplatin sensitivity and gastric cancer progression, we used starBase v2.0 online database to predict the miRNA associated with circ_0110805. As shown in Figure 4A, our results showed that circ_0110805 contained the targeting sites of miR-299-3p. Meanwhile, by dual-luciferase reporter assay, the luciferase activity of WT-circ_0110805+miR-299-3p group was dramatically decreased in AGS/DDP and HGC-27/DDP cells, but there was no obvious change after MUT-circ_0110805 and miR-299-3p transfection (Figure 4B and C). Subsequently, we detected the expression of miR-299-3p by qRT-PCR in cisplatin-resistant gastric cancer tissues (N=35) and cells. We observed that when compared with cisplatin-sensitivity gastric cancer tissues, miR-299-3p expression was significantly downregulated in cisplatin-resistant gastric cancer tissues (Figure 4D). We also found that miR-299-3p expression was dramatically decreased in AGS and HGC-27 cells as compared to GES-1 cells. Consistently, in AGS/DDP and HGC-27/DDP cells, the expression of miR-299-3p was significantly downregulated when compared with control groups (Figure 4E). Spearman correlation analysis showed that circ_0110805 expression was negatively correlated with miR-299-3p (Figure 4F). Overall, these data indicated that circ_0110805 plays a significant role as a sponge of miR-299-3p in AGS/DDP and HGC-27/DDP cells.
Circ_0110805 Knockdown Enhances Cisplatin Sensitivity and Represses Gastric Cancer Progression by Sponging miR-299-3p
To explore the effects between circ_0110805 and miR-299-3p on cisplatin sensitivity and gastric cancer progression, we first identified the interfering efficiency of anti-miR-299-3p. qRT-PCR detected that the expression of miR-299-3p was dramatically downregulated by anti-miR-299-3p (Figure 5A). Meanwhile, by qRT-PCR, we observed that circ_0110805 knockdown upregulated miR-299-3p expression in AGS/DDP and HGC-27/DDP cells, whereas this effect was decreased by an miR-299-3p inhibitor (Figure 5B).
Subsequently, CCK-8 assay showed that circ_0110805 silencing decreased the IC50 value of cisplatin in AGS/DDP and HGC-27/DDP cells, whereas this impact was partially attenuated by miR-299-3p deletion (Figure 5C). Similarly, the viability of AGS/DDP and HGC-27/DDP cells was inhibited due to knockdown of circ_0110805, and this effect was hindered by an miR-299-3p inhibitor (Figure 5D). Consistent with these observations, flow cytometry analysis unveiled that in AGS/DDP and HGC-27/DDP cells, miR-299-3p inhibitor partially restored the promotion effect of circ_0110805 inhibition on cell apoptosis (Figure 5E). We further performed transwell assay, which showed that anti-miR-299-3p restrained the inhibition impacts of circ_0110805 knockdown on cell migration and invasion in AGS/DDP and HGC-27/DDP cells (Figures 5F, G and S1B). Collectively, our findings demonstrated that circ_0110805 knockdown improved cisplatin sensitivity and hindered gastric cancer development by binding to miR-299-3p.
MiR-299-3p Targets ENDOPDI in AGS/DDP and HGC-27/DDP Cells
The target gene of miR-299-3p was verified in this part. StarBase v2.0 online database showed that there were binding sites of miR-299-3p in ENDOPDI 3ʹUTR (Figure 6A). Dual-luciferase reporter assay revealed that the luciferase activity of ENDOPDI 3ʹUTR-WT was dramatically weakened after miR-299-3p mimic transfection, whereas the luciferase activity of ENDOPDI 3ʹUTR-MUT was not significantly changed by miR-299-3p (Figure 6B and C). Subsequently, the expression level of ENDOPDI was detected by qRT-PCR or Western blot in cisplatin-resistant tissues (N=35) and cells. Results showed that ENDOPDI expression at mRNA levels was dramatically upregulated in cisplatin-resistant gastric cancer tissues when compared with cisplatin-sensitive gastric cancer tissues (Figure 6D). Spearman correlation analysis showed that miR-299-3p expression was negatively related to ENDOPDI expression (Figure 6E). These results revealed that miR-299-3p was associated with ENDOPDI in AGS/DDP and HGC-27/DDP cells.
MiR-299-3p Mimic Improves Cisplatin Sensitivity and Inhibits Gastric Cancer Progression by Targeting ENDOPDI
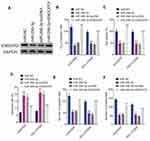
We further explored the underneath effects between miR-299-3p and ENDOPDI on cisplatin sensitivity and gastric cancer progression. QRT-PCR was firstly performed to detect the effects between miR-299-3p mimic and ENDOPDI overexpression on ENDOPDI expression in AGS/DDP and HGC-27/DDP cells. Results showed that ENDOPDI expression was dramatically downregulated by miR-299-3p in AGS/DDP and HGC-27/DDP cells (Figure 7A). Subsequently, CCK-8 assay showed that miR-299-3p mimic decreased the IC50 value of cisplatin, whereas this impact was reversed by ENDOPDI overexpression (Figure 7B). Cell viability was detected by CCK-8 assay, which revealed that ENDOPDI overexpression hindered the inhibition effects of miR-299-3p mimic in AGS/DDP and HGC-27/DDP cells (Figure 7C). Moreover, by Flow cytometry analysis, we found that miR-299-3p mimic induced cell apoptosis in AGS/DDP and HGC-27/DDP cells, and this effect was restrained by ENDOPDI overexpression (Figure 7D). In addition, transwell assay demonstrated that the migration and invasion of AGS/DDP and HGC-27/DDP cells were suppressed by miR-299-3p mimic; however, ENDOPDI overexpression can partially abolish these impacts (Figure 7E and F). All data illustrated that miR-299-3p regulated the sensitivity of gastric cancer cells to cisplatin sensitivity and cell proliferation, apoptosis, migration, invasion by targeting ENDOPDI.
Circ_0110805 Knockdown Downregulates ENDOPDI Expression by Sponging miR-299-3p
To further determine the relationship between circ_0110805, miR-299-3p and ENDOPDI, we revealed the effects between circ_0110805 knockdown and miR-299-3p inhibitor on ENDOPDI protein expression. Western blot was performed to show that the protein expression of ENDOPDI was significantly downregulated due to circ_0110805 silencing, whereas this effect was hindered by a miR-299-3p inhibitor (Figure 8). Overall, these data implicated that circ_0110805 regulated ENDOPDI expression by binding to miR-299-3p.
Discussion
Gastric cancer is one of the most commonly seen malignancies with the highest mortality in China, which takes up more than 50% of major gastrointestinal-related carcinoma in people over the age of 18.23 Despite cisplatin is a primary first-line drug in gastric cancer treatment, cisplatin resistance brings great changes in its therapy. Therefore, our study revealed the mechanism of cisplatin in its resistance to gastric cancer cells.
Circular RNAs (circRNAs) are single-stranded non-coding RNAs whose 3ʹ and 5ʹ ends bond covalently into a loop.3,24 Some reports have shown that CircRNAs are contributed to cancer development and cisplatin resistance. For example, Huang et al revealed that cirAKT3 enhances cisplatin resistance through upregulating PIK3R1 via miR-198 in gastric cancer.25 Similarly, cisplatin resistance can also be modulated by circRNACCDC66 via the miR-618/BCL2 axis in gastric cancer.26 Besides, circFN1 regulates cisplatin resistance in gastric cancer via sponging miR-182-5p.27
Circ_0110805 was found for the first time that it enhanced non-small cell lung cancer proliferation and metastasis.19 A previous study has also shown that circ_0110805 played an important role in gastric cancer progression.18 Song et al indicated circ_0110805 expression was increased in gastric cancer tissues and cells and promoted cell development.28
In our study, circ_0110805 was indicated to be upregulated in gastric cancer tissues and cells and promoted cell invasion. Besides, when compared with control groups, we found that circ_0110805 was upregulated in cisplatin-resistant tissues and cells. Our results also showed that circ_0110805 silencing induced cell apoptosis and repressed cell viability and migratory abilities in AGS/DDP and HGC-27/DDP cells. Furthermore, circ_0110805 sponged miR-299-3p in AGS/DDP and HGC-27/DDP cells.
MiR-299-3p was disclosed to be linked to cisplatin resistance in cancers and cancer development. Zhou et al unveiled that miR-299-3p mimic suppressed gastric cancer invasion by inhibiting HPSE.29 Meanwhile, Zhao et al, demonstrated that in ovarian cancer, cell viability was repressed by miR-299-3p.30 Dang et al indicated miR-299-3p was lowly expressed in hepatocellular carcinoma tissues and cells, and its inhibitor promoted cell migration and invasion.20 Consistent with these data, in our experiment, miR-299-3p expression was also downregulated in gastric cancer tissues and cells. MiR-299-3p inhibited cisplatin resistance, cell viability, migratory, and invasive abilities, and promoted cell apoptosis. Besides, miR-299-3p was also found to be downregulated in cisplatin-resistant gastric cancer tissues and cells, and miR-299-3p targeted ENDOPDI.
Previous studies indicated that ENDOPDI promoted gastric cancer development. For instance, Zhang et al displayed that ENDOPDI was upregulated in gastric cancer tissues, and its silencing restrained cell proliferative, migratory, and invasive abilities.31 Similarly, our findings showed that ENDOPDI was highly expressed in gastric cancer tissues and cells. Its overexpression repressed cell apoptosis, whereas promoted cell proliferation, migration and invasion. Furthermore, ENDOPDI was explained to repress gastric cancer sensitivity to cisplatin and to be upregulated in cisplatin-resistant gastric cancer tissues and cells in our study.
Collectively, in gastric cancer cells, cisplatin-resistant gastric cancer tissues or cells, our results provide direct experimental evidence that circ_0110805 and ENDOPDI were upregulated, and miR-299-3p was downregulated. We also showed that knockdown of circ_0110805 improved cisplatin sensitivity and repressed gastric cancer progression both in vitro and in vivo. Additionally, circ_0110805 served as a sponge of miR-299-3p to regulate ENDOPDI expression. Together, our results provided a novel theoretical basis in treating gastric cancer with cisplatin.
Data Sharing Statement
All data generated or analyzed during this study are included in this published article and its additional files.
Ethics Approval and Consent to Participate
The Ethics Committee of People’s Hospital of Jinhu consented this experiment. Gastric cancer patients related to this experiment agreed with the written informed consents. People’s Hospital of Jinhu Animal Care and Use Committee consented this study.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
Huai’an Science and Technology Planning Project (HAB201850); Science, Education and Health Promotion Project in Wujiang District, Suzhou (WWK201927).
Disclosure
The authors declare that they have no competing interests.
References
1. Battaglin F, Naseem M, Puccini A, Lenz HJ. Molecular biomarkers in gastro-esophageal cancer: recent developments, current trends and future directions. Cancer Cell Int. 2018;18:99. doi:10.1186/s12935-018-0594-z
2. Xue M, Li G, Fang X, Wang L, Jin Y, Zhou Q. hsa_circ_0081143 promotes cisplatin resistance in gastric cancer by targeting miR-646/CDK6 pathway. Cancer Cell Int. 2019;19:25. doi:10.1186/s12935-019-0737-x
3. Bao L, Zhong J, Pang L. Upregulation of circular RNA VPS13C-has-circ-001567 promotes ovarian cancer cell proliferation and invasion. Cancer Biother Radiopharm. 2019;34(2):110–118. doi:10.1089/cbr.2018.2641
4. Li Y, Zheng Q, Bao C, et al. Circular RNA is enriched and stable in exosomes: a promising biomarker for cancer diagnosis. Cell Res. 2015;25(8):981–984. doi:10.1038/cr.2015.82
5. Yin H, Cui X. Knockdown of circHIPK3 facilitates temozolomide sensitivity in glioma by regulating cellular behaviors through miR-524-5p/KIF2A-mediated PI3K/AKT pathway. Cancer Biother Radiopharm. 2020. doi:10.1089/cbr.2020.3575
6. Xu T, Wang M, Jiang L, et al. CircRNAs in anticancer drug resistance: recent advances and future potential. Mol Cancer. 2020;19(1):127.
7. Zhao Z, Ji M, Wang Q, He N, Li Y. Circular RNA Cdr1as upregulates SCAI to suppress cisplatin resistance in ovarian cancer via miR-1270 suppression. Mol Ther Nucleic Acids. 2019;18:24–33. doi:10.1016/j.omtn.2019.07.012
8. Zhang S, Cheng J, Quan C, et al. circCELSR1 (hsa_circ_0063809) contributes to paclitaxel resistance of ovarian cancer cells by regulating FOXR2 expression via miR-1252. Mol Ther Nucleic Acids. 2020;19:718–730. doi:10.1016/j.omtn.2019.12.005
9. Wang J, Liu S, Shi J, et al. The role of miRNA in the diagnosis, prognosis, and treatment of osteosarcoma. Cancer Biother Radiopharm. 2019;34(10):605–613. doi:10.1089/cbr.2019.2939
10. Yu Y, Cao XC. miR-190-5p in human diseases. Cancer Cell Int. 2019;19:257. doi:10.1186/s12935-019-0984-x
11. Qi Y, Wang X, Kong X, et al. Expression signatures and roles of microRNAs in inflammatory breast cancer. Cancer Cell Int. 2019;19:23. doi:10.1186/s12935-018-0709-6
12. Ma R, Zhao Y, He M, et al. Identifying a ten-microRNA signature as a superior prognosis biomarker in colon adenocarcinoma. Cancer Cell Int. 2019;19:360. doi:10.1186/s12935-019-1074-9
13. Qiao Z, Zou Y, Zhao H. MicroRNA-140-5p inhibits salivary adenoid cystic carcinoma progression and metastasis via targeting survivin. Cancer Cell Int. 2019;19:301. doi:10.1186/s12935-019-1018-4
14. Fadaka AO, Ojo BA, Adewale OB, Esho T, Pretorius A. Effect of dietary components on miRNA and colorectal carcinogenesis. Cancer Cell Int. 2018;18:130. doi:10.1186/s12935-018-0631-y
15. Qian Y, Wu X, Wang H, Hou G, Han X, Song W. MicroRNA-4290 suppresses PDK1-mediated glycolysis to enhance the sensitivity of gastric cancer cell to cisplatin. Braz J Med Biol Res. 2020;53(5):e9330. doi:10.1590/1414-431x20209330
16. Lin W, Miao Y, Meng X, Huang Y, Zhao W, Ruan J. miRNA-765 mediates multidrug resistance via targeting BATF2 in gastric cancer cells. FEBS Open Bio. 2020;10(6):1021–1030. doi:10.1002/2211-5463.12838
17. Zhang H, Deng T, Liu R, et al. CAF secreted miR-522 suppresses ferroptosis and promotes acquired chemo-resistance in gastric cancer. Mol Cancer. 2020;19(1):43. doi:10.1186/s12943-020-01168-8
18. Ma Y, Cong X, Zhang Y, Yin X, Zhu Z, Xue Y. CircPIP5K1A facilitates gastric cancer progression via miR-376c-3p/ZNF146 axis. Cancer Cell Int. 2020;20:81. doi:10.1186/s12935-020-1122-5
19. Chi Y, Luo Q, Song Y, et al. Circular RNA circPIP5K1A promotes non-small cell lung cancer proliferation and metastasis through miR-600/HIF-1alpha regulation. J Cell Biochem. 2019;120(11):19019–19030. doi:10.1002/jcb.29225
20. Dang S, Zhou J, Wang Z, Wang K, Dai S, He S. MiR-299-3p functions as a tumor suppressor via targeting Sirtuin 5 in hepatocellular carcinoma. Biomed Pharmacother. 2018;106:966–975. doi:10.1016/j.biopha.2018.06.042
21. Yu J, Yang M, Zhou B, et al. CircRNA-104718 acts as competing endogenous RNA and promotes hepatocellular carcinoma progression through microRNA-218-5p/TXNDC5 signaling pathway. Clin Sci (Lond). 2019;133(13):1487–1503. doi:10.1042/CS20190394
22. Bagrodia A, Lee BH, Lee W, et al. Genetic determinants of cisplatin resistance in patients with advanced germ cell tumors. J Clin Oncol. 2016;34(33):4000–4007. doi:10.1200/JCO.2016.68.7798
23. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
24. Wang B, Chen H, Zhang C, et al. Effects of hsa_circRBM23 on hepatocellular carcinoma cell viability and migration as produced by regulating miR-138 expression. Cancer Biother Radiopharm. 2018;33(5):194–202. doi:10.1089/cbr.2017.2424
25. Huang X, Li Z, Zhang Q, et al. Circular RNA AKT3 upregulates PIK3R1 to enhance cisplatin resistance in gastric cancer via miR-198 suppression. Mol Cancer. 2019;18(1):71. doi:10.1186/s12943-019-0969-3
26. Zhang Q, Miao Y, Fu Q, et al. CircRNACCDC66 regulates cisplatin resistance in gastric cancer via the miR-618/BCL2 axis. Biochem Biophys Res Commun. 2020;526(3):713–720. doi:10.1016/j.bbrc.2020.03.156
27. Huang XX, Zhang Q, Hu H, et al. A novel circular RNA circFN1 enhances cisplatin resistance in gastric cancer via sponging miR-182-5p. J Cell Biochem. 2020. doi:10.1002/jcb.29641
28. Song H, Xu Y, Xu T, et al. CircPIP5K1A activates KRT80 and PI3K/AKT pathway to promote gastric cancer development through sponging miR-671-5p. Biomed Pharmacother. 2020;126:109941. doi:10.1016/j.biopha.2020.109941
29. Zhou X, Hu M, Ge Z. Tumor-suppressive miR2993p inhibits gastric cancer cell invasion by targeting heparanase. Mol Med Rep. 2019;20(3):2151–2158.
30. Zhao R, Liu Q, Lou C. MicroRNA-299-3p regulates proliferation, migration and invasion of human ovarian cancer cells by modulating the expression of OCT4. Arch Biochem Biophys. 2018;651:21–27. doi:10.1016/j.abb.2018.05.007
31. Zhang L, Hou Y, Li N, Wu K, Zhai J. The influence of TXNDC5 gene on gastric cancer cell. J Cancer Res Clin Oncol. 2010;136(10):1497–1505. doi:10.1007/s00432-010-0807-x
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.