Back to Journals » Cancer Management and Research » Volume 12
Circ_0062020 Knockdown Strengthens the Radiosensitivity of Prostate Cancer Cells
Authors Li H, Zhi Y, Ma C, Shen Q, Sun F, Cai C
Received 24 July 2020
Accepted for publication 15 October 2020
Published 17 November 2020 Volume 2020:12 Pages 11701—11712
DOI https://doi.org/10.2147/CMAR.S273826
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Haitao Li,* Yunlai Zhi,* Chunyan Ma, Qianqian Shen, Fanghu Sun, Chengkuan Cai
Department of Urology, Lianyungang Clinical College of Nanjing Medical University/The First People’s Hospital of Lianyungang, Lianyungang, Jiangsu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Fanghu Sun
Department of Urology, Lianyungang Clinical College of Nanjing Medical University/The First People’s Hospital of Lianyungang, No. 6, Zhenghua East Road, Lianyungang, Jiangsu Province 222002, People’s Republic of China
Tel +86 18961326564
Email [email protected]
Background: Prostate cancer (PCa) is a major contributor to reduce the life quality of males. Circular RNAs were frequently reported to be associated with cancers. In the case of radiotherapy to PCa, the role of circ_0062020 was still inconclusive, which was further explored in this study.
Methods: Quantitative real-time polymerase chain reaction (qRT-PCR) was used to determine the expression of circ_0062020, miR-615-5p and thyroid hormone receptor interactor 13 (TRIP13) in PCa tissues and cells, as well as in normal tissues and cell. Meanwhile, the proliferation of PCa cells was evaluated by clone formation assay and cell counting kit 8 (CCK8) assay. Moreover, the metastasis of PCa cells was assessed by transwell and wound healing assays. Furthermore, the apoptosis of PCa cells was determined by flow cytometry assay. Besides, dual-luciferase reporter system was applied to verify the correlation between miR-615-5p and circ_0062020 or TRIP13, which was predicted by online tool CircRNA interactome or TargetScan. In addition, the protein expression of TRIP13 was measured by Western blot in PCa tissues and cells and normal tissues and cells. Finally, xenograft tumor assay was performed to further confirming the function of circ_0062020 in PCa in vivo.
Results: Circ_0062020 and TRIP13 were upregulated, while miR-615-5p was downregulated in PCa tissues and cells. Circ_0062020 knockdown or miR-615-5p overexpression inhibited the proliferation and metastasis, and promoted apoptosis, which could be reversed by miR-615-5p inhibitor or pc-TRIP13 in ionizing radiation (IR)-treated PCa cells. As expected, circ_0062020 sponged miR-615-5p to regulate TRIP13 expression in PCa cells. Circ_0062020 knockdown also suppressed PCa tumor growth in vivo.
Conclusion: Circ_0062020 suppressed the radiosensitivity by miR-615-5p/TRIP13 axis in PCa cells, which might provide insights into the radiotherapy for PCa.
Keywords: prostate cancer, circ_0062020, miR-615-5p, TRIP13, radiosensitivity
Introduction
Due to the high incidence (13.5%) in males, prostate cancer (PCa) has become the second ubiquitous cancer in men only to lung cancer (14.5%).1 The putative major risk factors to PCa were listed as chronic inflammation, genetic factors and the occurrence of metastatic castration-resistant prostate cancer.2 Despite the extraordinary progress in PCa treatment, the mortality of PCa remained in 6.7% worldwide.1 Multiple regimens of radiotherapy are the mainstay treatment for PCa, meanwhile, radical prostatectomy, androgen deprivation therapy (ADT) and expectant management are the restrictive options.3–5 For the treatment of localized PCa, half of the patients underwent radical prostatectomy and a quarter of patients suffered radiotherapy.4 Meanwhile, the combined application of radical prostatectomy and ADT, the five-year survival rate of PCa could reach up to 94%.6,7 Radiotherapy is characterized by noninvasive and broad application in early PCa, locally advanced PCa, postoperative PCa tumor residue and palliative therapy.8 As an effective therapeutical method, radiotherapy was recognized as an indispensable treatment in PCa and its curative effect correlates with the radiosensitivity of tumors; however, the signaling pathways in PCa to radiotherapy was still perplexing, which needs further investigate to improve the therapeutic effect.
Circular RNAs (circRNAs) are a kind of non-coding RNAs, which were documented to be associated with cancers process.9–12 The covalent closed loop structure of circRNAs provided more stability due to the lack of poly-A capture, which unleashed the potential of circRNAs used as biomarkers in the diagnosis of cancers.13 Recently, numerous studies have exposed the momentous role of circRNAs in PCa. CircLARP4 was downregulated and restrained the metastasis in PCa cells.14 The reduction of circITCH expression was widely discovered in PCa tissues and cells, and was relevant to promote PCa development.15 Has_circ_0001206 expression was also declined and inhibited the proliferation and metastasis in PCa cells.16 However, the upregulation of circ0005276 was discovered in PCa tissues and cells, and facilitate the proliferation and metastasis.17 CircFMN2 was highly expressed, and the inhibition of circFMN2 suppressed the proliferation in PCa cells.18 The excessive expression of circZNF609 was associated with the elevated proliferation and metastasis in PCa cells.19 These contradictory results highlighted the critical role of circRNAs in PCa, which still need further investigated. This study looked at the regulatory pathway of circ_0062020 to fill the potential role of circRNAs in PCa.
To better understanding the role of circ_0062020 in PCa receives radiotherapy, PCa cells were primarily treated by ionizing radiation (IR). The expression of circ_0062020 was elevated in PCa tissues and cells, especially in radioresistant PCa tissues. Meanwhile, circ_0062020 knockdown strengthens the radiosensitivity by regulating miR-615-5p/thyroid hormone receptor interactor 13 (TRIP13) axis in PCa cells. Besides, circ_0062020 knockdown inhibited PCa tumor growth in vivo. These finding might contribute to the improvement of PCa therapy.
Materials and Methods
Clinical Samples
PCa tumor samples containing 30 radiosensitive samples and 30 radioresistant samples were obtained from PCa patients suffered radiotherapy and the normal prostate tissues (n=30) were provided by Lianyungang Clinical College of Nanjing Medical University. All the samples were temporarily stored in liquid nitrogen. Prior of taking up the study, all the participators were signed the informed consent forms. Meanwhile, this study applied and received the authorization of the Ethics Committee of Lianyungang Clinical College of Nanjing Medical University. PCa patients were separated into high expressed group and low expressed group based on the median value of circ_0062020 expression and the clinical characteristics of PCa patients are shown in Table 1.
 |
Table 1 Correlation Between Circ_0062020 Expression and Clinical Clinicopathological Parameters of PCa |
Cell Culture and Treatment
Prostate epithelial cells (WPMY-1) and PCa cell lines (DU145, LNCaP) used in this research were purchased from American Type Culture Collection (Rockville, MD, USA). DU145 cell line do not express prostate antigen while LNCaP exhibits androgen dependence, which is the suitable vitro model for studying androgen independent or androgen-responsiveness of human prostate cancer. DU145 and LNCaP cells were cultured in RPMI 1640 medium (Gibco, Grand Island, NY, USA) containing 10% fetal bovine serum (FBS, PAN, Bavaria, Germany), WPMY-1 cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM, Gibco) containing 10% FBS (PAN). For the establishment of post-radiotherapy PCa cell model, Caesium-137 irradiator (MSD Nordion, Ottawa, Ontario, Canada) was applied to irradiate cells. Cells were cultured in an incubator with the setting of 5% CO2 at 37°C.
RNA Isolation and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
QRT-PCR was applied to determine the expression of circ_0062020, miR-615-5p and TRIP13 in PCa tissues and cells. Total RNA was isolated from PCa cells and tissues as well as the normal tissues and cells by using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). The reverse transcription was accomplished by cDNA reverse transcription kit (Applied Biosystems, Foster City, CA, USA) and All-in-OneTM miRNA First-Strand cDNA Synthesis Kit 2.0 (Genecopoeia, Guangzhou, China). Then, Platinum SYBR Green qPCR SuperMix UDG (Invitrogen) and All-in-OneTM miRNA qPCR Kit (Genecopoeia, Guangzhou, China) was used to conduct qRT-PCR. The relative expression was calculated using the 2−ΔΔCt method. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and U6 small nuclear RNA (U6) served as the references control. The primer sequences were shown as below: circ_0062020, forward: 5ʹ-ACTTAAGTGAGGCACAGCGT-3ʹ, reverse: 5ʹ-GGGTGATGAAGCTCTCCCCT-3ʹ; miR-615-5p, forward: 5ʹ-GCATTTAGCAGCGAGACAA-3ʹ, reverse: 5ʹ-AGCGACACGTGCGAATGTTCT-3ʹ; TRIP13, forward: 5ʹ-ACTGTTGCACTTCACATTTTCC-3ʹ, reverse: 5ʹ-TCAGTTTAGGGTAGAGGAGCT-3ʹ; U6, forward: 5ʹ-GCTTCGGCAGCACATATACTAAAAT-3ʹ, reverse: 5ʹ-CGCTTCAGAATTTGCGTGTCAT-3ʹ; GAPDH, forward: 5ʹ-CGCTCTCTGCTCCTCCTGTTC-3ʹ, reverse: 5ʹ-ATCCGTTGACTCCGACCTTCAC-3ʹ.
Transient Transfection
Short hairpin RNAs (shRNAs) stably knocked out circ_0062020 (sh-circ_0062020), small interfering RNAs (siRNAs) specifically inhibited circ_0062020 (si-circ_0062020) expression, miR-615-5p inhibition (miR-615-5p inhibitor), miR-615-5p overexpression (miR-615-5p mimic), TRIP13 overexpression (pc-TRIP13), and the corresponding negative controls (sh-NC, si-NC, inhibitor NC, miRNA NC, pc-NC) were synthesized and provided by GenePharma (Shanghai, China). The transfection was performed by Lipofectamine2000 (Invitrogen) to DU145 and LNCaP cells according to the manufacturer’s protocol.
Clone Formation Assay
DU145 and LNCaP cells were transfected with si-circ_0062020 or si-NC and cultured in 6-well plates. Once the cells attached to petri dish, cells were divided into five groups and irradiated at 0, 2, 4, 6, and 8 Gy by Caesium-137 irradiator (MSD Nordion), respectively. After incubation for 24 h at 37°C with 5% CO2, cells were fixed with 600 μL of 4% paraformaldehyde (Sigma-Aldrich, St. Louis, MO, USA) for 20 min, and stained with crystal violet (Sigma-Aldrich) for 30 min. The colonies were counted and photographed (Carl Zeiss AG, Heidenheim, Germany) by an inverted microscope.
Cell Counting Kit 8 (CCK8) Assay
CCK8 assay was used to assess the viability of PCa cells by Cell Counting Kit-8 kit (Beyotime Biotechnology, Nantong, China). Briefly, cells were cultured in 96-well plates at a density of 2×103 per well with 2 Gy IR for 24 h. Then, cells were incubated with 10 μL CCK8 solution at 37°C with 5% CO2 for 2 h. A spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) was used to measure the absorbance at 450 nm.
Transwell Assay
The 24-well transwell chamber (8 μm pore size, Corning, Corning, NY, USA) with or without Matrigel (BD Biosciences; Bedford, MA, USA) was used to perform the invasion or migration assay correspondingly. Cells were suspended in DMEM (Gibco) medium without serum in the upper chamber, and DMEM (Gibco) together with FBS (PAN) were used to culture cells in the lower chamber. After being cultured for 2 h, cells were fixed with methanol (Sigma-Aldrich) for 30 min, and stained in crystal violet (Sigma-Aldrich) for another 30 min. An inverted microscope (×100) (CarlZeiss, Hallbergnoos, Germany) was used to count the number of cells with migration or invasion.
Wound Healing Assay
DU145 and LNCaP cells were seeded into 6-well plates with 2 Gy IR and cultured for 48 h to gain the adherent cells. A scratch wound was artificially made by 10 μL pipettes tips. The images were taken by an inverted microscope (×40) (Olympus, Tokyo, Japan) at the pre-determined time (0 h and 24 h) after scratch.
Flow Cytometry Assay
DU145 and LNCaP cells were cultured in RPMI 1640 medium (Gibco) with 2 Gy IR treatment. Annexin V-Fluorescein isothiocyanate (FITC)/Propidium iodide (PI) staining kit (BD Biosciences) was used to detect the apoptosis cells numbers of PCa cells following the manufacturer’s protocol. The result was determined by BD FACS Canto™ II flow cytometer (BD Biosciences).
Dual-Luciferase Reporter Assay
DU145 and LNCaP cells were cultured in 96-well plates for 24 h in prior of being co-transfected with dual-luciferase reporter vector and miR-615-5p mimic or miRNA NC using Lipofectamine 3000 (Invitrogen). For the construction of dual-luciferase reporter vectors, the sequences with the wide type or mutant type binding sites of circ_0062020 in miR-615-5p were respectively inserted into pMIR-Report vectors (Promega, Madison, WI, USA) and named as WT-circ_0062020 and MUT-circ_0062020. Similarly, WT-TRIP13-3ʹuntranslated region (WT-TRIP13-3ʹUTR) and MUT-TRIP13-3ʹUTR represents the pMIR-Report vectors (Promega) contained the sequences with wild type or mutant type binding sites of miR-615-5p in TRIP13 3ʹUTR. Finally, the luciferase activity was tested by a Dual-Luciferase® Reporter Assay System (Promega). Renilla luciferase activity served as an internal normalization of firefly activity values.
Western Blot Assay
WPMY-1, DU145 and LNCaP cells were lysed by RIPA buffer (Beyotime). The lysate were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE, Beyotime) and electrotransferred into the polyvinylidene difluoride membrane (PVDF, Millipore, Danvers, MA, USA). After washing by Phosphate Buffered Saline and Tween (PBST, Sigma-Aldrich) for 3 times, the membranes were blocked with 5% fat-free milk for 1 h, and then incubated with the primary antibodies and corresponding secondary antibody. The blots was visualized with the ECL Detection Reagents (Amersham Biosciences, Piscataway, NJ, USA). The primary antibodies were anti-GAPDH (1:2500, Cell Signaling Technologies, Danvers, MA, USA), anti-TRIP13 (1:200, Sigma-Aldrich), and the secondary antibody was anti-rabbit IgG (1:20, 000, Abcam, Cambridge, UK). GAPDH used as an internal control.
Xenograft Tumor Assay
The xenograft tumor assay was approved by the Experimental Animal Care Commission of Lianyungang Clinical College of Nanjing Medical University and performed by using LNCaP cells which were transfected with sh-circ_0062020 or sh-NC. Animal studies were performed in compliance with the ARRIVE guidelines and the Basel Declaration. All animals received humane care according to the National Institutes of Health (USA) guidelines. Twelve nude mice (male, 6 weeks old) were provided by Shanghai Laboratory Animal Co, Ltd. (Shanghai, China) and housed in an ideal sterile environment. Cells (2×106) transfected with sh-circ_0062020 or sh-NC were subcutaneously injected into the nude mice and divided into two groups. The nude mice were then housed for 28 days before being euthanatized for measuring the tumor weight. Tumor volume was recorded every 7 days.
Statistical Analysis
SPSS software (version 24.0 SPSS, Chicago, IL, USA) was applied to analyze data. All data were set of three replicates and presented by mean ± standard deviation (SD). The comparison between two or more groups was performed by Student’s t test or the one-way analysis of variance (ANOVA) test. P<0.05 was regarded as statistically significant.
Results
IR Treatment Induced the Downregulation of Circ_0062020 in PCa Cells
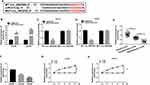
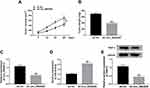
To investigate the role of circ_0062020 in PCa, the expression of circ_0062020 in PCa tissues were detected. The upregulation of circ_0062020 was detected in PCa tissues including radiosensitive and radioresistant tissues in contrast to adjacent normal tissues, especially in radioresistant tissues (Figure 1A). Meanwhile, the significant increase of circ_0062020 expression was also detected in PCa cells compared with normal cells (Figure 1B). Due to the marked increase of circ_0062020 expression in radioresistant PCa tissues compared with radiosensitive PCa tissues, the effect of IR on circ_0062020 expression was further explored in PCa cells. A noticeable decrease of circ_0062020 expression was detected in PCa cells with IR treatment (Figure 1C and D). Besides, we also found that the expression of circ_0062020 also associated with Gleason score, Tumor size, TNM stages and Lymphatic metastasis (Table 1). These findings illustrated that the upregulation of circ_0062020 could be restrained by IR treatment in PCa cells.
Circ_0062020 Knockdown Stimulated the Radiosensitivity in PCa Cells in vitro
Considering the relationship between circ_0062020 and IR treatment, the function of circ_0062020 was further investigated in IR-treated PCa cells. The noticeable decrease of circ_0062020 expression was detected in IR-treated PCa cells transfected with si-circ_0062020, which reflected the transfection efficiency of si-circ_0062020 (Figure 2A). Moreover, IR caused a dose-dependent decrease in viability in PCa cells, and the deficiency of circ_0062020 further suppressed the viability in IR-treated PCa cells (Figure 2B and C), which was coincided with the result of Figure 2D. Besides, the migration and invasion capacities of cells were inhibited by IR treatment, while si-circ_0062020 amplified this inhibition effect in PCa cells (Figure 2E–G). Meanwhile, circ_0062020 knockdown aggravated the promotion effect of apoptosis in PCa cells that induced by IR treatment (Figure 2H). These data indicated that circ_0062020 knockdown increased the radiosensitivity.
MiR-615-5p Participated in PCa Progression by Interacting with Circ_0062020
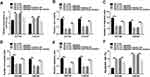
Bioinformatics analysis suggested the existence of presumptive binding sites of circ-0062020 in miR-615-5p (Figure 3A). To validate this assumption, dual-luciferase reporter assay was performed. The striking increase of miR-615-5p expression indicated the successful transfection of miR-615-5p mimic in PCa cells (Figure 3B). The significant decrease in luciferase activity in WT-circ_0062020 exposed the existence of combination between miR-615-5p and circ_0062020 in PCa cells (Figure 3C and D). Moreover, the expression of miR-615-5p was strikingly decreased in PCa tissues and cells compared with normal tissues and cells, especially in radioresistant PCa tissues (Figure 3E and F). In addition, IR treatment induced a time-dependent increase in miR-615-5p expression in PCa cells (Figure 3G and H). Thus, these data exposed that miR-615-5p was sponged by circ_0062020 and IR treatment elevated the expression of miR-615-5p in PCa cells.
Circ_0062020 Sponged miR-615-5p to Modulate the Radiosensitivity in PCa Cells
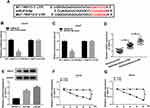
Given the interaction between miR-615-5p and circ-0062020, we further confirmed whether miR-615-5p participates in the regulating effect of circ-0062020 in PCa cells. Functionally, si-circ-0062020 led to a significant increase in miR-615-5p expression, which could be reversed by miR-615-5p inhibitor in IR-treated PCa cells (Figure 4A). Besides, miR-615-5p inhibitor could reverse the inhibition effects on proliferation, metastasis and the promotion effect on apoptosis that induced by si-circ-0062020 in IR-treated PCa cells (Figure 4B–F). These results highlighted that circ-0062020 regulated proliferation, metastasis, and apoptosis by sponging miR-615-5p in PCa cells.
MiR-615-5p Targeted TRIP13 3ʹUTR
The sequences containing predicted binding sites or matched mutated sites of TRIP13 3ʹUTR in miR-615-5p is shown in Figure 5A. Dual-luciferase reporter system was subsequently used to confirm the prediction. The decreased luciferase activity in cells with miR-615-5p mimic transfection was found in WT-TRIP13-3ʹUTR group, rather than MUT-TRIP13-3ʹUTR group (Figure 5B and C). TRIP13 mRNA was obviously increased in PCa tissues, especially in radioresistant PCa tissues, contrasted with normal tissues (Figure 5D). Meanwhile, the upregulation of TRIP13 protein level was detected in PCa cells (Figure 5E). Interestingly, IR treatment-induced a time-dependent suppression in TRIP13 mRNA expression in PCa cells (Figure 5F and G). These data supposed that miR-615-5p bound to TRIP13 3ʹUTR and the expression of TRIP13 was closely related to IR treatment.
MiR-615-5p Contributed to the Radiosensitivity by Targeting TRIP13 in PCa Cells
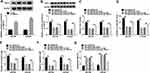
According to the relationship between miR-615-5p and TRIP13, we wondered whether miR-615-5p exerted its role by interacting with TRIP13. To test this assumption, gain-of-function assays were performed. Transfection of pc-TRIR13 led to an obvious increase in TRIP13 protein level in PCa cells (Figure 6A). MiR-615-5p mimic downregulated the protein level of TRIP13, however, this downregulation effect was reversed by pc-TRIP13 in IR-treated PCa cells (Figure 6B). Furthermore, miR-615-5p mimic induced suppression in proliferation, metastasis, and promotion in apoptosis, which could be relieved by transfecting pc-TRIP13 in IR-treated PCa cells (Figure 6C–G). These data manifested that miR-615-5p targeted to TRIR13 3ʹUTR to regulate TRIR13 expression and the radiosensitivity in PCa cells.
Circ_0062020 Sponged miR-615-5p to Regulate TRIR13 Expression
Given the relationship between miR-615-5p and circ_0062020 or TRIR13, the potential regulatory pathway was further explored in IR-treated PCa cells. As expected, miR-615-5p inhibitor partly reversed si-circ_0062020 induced restraint in TRIR13 protein expression (Figure 7A). Thus, circ_0062020/miR-615-5p/TRIR13 axis correlated with IR-treated PCa cells.
Circ_0062020 Knockdown Suppressed Tumor Growth in vivo
To further investigate the function of circ_0062020, LNCaP transfected with sh-circ_0062020 or sh-NC was injected into the nude mice to establish the transplantation tumor models of PCa. By measuring tumor volume and weight, the suppression effect of sh-circ_0062020 in tumor growth was fully exposed (Figure 8A and B). Meanwhile, sh-circ_0062020 triggered the repression effects on the expression of circ_0062020 and TRIR13 protein and the facilitation effect on miR-615-5p expression in vivo (Figure 8C–E). These findings further confirmed the carcinogenesis role of circ_0062020 in PCa.
Discussion
PCa as a malignant tumor, causes considerable pain to mental and physical health of males. Radiation, an efficient therapy, is widely applied in the treatment of different stages of PCa.20 Numerous studies have illustrated the underlying regulatory mechanism of RNAs in the radiosensitivity of PCa. Yang et al exposed that long noncoding RNA (lncRNA) GAS5 sponged miR-18a to regulate the radiosensitivity in PCa cells.21 The dysregulation of lncRNA UCA1 was involved in the radiosensitivity through interacting with Akt signal pathway in PCa cells.22 MiR-145 facilitated the radiosensitivity by inhibiting DNA repair pathway in PCa.23 However, the role of circRNAs in radiosensitivity in PCa has hardly been reported, which attracted us to further study. In this study, the expression of circ_0062020 was elevated in PCa tissues and cells, and was significantly increased in radioresistant PCa tissues compared with radiosensitive PCa tissues. Meanwhile, IR treatment-induced the inhibition of circ_0062020 expression in PCa cells. Besides, patients who high expressed circ_0062020 tend to have a larger size tumor, higher malignancy and worse prognosis. Functionally, circ_0062020 knockdown led to the repression of proliferation and metastasis, and the promotion of apoptosis in IR-treated PCa cells in vitro. CircRNA_100367 was upregulated, and this dysregulation induced the suppression in radiation sensitivity in ESCC cells.24 Has_circRNA_006660 was upregulated and sponged miR-1276 to increase the radiation sensitivity in nasopharyngeal carcinoma.25 The effect of circRNAs dysregulation on the radiation sensitivity of cancers remained disunited; however, the upregulation of circ_0062020 further contributed to the facilitation of radiation sensitivity in PCa.
In the sense of the regulatory pathway of circRNAs in cancers, circRNAs/micro RNAs (miRNAs)/message RNAs (mRNAs) axis was the principal route of research. For example, circATP8B4 was documented to restrain the radiation sensitivity by sponging miR-776 in glioma.26 CircVRK1 enhanced the radiosensitivity by miR-624-3p/PTEN/PI3K/Akt pathway in esophageal cancer.27 In our research, the interaction of circ_0062020 on miR-615-5p was not just mechanically embodied in the existence of combination, but also functionally reflected in the reversion effect of miR-615-5p inhibitor on si-circ_0062020-induced inhibition in proliferation and metastasis, promotion in apoptosis in IR-treated PCa cells. Circ_VCAN harbored miR-1183 to repress the proliferation, metastasis and facilitate apoptosis in IR-treated glioma cells.28 Similarly, has_circ_0001313 inhibited the radiosensitivity by regulating miR-338-3p in colon cancer cells.29 MiR-615-5p was a vital media for circ_100146 to participate in non-small cell lung cancer progression.30 In addition, miR-615-5p bound to TRIP13, which was further exposed in the abirritation of pc-TRIP13 to miR-615-5p mimic-mediated suppression in proliferation and metastasis, facilitation in apoptosis in IR-treated PCa cells. TRIP13 was targeted by miR-515-5p to defer the development of PCa.31 Circ_LAMP1 harbored miR-615-5p to modulate DDR2 expression to accelerate the process of T-cell lymphoblastic lymphoma.32 Moreover, circ_0062020 inhibited the radiosensitivity by miR-615-5p/TRIP13 pathway in PCa cells. Besides, the carcinogenesis of circ_0062020 was further verified in vivo.
To sum up, the downregulation of circ_0062020 was associated with the inhibition of radiosensitivity in PCa tissues and cells. Circ_0062020 inhibition restrained PCa tumor growth in vivo and suppressed the proliferation and metastasis, triggered the apoptosis in IR-treated PCa cells in vitro. Furthermore, circ_0062020 regulated radiosensitivity by miR-615-5p/TRIP13 axis in PCa cells. Our data highlighted the regulatory pathway of circ_0062020 in the radiosensitivity of PCa cells, which might contribute to the improvement of therapeutic approach of PCa.
Funding
This work was supported by Chengdu Municipal Health Commission Project (No: 2019107).
Disclosure
The authors declare that they have no financial or non-financial conflicts of interest for this work.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. de Bono JS, Guo C, Gurel B, et al. Prostate carcinogenesis: inflammatory storms. Nat Rev Cancer. 2020. doi:10.1038/s41568-020-0267-9
3. Cooperberg MR, Vickers AJ, Broering JM, Carroll PR. Comparative risk-adjusted mortality outcomes after primary surgery, radiotherapy, or androgen-deprivation therapy for localized prostate cancer. Cancer. 2010;116(22):5226–5234. doi:10.1002/cncr.25456
4. Cooperberg MR, Broering JM, Carroll PR. Time trends and local variation in primary treatment of localized prostate cancer. J Clin Oncol. 2010;28(7):1117–1123. doi:10.1200/JCO.2009.26.0133
5. Filson CP, Marks LS, Litwin MS. Expectant management for men with early stage prostate cancer. CA Cancer J Clin. 2015;65(4):265–282. doi:10.3322/caac.21278
6. Rodrigues DN, Butler LM, Estelles DL, de Bono JS. Molecular pathology and prostate cancer therapeutics: from biology to bedside. J Pathol. 2014;232(2):178–184. doi:10.1002/path.4272
7. D’Amico AVMJ, Loffredo M, Renshaw AA, DellaCroce A, Kantoff PW. 6-Month Androgen Suppression Plus Radiation Therapy vs Radiation Therapy Alone for Patients With Clinically Localized Prostate Cancer. JAMA. 2004;292(7):821–827.
8. Al-Mamgani A, Heemsbergen WD, Peeters ST, Lebesque JV. Role of intensity-modulated radiotherapy in reducing toxicity in dose escalation for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2009;73(3):685–691. doi:10.1016/j.ijrobp.2008.04.063
9. Xia T, Pan Z, Zhang J. CircSMC3 regulates gastric cancer tumorigenesis by targeting miR-4720-3p/TJP1 axis. Cancer Med. 2020. doi:10.1002/cam4.3057
10. Chen H, Liu S, Li M, Huang P, Li X. <p>circ_0003418 Inhibits Tumorigenesis And Cisplatin Chemoresistance Through Wnt/β-Catenin Pathway In Hepatocellular Carcinoma. Onco Targets Ther. 2019;12:9539–9549. doi:10.2147/OTT.S229507
11. Zhang Z, Lin W, Gao L, et al. Hsa_circ_0004370 promotes esophageal cancer progression through miR-1294/LASP1 pathway. Biosci Rep. 2019;39(5). doi:10.1042/BSR20182377
12. Dong Y, Xu T, Zhong S, et al. Circ_0076305 regulates cisplatin resistance of non-small cell lung cancer via positively modulating STAT3 by sponging miR-296–5p. Life Sci. 2019;239:116984. doi:10.1016/j.lfs.2019.116984
13. Memczak S, Papavasileiou P, Peters O, Rajewsky N, Pfeffer S. Identification and Characterization of Circular RNAs As a New Class of Putative Biomarkers in Human Blood. PLoS One. 2015;10(10):e0141214. doi:10.1371/journal.pone.0141214
14. XD YT W, Liu CL. Circular RNA_LARP4 inhibits cell migration and invasion of prostate cancer by targeting FOXO3A. Eur Rev Med Pharmacol Sci. 2020;24:5303–5309.
15. Wang X, Wang R, Wu Z, Bai BP. Circular RNA ITCH suppressed prostate cancer progression by increasing HOXB13 expression via spongy miR-17-5p. Cancer Cell Int. 2019;19(1):328. doi:10.1186/s12935-019-0994-8
16. Song Z, Zhuo Z, Ma Z, Hou C, Chen G, Xu G. Hsa_Circ_0001206 is downregulated and inhibits cell proliferation, migration and invasion in prostate cancer. Artif Cells Nanomed Biotechnol. 2019;47(1):2449–2464. doi:10.1080/21691401.2019.1626866
17. Feng Y, Yang Y, Zhao X, et al. Circular RNA circ0005276 promotes the proliferation and migration of prostate cancer cells by interacting with FUS to transcriptionally activate XIAP. Cell Death Dis. 2019;10(11):792. doi:10.1038/s41419-019-2028-9
18. Shan G, Shao B, Liu Q, et al. circFMN2 Sponges miR-1238 to Promote the Expression of LIM-Homeobox Gene 2 in Prostate Cancer Cells. Mol Ther Nucleic Acids. 2020;21:133–146. doi:10.1016/j.omtn.2020.05.008
19. Jin C, Zhao W, Zhang Z, Liu W. Silencing circular RNA circZNF609 restrains growth, migration and invasion by up-regulating microRNA-186-5p in prostate cancer. Artif Cells Nanomed Biotechnol. 2019;47(1):3350–3358. doi:10.1080/21691401.2019.1648281
20. Martin NE, D’Amico AV. Progress and controversies: radiation therapy for prostate cancer. CA Cancer J Clin. 2014;64(6):389–407. doi:10.3322/caac.21250
21. Yang J, Hao T, Sun J, Wei P, Zhang H. Long noncoding RNA GAS5 modulates α-Solanine-induced radiosensitivity by negatively regulating miR-18a in human prostate cancer cells. Biomed Pharmacother. 2019;112:108656. doi:10.1016/j.biopha.2019.108656
22. Ghiam AF, Taeb S, Huang X, et al. Long non-coding RNA urothelial carcinoma associated 1 (UCA1) mediates radiation response in prostate cancer. oncotarget. 2017;8(3):4668–4689. doi:10.18632/oncotarget.13576
23. Gong P, Zhang T, He D, Hsieh J-T. MicroRNA-145 Modulates Tumor Sensitivity to Radiation in Prostate Cancer. Radiat Res. 2015;184(6):630–638. doi:10.1667/RR14185.1
24. Junqi Liu J, Xue N, Guo Y, Niu K, Gao L, Zhang S. Di Zhao, Ruitai Fan. CircRNA_100367 regulated the radiation sensitivity of esophageal squamous cell carcinomas through miR-217/Wnt3 pathway. AGING. 2019;11(24):12412–12427. doi:10.18632/aging.102580
25. Zhu D, Shao M, Yang J, et al. Curcumin Enhances Radiosensitization of Nasopharyngeal Carcinoma via Mediating Regulation of Tumor Stem-like Cells by a CircRNA Network. J Cancer. 2020;11(8):2360–2370. doi:10.7150/jca.39511
26. Zhao M, Xu J, Zhong S, et al. Expression profiles and potential functions of circular RNAs in extracellular vesicles isolated from radioresistant glioma cells. Oncol Rep. 2019;41(3):1893–1900. doi:10.3892/or.2019.6972
27. He Y, Mingyan E, Wang C, Liu G, Shi M, Liu LS. CircVRK1 regulates tumor progression and radioresistance in esophageal squamous cell carcinoma by regulating miR-624-3p/PTEN/PI3K/AKT signaling pathway. Int J Biol Macromol. 2019;125:116–123. doi:10.1016/j.ijbiomac.2018.11.273
28. Zhu C, Mao X, Zhao H. The circ_VCAN with radioresistance contributes to the carcinogenesis of glioma by regulating microRNA-1183. Medicine. 2020;99(8):e19171. doi:10.1097/MD.0000000000019171
29. Wang L, Peng X, Lu X, Wei Q, Chen M, Liu L. Inhibition of hsa_circ_0001313 (circCCDC66) induction enhances the radio-sensitivity of colon cancer cells via tumor suppressor miR-338-3p: effects of cicr_0001313 on colon cancer radio-sensitivity. Pathol Res Pract. 2019;215(4):689–696. doi:10.1016/j.prp.2018.12.032
30. Chen L, Nan A, Zhang N, et al. Circular RNA 100146 functions as an oncogene through direct binding to miR-361-3p and miR-615-5p in non-small cell lung cancer. Mol Cancer. 2019;18(1):13. doi:10.1186/s12943-019-0943-0
31. Zhang X, Zhou J, Xue D, Li Z, Liu Y, Dong L. MiR-515-5p acts as a tumor suppressor via targeting TRIP13 in prostate cancer. Int J Biol Macromol. 2019;129:227–232. doi:10.1016/j.ijbiomac.2019.01.127
32. Deng L, Liu G, Zheng C, Zhang L, Kang Y, Yang YF. Circ-LAMP1 promotes T-cell lymphoblastic lymphoma progression via acting as a ceRNA for miR-615-5p to regulate DDR2 expression. Gene. 2019;701:146–151. doi:10.1016/j.gene.2019.03.052
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.