Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 17
Chronic Obstructive Pulmonary Disease Prevalence and Associated Risk Factors in Adults Aged 40 Years and Older in Southeast China: A Cross-Sectional Study During 2019–2020
Authors Chen J , Yin Y, Zhang Y, Lin X, Chen T, Yang Z, Wang D, Zhong W
Received 14 June 2022
Accepted for publication 9 September 2022
Published 17 September 2022 Volume 2022:17 Pages 2317—2328
DOI https://doi.org/10.2147/COPD.S377857
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Jingyu Chen, Yanrong Yin, Yefa Zhang, Xiuquan Lin, Tiehui Chen, Ze Yang, Dengwei Wang, Wenling Zhong
Fujian Provincial Center for Disease Control and Prevention, Fuzhou, Fujian Province, People’s Republic of China
Correspondence: Wenling Zhong, Fujian Provincial Center for Disease Control and Prevention, No. 386 Chong’an Road, Jin’an District, Fuzhou, Fujian Province, 350012, People’s Republic of China, Tel +86-591-87539007, Email [email protected]
Purpose: Chronic obstructive pulmonary disease (COPD) is one of many major public health problems in China, and its prevalence and associated risk factors in the southeast of China need to be determined to facilitate disease control and prevention.
Methods: A multistage stratified cluster sampling method was used to select 5486 participants aged ≥ 40 years from nine COPD monitoring districts in Fujian Province during 2019– 2020. Participants were interviewed using a laptop-based questionnaire and underwent pulmonary function tests. COPD was diagnosed according to the 2019 Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria.
Results: Final analysis was conducted using data from 4999 participants with qualified post-bronchodilator results. The prevalence of COPD was 11.6% (95% confidence interval [CI]: 10.5– 12.7). Risk factors for COPD in the logistic regression model were being male (odds ratio [OR] = 2.83, 95% CI: 2.01– 3.98), > 70 years old (OR = 16.16, 95% CI: 8.14– 32.08), having a low body mass index (BMI) (OR = 1.81, 95% CI: 1.13– 2.89), parental history of respiratory disease (OR = 1.78, 95% CI: 1.50– 2.10), being a current (OR = 2.82, 95% CI: 1.83– 4.36) or former (OR = 2.47, 95% CI: 1.45– 4.19) smoker, and indoor exposure to biomass (OR = 1.28, 95% CI: 1.05– 1.58).
Conclusion: The estimated prevalence of COPD in southeast China is high. COPD was strongly associated with sex, aging, a low BMI, parental history of respiratory diseases, smoking, and indoor exposure to biomass in adults aged ≥ 40 years. The government should urgently implement comprehensive measures to reduce the risk factors for COPD.
Keywords: COPD, logistic models, biomass, public health, smoking, surveys and questionnaires
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by airflow limitation and persistent respiratory symptoms and common among people aged ≥ 40 years.1 In 2019, the World Health Organization stated that COPD was the third-leading cause of death, with 3.23 million deaths worldwide.2 The prevalence of COPD was estimated as 10.3%, with 391 million patients globally, and > 80% of cases lived in developing countries and areas.3 The prevalence of COPD in China was estimated as 13.7%, equating to approximately 99.9 million patients with COPD.4 With increases in life expectancy and lifestyle changes, COPD will continue to be a significant cause of morbidity and mortality, with serious economic and social burden impacts on public health.
Although COPD is a preventable and treatable disease, a large percentage of patients in developing countries and areas continue to be underdiagnosed, and unaware of COPD is.3,5 Cough, phlegm production, and breathlessness are the main symptoms of COPD;1 however, patients with COPD often do not recognize that their symptoms are signs of disease and consequently do not seek medical help. Many patients are diagnosed without being given any information about the disease, and most health information systems and clinics do not pay sufficient attention to COPD. Underestimation of COPD prevalence is common in some rural areas of China;3 therefore, the diagnosis, outpatient treatment, and admission rates of patients with COPD are extremely low.6
As COPD is a relatively neglected disease, there is an urgent need to collect data to assist in understanding the dynamic trends and various risk factors associated with the condition in different regions of China, to promote specific prevention and early screening. To help fill the evidence gap, we conducted a survey, with the aim of including a large population, and used spirometry and quality-assured studies to estimate the prevalence of COPD and its associated risk factors in southeast China. In our conclusions, we recommend that the government take effective action and implement preventive measures in a timely manner.
Methods
Study Design
The participants in this cross-sectional study were aged ≥ 40 years and from local residences in Fujian. A complex, multistage stratified cluster random sampling method was used to select a representative sample population (Figure 1). Nine COPD monitoring districts located in nine administrative regions in Fujian Province were randomly selected, to obtain a representative sample of the Fujian provincial population. The nine COPD monitoring districts were: Taijiang District in Fuzhou, Jiaocheng District in Ningde, Licheng District in Putian, Fengze District and Jinjiang city in Quanzhou, Ninghua County in Sanming, Jian’ou County in Nanping, Zhangpu County in Zhangzhou, and Liancheng County in Longyan.
 |
Figure 1 Flowchart of multistage stratified cluster random sampling. COPD, chronic obstructive pulmonary disease; PPS, probability proportional sampling. |
A probability proportional sampling method was applied. First, three rural towns/urban streets in each monitoring district, including 27 rural towns/urban streets, were randomly selected. Second, in each rural town/urban street, two rural villages/urban neighborhood committees, which included 54 rural villages/urban neighborhood committees, were randomly selected. Subsequently, in each rural village/urban neighborhood committee, 150 households that included residents aged ≥ 40 were randomly selected. Finally, the Kish grid method was used to select one of family member aged ≥ 40 years from each of the 100 households as eligible participants in this study.
Participants
Participants in this study were permanent Chinese residents aged ≥ 40 years who had lived in the monitoring districts for more than six months before this survey. Exclusion criteria were: (1) lived in a communal residence, for example, a university dormitory, military, or nursing home; (2) were pregnant or lactating women; (3) had any mental illness, chronic disease, or physical disability that prevented them from completing the questionnaire and spirometry function tests; (4) did not provide written informed consent. Additionally, individuals with a resting heart rate > 100 bpm and those who were allergic to salbutamol were not permitted to undergo the post-bronchodilator examination.
Ethics Statement
We confirm that our study complied with the Declaration of Helsinki. As a part of the national COPD surveillance in China, the protocol of this survey was approved by the Ethics Review Committee of the National Center for Chronic and Non-communicable Disease Control and Prevention (No. 201901). All participants signed written informed consent before completing the survey.
Survey Procedures
Each eligible adult participated in a face-to-face interview. Trained medical staff from local centers for disease control and prevention (CDCs) and health centers administered a laptop-based questionnaire, designed by the Chinese CDC, to collect the data, which included personal demographic characteristics, personal and family respiratory disease history, respiratory symptoms, and risk factors related to COPD.7
After the interview, trained medical staff measured the body weight, height, and blood pressure of participants. Height was measured without shoes, up to a range of 2 m, and recorded to the nearest 0.1 cm. Weight was measured with participants wearing light clothes, using a scale with a maximum capacity of 150 kg, and recorded to the nearest 0.1 kg. After at least 5 min of rest, participant blood pressure was measured three times. Subsequently, BMI was calculated using the formula BMI = weight (kg)/height2 (m)2.
Trained medical staff conducted spirometry function tests on all eligible participants using the same brand of digital portable spirometer (MasterScreen Pneumo; CareFusion, Jaeger, Höchberg, Germany), based on GOLD guidelines.1 Before participants underwent the spirometry function tests, the exhale and inhale procedures were explained to them, and they practiced blowing several times to achieve the criteria. Three acceptable measurements with the three highest values in the spirometry function tests were considered the best records for inclusion in the final data analysis.
After the spirometry function tests, a trained physician or nurse gave the participants a total dose of 400 μg of salbutamol (Ventolin, GlaxoSmithKline, Middlesex, UK) for inhalation. Spirometry was repeated within 15 to 25 min after the last spray of salbutamol. The parameters, forced expiratory volume in 1 s (FEV1) and forced vital capacity (FVC) were recorded in both pre-bronchodilator and post-bronchodilator examinations.
After completing the lung function tests, spirometer reports were checked for acceptability and repeatability by the trained physician performing the tests, and again by a senior pulmonary function technician.7 To obtain acceptable curves and repeatable data from the lung function test results, quality assessments of lung function reports were conducted for each participant within 24 h. The qualified rates for pre-bronchodilator and post-bronchodilator examinations were 97.84% (5107/5128) and 97.87% (4999/5108), respectively.
Study Variables
Outcome Variables
In accordance with the 2019 GOLD guidelines, all eligible participants underwent post-bronchodilator pulmonary function tests to diagnose COPD.1 COPD was diagnosed in patients with post-bronchodilator FEV1/FVC < 0.70, combined with exposure to risk factors and respiratory symptoms, such as chronic cough, expectoration, and dyspnoea.1 Participants with a post-bronchodilator of FEV1/FVC < 0.70 were offered extra chest radiography, to exclude other pulmonary diseases.
Independent Variables
Analyzed independent variables were demographic characteristics, including: sex (male and female), age group (40–49, 50–59, 60–69, and ≥ 70 years), educational status (primary school and below, secondary school, college and above), residence (urban/rural), BMI (underweight, normal, overweight, obese), and parental history of chronic respiratory diseases (yes/no). Environmental risk factors assessed included: smoking (never smoked, former smoker, current smoker), cooking area (outdoor, separate room, living room), kitchen ventilation (yes/no), indoor exposure to biomass for cooking or heating (yes/no), and exposure to dust or chemicals in the workplace (yes/no).
Statistical Analysis
Standardized COPD prevalence with 95% confidence interval (CI) was estimated for the overall population. Sample weight was calculated using a stratified, complex cluster sampling method based on data from the 2010 census of the Chinese population aged ≥ 40 years. The Rao–Scott chi-square test was applied to compare the prevalence of COPD between chosen subgroups. Survey logistic regression was applied to assess the relationships between the prevalence rate of COPD and relevant risk factors. Statistical significance was set at P < 0.05 (two-sided). Data were analyzed using SAS version 9.4.
Results
The study was conducted from September 2019 to December 2020 in Fujian Province, China.
A total of 5486 adults (2844 men/2642 women) were enrolled in the study, and 4999 adults (2589 men/2410 women) completed all the required procedures. The response rate was 91.12%. A total of 487 adults (255 men/232 women) were excluded, 358 due to contraindications in the spirometry function examination, 20 due to completing only the pre-bronchodilator test, and 109 due to unqualified pulmonary function examinations (Figure 2).
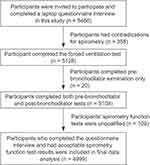 |
Figure 2 Flowchart of study participant selection. |
Analysis of the excluded population (n = 487) to investigate whether it differed from the population included in the final data analysis demonstrated that included adults were younger (mean age 58.1 vs 62.3 years, P < 0.01), had a higher educational status (36.2% vs 30.4%, P = 0.02), were more likely to have a BMI ≥ 24.0 kg/m2 (49.6% vs 38.3%, P < 0.01), and were more likely to use kitchen ventilation (82.0% vs 77.6%, P = 0.03). The distributions of sex, residence, and other factors did not differ significantly between included and excluded subjects (all P > 0.05, Table 1).
 |
Table 1 Comparison of Included and Excluded Participants in Analysis* |
Descriptive Analysis
Using a multistage stratified sampling weight formulae, the evaluated sample size was equivalent to a population of 8,958,825 in Fujian Province. The mean age of the total included population was 58.1 years (range of 40–94 years), of which 40–49, 50–59, 60–69, and ≥ 70-year-old age groups constituted 22.9%, 33.2%, 31.7%, and 12.3%, respectively. The majority of participants were male (53.5%), 67.8% had received a primary school education and below, 55.2% lived in urban areas, 48.7% had a normal BMI, 83.9% did not have a parental history of respiratory disease, 57.6% had never smoked, 95.6% cooked in a separate room, 80.8% used kitchen ventilation, 77.6% were not exposed to indoor biomass fuels for cooking or heating, and 63.4% were not exposed to dust or chemicals in the workplace. The demographic information and risk factors of participants included in the final analysis are presented in Table 2.
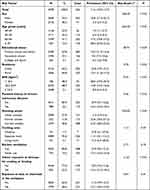 |
Table 2 Characteristics and Risk Factors for COPD in the Population of Southeast China During 2019–2020 |
Univariate Analysis
Among the 4999 eligible participants, 542 were defined as patients with COPD, based on the 2019 GOLD guidelines. Under the complex sampling weighting estimation, the prevalence of COPD in adults aged ≥ 40 years in southeast China was estimated as 11.6% (95% CI: 10.5–12.7). The prevalence of COPD was significantly higher in men (18.8%, 95% CI: 17.0–20.7) than in women (3.3%, 95% CI: 2.5–4.2) (P < 0.01). Further, the prevalence of COPD rose significantly with increasing age from 1.9% (95% CI: 1.1–2.7) in the 40–49-year age group to 28.6% (95% CI: 23.8–33.3) in the ≥ 70 years group (P < 0.01). More specifically, when the different characteristics and risk factors of participants with and without COPD were compared, the distributions of sex, age group, educational status, residence, BMI, parental history of respiratory diseases, smoking, and indoor exposure to biomass for cooking or heating differed significantly (all P < 0.05). The results of univariate analysis of risk factors and categories are shown in Table 2.
Survey Logistic Regression Model
Next, we identified six factors that are potential risk factors for COPD in southeast China by adjusted analysis using a multiple logistic regression model. Specifically, individuals aged 50–59 years (OR = 3.41, 95% CI: 1.94–5.99), 60–69 years (OR = 8.91, 95% CI: 5.23–15.17), and ≥ 70 years (OR = 16.16, 95% CI: 8.14–32.08) were more likely to develop COPD than participants aged 40–49 years. Additionally, male sex (OR = 2.83, 95% CI: 2.01–3.98), a lower BMI (OR = 1.81, 95% CI: 1.13–2.89), a parental history of respiratory disease (OR = 1.78, 95% CI: 1.50–2.10), being a current (OR = 2.82, 95% CI: 1.83–4.36) or former (OR = 2.47, 95% CI: 1.45–4.19) smoker, and indoor exposure to biomass for cooking or heating (OR = 1.28, 95% CI: 1.05–1.58) were significantly associated with development of COPD (all P < 0.05) and were regarded as substantial risk factors for COPD in the southeast of China (Table 3).
 |
Table 3 Associations Between Risk Factors and COPD Determined Using a Logistic Regression Model |
Discussion
This survey was conducted using a large and random sample of a local population aged ≥ 40 years to assess the provincial representative prevalence and risk factors associated with COPD in southeast China. Using the 2019 GOLD definition for diagnosing COPD by spirometry, the prevalence of COPD in Fujian Province was 11.6% (95% CI: 10.5–12.7) during 2019−2020, compared with two other large national representative studies in China in 2015, where prevalence rates were estimated as 13.6% (95% CI: 12.0–15.2) and 13.7% (95% CI: 12.1–15.5), respectively.4,7 The prevalence rates of COPD in Sichuan Province (25.4%, 95% CI: 24.0–26.9), Chongqing City (18.7%, 95% CI: 16.7–20.6),7 and 17.01% in Kashi Region8 were higher than that in Fujian Province; however, the prevalence rates in Jiangsu (11.9%)9 and Jiangxi (10.6%) Provinces,10 which are similar geographic areas close to Fujian Province, were similar and slightly lower, and those in Anhui Province (9.8%),11 Hebei Province (9.6%)12 and Inner Mongolia Autonomous Region (9.3%)13 were lower still. Our study also confirms that COPD has a high prevalence in southeastern China, indicating that it represents a serious public health burden.
Risk factors for COPD may differ around the world, due to individual circumstances, living environments, living standards, and lifestyle choices. Being diagnosed with COPD has been established to be the result of interactions between genetic and environmental factors for many years.1,14 The risk factors identified in our study, including age, sex, weight, parental history of respiratory disease, smoking, and indoor biomass exposure, have previously been identified as substantial risk factors for COPD.1,3 Understanding the impacts of different risk factors leading to COPD at different life stages and by various mechanisms will be necessary to prevent this chronic respiratory disease.
Several previous studies have demonstrated that the prevalence of COPD is significantly higher in males than females, with a rising trend in prevalence as individuals grow older, and these findings were confirmed in our survey.3,4,7 A reasonable explanation for this is that more males smoked and were occasionally exposed to dust and chemicals in their daily lives. In China, 74.3% of men have smoked compared to 3.2% of women,15 and cigarette smoking is currently regarded as the most significant risk factor for COPD.1,16 A recent study found that the smoking rate in the whole population of mainland of China was approximately 25.1% (95% CI: 23.4–26.8%), comprising 47.6% (95% CI: 44.6–50.5) in men and 1.9% (95% CI: 1.3–2.6) in women.17 Unfortunately, almost half of current smokers were dependent on tobacco (males: 49.7%/females: 50.8%), and tobacco dependence was strongly associated with aging. Hence, male sex and aging are two significant risk factors associated with smoking, leading to a high prevalence of COPD in older men.
We found that the prevalence of COPD was 21.0% in current smokers, 22.8% in former smokers, and 4.3% in those who had never smoked (Table 2). Similarly, Fang et al reported a prevalence of COPD of 20.4% in current smokers, 22.6% in former smokers, and 8.7% in those who had never smoked.7 Hence, there is a significant relationship between smoking status and the prevalence of COPD. Controversially, although quitting smoking early can result in additional health benefits,14 former smokers appear to exhibit a higher prevalence of COPD compared to current smokers. One reasonable explanation for this phenomenon is that former smokers may have other chronic health problems; therefore, such patients may be more likely to take medical advice to quit smoking, due to heightened awareness of the risks associated with smoking.15 Most cigarette smokers are unaware of the serious health implications of the early symptoms of disease and do not want to give up smoking until they develop a serious health problem.15 Additionally, we observed that the prevalence of COPD among both current (OR = 2.82, 95% CI: 1.83–4.36) and former (OR = 2.47, 95% CI: 1.45–4.19) smokers was more than twice that in never smokers, consistent with the findings of previous studies.4,7,18
Other than cigarette smoking, indoor exposure to biomass for cooking or heating (OR = 1.28, 95% CI: 1.05–1.58) is a risk factor because of the effects of indoor air pollutants, which lead to the development of COPD.3,6,16,19,20 The complex mixture of carbon-based particles and irritant gases produced by inefficient burning can lead to airway damage and result in the development of COPD.1 Low-priced and easily available indoor biomass fuels, for example, wood, crops, and animal dung, are widely used in low- and medium-income countries for cooking and heating, which affects approximately 2.8 billion people globally and is a leading risk factor for COPD.21 In particular, the risks of using indoor biomass for cooking or heating vary in Africa (OR = 3.19), Asia (OR = 2.88), Europe (OR = 2.30), and South America (OR = 2.15).22 Moreover, the use of indoor biomass fuels may increase the possibility of developing chronic lung diseases by 30%, as well as elevating the possibility of respiratory disease exacerbation by up to 95%.23
We found that low BMI was significantly associated with COPD prevalence in the southeast of China (OR = 1.81, 95% CI: 1.13–2.89), consistent with the results of previous studies.3,4,7,9 As this was a cross-sectional study, we cannot attribute causality, but speculate that malnutrition may affect the structure and exercise capacity of respiratory muscle, increasing the likelihood of muscle fatigue and leading to ventilation dysfunction.8,24–26 A lack of protein can also impact immunoglobulin and complement production, leading to decreased immune function, increasing the likelihood of infection, and aggravating respiratory dysfunction among the underweight population.25–27 These factors may contribute to the association between low BMI and a higher prevalence of COPD.
Our data also indicate that individuals with a parental history of respiratory disease were more likely to have COPD (OR = 1.78, 95% CI: 1.50–2.10). Similar to findings worldwide, individuals with a parental history of obstructive airway disease are at risk of COPD both in low- and middle-income (OR = 1.7, 95% CI:1.5–2.0) and high-income (OR = 1.5, 95% CI: 1.1–2.0) countries.3 In two Chinese national surveys with large sample sizes, family history of respiratory disease was significantly associated with increased risk of developing COPD in mainland China, including Anhui and Hebei Province.4,7,11,12 Genetic polymorphisms associated with familial clustering are related to a higher risk of COPD.28 Further, living with family members who adopt COPD risk behaviors (such as smoking) in childhood may influence the prevalence of COPD in adulthood.29,30
An estimated 14% of COPD worldwide is attributable to occupational exposures.31 A significant relationship between COPD prevalence and occupational exposure was observed in a national study, as well as in Jiangsu, Jiangxi, and Hebei Provinces;7,9,10,12 however, we did not detect a significant association with occupational exposure in our study, consistent with findings from Anhui Province and the Inner Mongolia Autonomous Region.11,13 Industrial structures, occupational components, and protective measures vary among different areas and types of work. Production methods have improved in recent years, and workers understand how to protect themselves from occupational dust in the workplace, which may explain our findings.
In addition, we did not find significant associations between COPD prevalence and educational status or residential area, possibly because the majority of participants had low educational status and lived in urban areas. The lifestyle gap between rural and urban areas of Fujian Province may be diminishing due to economic development; however, educational status can directly impact the occupation and lifestyle of an individual and can be a predictor of COPD.32,33 Individuals with a higher educational level may have higher socioeconomic status; therefore, they might find it easier to access health knowledge and medical care, as they have higher self-management skills.34 Further in-depth research is needed to confirm this hypothesis.
Our study had several strengths: it was the first and largest epidemiological population survey of a provincial representative sample for adults aged ≥ 40 years to estimate the prevalence of COPD and relevant risk factors in the southeast of China. Additionally, we used a questionnaire with a laptop-based data collection system, which could promptly record participant responses and support quality control. Furthermore, pre-bronchodilator and post-bronchodilator lung function were measured using a high-quality assessment, with respiratory symptoms and risk factors considered in COPD diagnosis in routine clinical institutions in this large epidemiological investigation.
Nevertheless, our study also had several limitations. First, the cross-sectional design meant that we could not confirm causal inferences. Second, as the interview used a self-reported questionnaire, our findings may have been influenced by recall bias of personal memory. Furthermore, we did not consider outdoor air pollution and traffic-related exposure in the design stage, which could be relevant factors influencing COPD prevalence. The target population was ≥ 40 years old; therefore, data were not collected from individuals < 40 years. Finally, we applied two definitions for COPD, with different cut-off values: one was the GOLD criteria and the other used the lower limit of normal for differential diagnosis; therefore, COPD prevalence may have been overestimated in the elderly population and underestimated in the younger population.3
Recommendations
Reductions in exposure to the risk factors for COPD in daily life are required. The most effective strategy for reducing the COPD disease burden is reported to be supporting smoking cessation,35 which will reduce the risk of COPD and can also lower the risks of cardiovascular disease, diabetes, and cancers.7 To reduce tobacco consumption, many comprehensive social efforts have been made by physicians and health workers, as well as counseling and support groups for smoking cessation to reduce tobacco dependence. Furthermore, the government should promote tobacco control legislation, increase the price of cigarettes, endorse anti-smoking campaigns on different media, and improve smoking cessation support. Additionally, efforts should be made to decrease indoor exposure to biomass fuels and use cleaner fuels and new technology when cooking or heating in rural areas, and to guide policy and interventions to improve public health.16,19
Finally, the lack of awareness and underestimation of COPD needs to be addressed urgently. The Chinese central government issued a national health policy, Healthy China 2030, aiming to improve the awareness of COPD among the Chinese population and reach a 20% target of lung function tests among the population aged ≥ 40 years by 2030.36 Using a spirometer to screen older adults and populations at high risk of developing COPD should be incorporated into primary care provision.
Conclusion
In conclusion, COPD was prevalent among adults in southeastern China in 2019–2020. Smoking and indoor exposure to biomass for cooking or heating were the main risk factors associated with COPD. More comprehensive measurements of COPD risk factors should be urgently undertaken to reduce the potential disease burden on the public in the future.
Abbreviations
BMI, Body Mass Index; CDC, Center for Disease Control and Prevention; CI, confidence interval; COPD, Chronic Obstructive Pulmonary Disease; FEV1, Forced Expiratory Volume in 1s; FVC, Forced Vital Capacity; GOLD, Global Initiative for Chronic Obstructive Lung Disease; OR, Odds Ratio; PPS, Probability Proportional Sampling; WHO, World Health Organization.
Data Sharing Statement
The datasets used during the current study are available from the corresponding author on reasonable request.
Acknowledgments
The authors would like to thank all the health staff members in CDC and health institutions joined in this survey in nine COPD monitoring districts in Fujian Province. Special thanks to all the study individuals who agreed to participate in this study. We also thank Chronic Disease Center of China CDC for supporting this research.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
This work was supported by grants from Fujian Province Pilot Project (2020Y0060), Scientific and Technology Innovation Platform Construction of Fujian Province (2019Y2001), Fujian Provincial Health Youth Project (2017-2-13), Fujian Provincial Center for Disease Control and Prevention Science and Technology Project. The funder of this survey had no role in study design, data collection, data analysis, data interpretation or writing this manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease; 2019. Available from: https://goldcopd.org/wp-content/uploads/2018/11/GOLD-2019-v1.7-FINAL-14Nov2018-WMS.pdf.
2. WHO. Chronic obstructive pulmonary disease (COPD); 2022. Available from: https://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd).
3. Adeloye D, Song P, Zhu Y, et al. Global, regional, and national prevalence of, and risk factors for chronic obstructive pulmonary disease (COPD) in 2019: a systematic review and modelling analysis. Lancet Respir Med. 2022;10:447–458. doi:10.1016/S2213-2600(21)00511-7
4. Wang C, Xu J, Yang L, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet. 2018;391:1706–1717. doi:10.1016/S0140-6736(18)30841-9
5. Lamprecht B, Soriano JB, Studnicka M, et al. Determinants of underdiagnosis of COPD in national and international surveys. Chest. 2015;148:971–985. doi:10.1378/chest.14-2535
6. Zhu B, Wang Y, Ming J, et al. Disease burden of COPD in China: a systematic review. Int J Chron Obstruct Pulmon Dis. 2018;13:1353–1364. doi:10.2147/COPD.S161555
7. Fang L, Gao P, Bao H, et al. Chronic obstructive pulmonary disease in China: a nationwide prevalence study. Lancet Respir Med. 2018;6:421–430. doi:10.1016/S2213-2600(18)30103-6
8. Li L, Zhong X, Zheng A, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in Kashi Region, Northwestern China. Int J Chron Obstruct Pulmon Dis. 2021;655–663. doi:10.2147/COPD.S289620
9. Su J, Tao R, Liu J, et al. Prevalence and influencing factors of chronic obstructive pulmonary disease among residents aged 40 and above in Jiangsu province. Chin J Public Health. 2021;37:1626–1630. in Chinese. doi:10.11847/zgggws1129731
10. Luo L, Yu J, Guo S, et al. Prevalence and influencing factors of chronic obstructive pulmonary disease among middle-aged and elderly residents in Jiangxi province. Chin J Public Health. 2019;35:1482–1486. in Chinese. doi:10.11847/zgggws1120324
11. Zha Z, Leng R, Xu W, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in Anhui Province, China: a population-based survey. BMC Pulm Med. 2019;19:102. doi:10.1186/s12890-019-0864-0
12. Shi W, Liu Y, Zhang F, et al. Survey on the prevalence and risk factors of chronic obstructive pulmonary disease in the population over 40 years in parts of areas of Hebei province. Hebei Med J. 2018;40:1419–1422. in Chinese. doi:10.3969/j.1002-7386.2018.09.035
13. Liu H, Qiao L, Xi Y, et al. Prevalence and influencing factors of chronic obstructive pulmonary disease in residents (≥ 40 years old) of Inner Mongolia in 2019. Chin J Prev Control Chronic Dis. 2022;30:192–195. in Chinese. doi:10.16386/j.cjpccd
14. Huang X, Mu X, Deng L, et al. The etiologic origins for chronic obstructive pulmonary disease. Int J Chronic Obstr Dis. 2019;14:1139–1158. doi:10.2147/COPD.S203215
15. Kurmi OP, Li L, Wang J, et al. COPD and its association with smoking in the mainland China: a cross-sectional analysis of 0.5 million men and women from ten diverse areas. Int J Chron Obstruct Pulmon Dis. 2015;10:655–665. doi:10.2147/COPD.S75454
16. Ko FWS, Hui DSC. Air pollution and chronic obstructive pulmonary disease. Respirology. 2012;17:395–401. doi:10.1111/j.1440-1843.2011.02112.x
17. Liu Z, Li Y, Cui Z, et al. Prevalence of tobacco dependence and associated factors in China: findings from nationwide China Health Literacy Survey during 2018–19. Lancet Reg Health West Pac. 2022;24:100464. doi:10.1016/j.lanwpc.2022.100464
18. Bird Y, Moraros J, Mahmood R, et al. Prevalence and associated factors of COPD among Aboriginal peoples in Canada: a cross-sectional study. Int J Chron Obstrct Pulmon Dis. 2017;12:1915–1922. doi:10.2147/COPD.S138304
19. Kurmi OP, Semple S, Simkhada P, et al. COPD and chronic bronchitis risk of indoor air pollution from solid fuel: a systematic review and meta-analysis. Thorax. 2010;65:221–228. doi:10.1136/thx.2009.124644
20. Smith M, Li L, Augustyn M, et al. Prevalence and correlates of airflow obstruction in 317,000 never-smokers in China. Eur Respir J. 2014;44:66–77. doi:10.1183/09031936.00152413
21. Sood A, Assad NA, Barnes PJ, et al. ERS/ATS workshop report on respiratory health effects of household air pollution. Eur Respir J. 2018;51:1700698. doi:10.1183/13993003.00698-2017
22. Pathak U, Gupta NC, Suri JC. Risk of COPD due to indoor air pollution from biomass cooking fuel: a systematic review and meta-analysis. Int J Environ Health Res. 2020;30:75–88. doi:10.1080/09603123.2019.1575951
23. Liu J, Hou B, Ma X, et al. Solid fuel use for cooking and its health effects on the elderly in rural China. Environ Sci Pollut R. 2018;25:3669–3680. doi:10.1007/s11356-017-0720-9
24. Benz E, Trajanoska K, Lahousse L, et al. Sarcopenia in COPD: a systematic review and meta-analysis. Eur Respir Rev. 2019;28:190049. doi:10.1183/16000617.0049-2019
25. Li J, Zhu L, Wei Y, et al.; China Kadoorie Biobank Collaborative Group. Association between adiposity measures and COPD risk in Chinese adults. Eur Respir J. 2020;55:1901899. doi:10.1183/13993003.01899-2019
26. Lee SJ, Kim SW, Kong KA, et al. Risk factors for chronic obstructive pulmonary disease among never-smokers in Korea. Int J Chron Obstruct Pulmon Dis. 2015;10:497–506. doi:10.2147/COPD.S77662
27. Dobner J, Kaser S. Body mass index and the risk of infection - from underweight to obesity. Clin Microbiol Infect. 2018;24:24–28. doi:10.1016/j.cmi.2017.02.013
28. Yu Z, Wang B, Cheng Z. The association of genetic polymorphisms of hypoxia inducible factor-1 alpha and vascular endothelial growth factor with increased risk of chronic obstructive pulmonary disease: a case-control study. Kaohsiung J Med Sci. 2017;33:433–441. doi:10.1016/j.kjms.2017.05.014
29. Li LSK, Williams MT, Johnston KN, et al. Parental and life-course influences on symptomatic airflow obstruction. ERJ Open Res. 2020;6:00343–2019. doi:10.1183/23120541.00343-2019
30. Sikjær M, Klitgaard A, Hilberg O, et al. Parental COPD as a risk factor for the development of COPD and disease severity in offspring: a systematic scoping review. Int J Chronic Obstr Dis. 2022;17:1323–1338. doi:10.2147/COPD.S364899
31. Ruvuna L, Akshay S. Epidemiology of chronic obstructive pulmonary disease. Clin Chest Med. 2020;41:315–327. doi:10.1016/j.ccm.2020.05.002
32. Zhang D, Liu J, Ye Q, et al. Association between socioeconomic status and chronic obstructive pulmonary disease in Jiangsu province, China: a population-based study. Chin Med J-Peking. 2021;134:1552–1560. in Chinese. doi:10.1097/CM9.0000000000001609
33. Eisner MD, Blanc PD, Omachi TA, et al. Socioeconomic status, race and COPD health outcomes. J Epidemiol Community Health. 2011;65:26–34. in Chinese. doi:10.1136/jech.2009.089722
34. Omachi TA, Sarkar U, Yelin EH, et al. Lower health literacy is associated with poorer health status and outcomes in chronic obstructive pulmonary disease. J Gen Intern Med. 2013;28:74–81. doi:10.1007/s11606-012-2177-3
35. Adeloye D, Agarwal D, Barnes PJ, et al. Research priorities to address the global burden of chronic obstructive pulmonary disease (COPD) in the next decade. J Glob Health. 2021;11:15003. doi:10.7189/jogh.11.15003
36. CPC Central Committee. The plan for “Healthy China 2030; 2016. Available from: http://www.gov.cn/xinwen/2016-10/25/content_5124174.htm.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
