Back to Journals » Adolescent Health, Medicine and Therapeutics » Volume 14
Chronic Fatigue Syndrome/Myalgic Encephalomyelitis (CFS/ME) in Adolescents: Practical Guidance and Management Challenges
Authors Rowe K
Received 2 November 2022
Accepted for publication 21 December 2022
Published 4 January 2023 Volume 2023:14 Pages 13—26
DOI https://doi.org/10.2147/AHMT.S317314
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Alastair Sutcliffe
Katherine Rowe
Department of General Medicine, Royal Children’s Hospital, Melbourne, Victoria, Australia
Correspondence: Katherine Rowe, Department of General Medicine, Royal Children’s Hospital, Flemington Road Parkville, Melbourne, Victoria, 3052, Australia, Tel +61 412059283, Email [email protected]
Abstract: This paper reviews the current understanding of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), and whether any treatment strategies have been effective. ME/CFS is a condition of as yet unknown etiology that commonly follows an infective process. It includes a new onset of fatigue (of more than 3– 6 month duration and not relieved by rest), post-exertional malaise, cognitive difficulties and unrefreshing sleep, and frequently orthostatic intolerance, somatic symptoms and pain. Long COVID has renewed interest in the condition and stimulated research with findings suggestive of a multisystem neuroimmune disease. There are no definitively effective treatments. Despite earlier recommendations regarding graded exercise therapy and cognitive behavior therapy, the current recommendations are managing symptoms, with lifestyle management and supportive care. This paper provides an outline of strategies that young people and their families have reported as helpful in managing a chronic illness that impacts their life socially, physically, emotionally, cognitively and educationally. As the illness frequently occurs at a time of rapid developmental changes, reducing these impacts is reported to be as important as managing the physical symptoms. Young people face a mean duration of 5 years illness (range 1– 16 years) with a likely residual 20% having significant restrictions after 10 years. Their feedback has suggested that symptom management, self-management strategies, advocacy and educational liaison have been the most helpful. They value professionals who will listen and take them seriously, and after excluding alternative diagnoses, they explain the diagnosis, are supportive and assist in monitoring their progress. Remaining engaged in education was the best predictor of later functioning. This allowed for social connections, as well as potential independence and fulfilling some aspirations. The need to consider the impact of this chronic illness on all aspects of adolescent development, as part of management, is highlighted.
Keywords: chronic fatigue syndrome, chronic illness, interventions, self-management
Plain Language Statement
This paper reviews the current understanding of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), and whether any treatment strategies in adolescents have been effective. ME/CFS has been reported in the literature for more than 150 years but with different names depending on the supposed etiology. Similarities with long COVID have renewed interest in the condition and stimulated research with findings suggestive of a multisystem neuroimmune disease. There are currently no definitively effective treatments. Despite earlier recommendations regarding graded exercise therapy and cognitive behavior therapy, the current recommendations are managing symptoms, with lifestyle management and supportive care. This paper provides an outline of strategies that young people and their families have reported as helpful in managing a chronic illness that impacts their life socially, physically, emotionally, cognitively and educationally. Their feedback has suggested that symptom management, self-management strategies, advocacy and educational liaison have been the most helpful. The need to consider the impact of this chronic illness on all aspects of adolescent development, as part of management, is highlighted
Introduction
Chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) is a condition of as yet unknown etiology that commonly follows an infective process in young people. There is a new onset of fatigue that lasts for at least 3–6 months that is not relieved by rest and is not explained by other medical conditions. Post-exertional malaise, cognitive difficulties and unrefreshing sleep are present. In addition, a variety of somatic symptoms are commonly present such as headache, abdominal or muscle pain, as well as flu-like symptoms without fever, and symptoms associated with orthostatic intolerance.1–5 The key features and symptom patterns in young people are similar to the symptoms in adults and have been consistent, but there are generally fewer alternative diagnoses to consider. Infections are the most common trigger.6–9
Background
Chronic Fatigue syndrome is not a new condition. It has been described in the literature as occurring either sporadically or in epidemics for more than 150 years. It has had a variety of names depending on the supposed etiology, such as Neurasthenia,10,11 Da Costa’s syndrome12–14 chronic Epstein Barr Virus (EBV),15 overtraining syndrome16 or named epidemics, for example, Icelandic disease.17,18 It was frequently noted that infectious diseases such as polio, influenza and EBV11,18–22 preceded the illness in previously well individuals. A well-known outbreak occurred in the Royal Free Hospital in 1955, when the hospital had to be closed as so many staff became ill.23 Controversy occurred when it was later suggested that, as the majority of sufferers were nursing or front-line staff, it had a psychological basis or was hysteria.24 This was disputed by those physicians who had examined the staff at the time, but this has contributed to sensitivity around etiology and management.25
In young people, the condition has been late in being accepted and diagnoses such as somatization disorder,26 school refusal, pathological parenting27 or likely depression28 were often utilized. This is despite children and young people being included in the reported cases during the epidemics18 or described following EBV infection.19,21
More recently, the emergence of a subset of patients suffering prolonged symptoms after contracting COVID-19 (Severe Acute Respiratory Syndrome Corona virus-2 [SARS-CoV-2]) during the pandemic has highlighted the similarity of this post-viral syndrome with ME/CFS.29–31 This has had the benefit of acknowledging the validity of the syndrome as well as stimulating research into the underlying pathophysiology.32 As the symptoms of ME/CFS can occur even following a relatively mild illness,33 it should be differentiated from recognised organ damage such as pulmonary, hepatic, renal, cardiac or brain pathology following severe disease with COVID-19,34 or the post-intensive care syndrome.35 Research has focussed on genetic differences in those affected,36 and the immunological and physiological changes during the illness.37–39 The proportion developing ME/CFS symptoms following infection has depended on the particular strain,40 vaccination status41 and number of exposures, but nonetheless the overall numbers due to the large proportion of the population infected has highlighted the need to increase the recognition of the condition, manage symptoms and consider appropriate ways to assist patients.33,42 Clinicians who are experienced with managing ME/CFS are very few and it has become clear that many more clinicians and services will be needed to meet the needs.
Prevalence
Prior to COVID-19, estimates of prevalence of ME/CFS varied depending on the case definition, geographical distribution, ascertainment of information, and country.43–47 The prevalence of CFS among adolescents was estimated at 0.1–1.9% in the US, depending on the applied case definition.48 Several studies indicate that in adolescents, 11–13% experienced a prolonged recovery following common illnesses such as EBV.19,21 It was similar for Q fever (Coxiella burnetii) and for Ross River virus19 and between 10% and 30% following COVID-19.33
Pathophysiology
The pathophysiology of ME/CFS remains largely unknown and the mechanisms and clear diagnostic markers, have not yet been found. Immunological mechanisms have been extensively researched, but results are inconclusive. Some studies have found low-grade systemic inflammation49,50 and attenuated natural killer (NK) cell function.51 However, these findings are not consistent.52 Recent findings of higher levels of immunosuppressive cytokines especially TGF-β,53 an altered gut microbiome,54 and nanoelectric markers for potential biomarkers55 show promise. In addition, studies investigating metabolomics, gut microbiota, the endocrine and nervous systems have been conducted.56 Findings are expected to confirm a complex, multisystem neuroimmune disease.57 Autonomic dysfunction with clinical orthostatic intolerance is common in adolescents.1,5,58 Neuropsychological studies suggest impairment of executive function, concentration and alterations in brain blood flow.39,58–61 ME/CFS patients experience more sleep difficulties than healthy individuals.62
In a large Australian cohort of adolescents with ME/CFS, depression was only mildly increased in prevalence compared with age-matched population levels, and generally did not precede the illness. Its presence was associated with delay in diagnosis, severity of illness, not being believed by family, school or medical practitioners, or social isolation.63 Reported anxiety was higher than the age-matched norms.8,9,63 A similar proportion with anxiety was noted in a United Kingdom cohort, although there was no control group there for comparison.64 Feelings of anxiety, including panic attacks, are commonly associated with orthostatic intolerance and may be an explanation for the increase in prevalence of reported anxiety with ME/CFS.65
Duration
There are very few long-term follow-up studies and comparisons are problematic due to differences in identification (chronic fatiguing illness rather than ME/CFS),45,66–68 numbers studied,67 duration of follow-up69 and retention rates.70 Retention rates in particular may be affected by those that avoid contact with researchers or medical professionals as they are discouraged, or they may not wish to revisit a painful period of their life if they feel they have recovered. Young adults are notoriously mobile, and tracing can be difficult.
In addition, how recovery is documented and how it is defined adds further complexity.71 Comparisons are difficult as recovery has been documented in terms of fatigue,72 self-report,69 presence of symptoms and functional outcomes,73,74 or a combination of global functioning and self-report have been used.75,76 Parslow et al76 investigated what aspects of defining recovery are important to young people with ME/CFS. When chronic illness affects every aspect of their development (social, emotional, physical and educational), it can be very difficult to interpret outcomes meaningfully when there may be differing severity of impact on each of the areas, and hence there are problems defining what “recovery” means. Similarly, young peoples’ perception of recovery may require them to have ‘no problems’ or they may be so relieved to be able to participate in life that they overlook the fact that they may be modifying their activities. An additional complicating factor that young people recognized, was that they did not have the normal progression in changing the ways they performed tasks as they grew older. They could only remember how they were as a fifteen-year old before they became ill. They were not sure what was normal functioning for their age.77 Nonetheless, the few long-term studies suggest that by 10 years the majority report improvement or recovery, but about 20–25% still had significant morbidity and reduced function.63,75,76,78 The average duration for clinically diagnosed ME/CFS in the Australian cohort was 5 years (range 1–16 years)63 for those that reported recovery. For the follow-up of the more broadly defined fatiguing illness (identified by questionnaire only), the duration was 2–3 years.45
Interventions
There is currently no definitively effective treatment. A double-blind study using intravenous immunoglobulin in adolescents showed promise,79 but studies in adults80,81 were inconclusive and the study has not been replicated in adolescents. Numerous approaches with various interventions82 including antivirals and monoclonal antibody rituximab83 have not shown promising results.
A large-scale clinical study (PACE trial)84 suggested that graded exercise therapy and cognitive behavior therapy were useful interventions and were initially recommended as effective therapy. However, these findings have been criticized by both patients and scientists. Doubt has been cast on the methodology suggesting that the findings are not as conclusive as reported.85–88 Many sufferers felt that these recommendations dismissed their experience of exacerbation of symptoms with exercise and disrespected their efforts to cope. Unfortunately, sensitivity to the fallout from the Royal Free experience has prejudiced cognitive behavior therapy’s usefulness, and a more measured individualized approach has been reported as more helpful.78,89,90 Acceptance and commitment therapy has been proposed as an alternative.91 However, current evidence for specific interventions, psychological or rehabilitation is limited57,91–94 At present, the Centers for Disease Control and Prevention (CDC) has proposed symptomatic treatments as an alternative.95
Predictors for Recovery
There are no known predictors for recovery. In a large follow-up study, severity of initial illness, nature of infectious trigger, presence of depression at initial visit or duration of illness prior to being seen were not predictive.63 There were, however, several factors that were identified as being helpful or unhelpful in management.63,78 Although the evidence for specific interventions is limited,57,91–94 feedback suggested that an individualized approach where the young person was more actively involved in the management plan was more satisfactory.
Management Strategies
Childhood and adolescence are periods of significant developmental changes. Consequently, if a chronic illness such as ME/CFS disrupts educational, emotional, social and physical activities in addition to the symptoms, there are significant challenges for young people and their families.96,97 In contrast, other chronic illnesses may impact only one or two of these areas.98,99 ME/CFS is the most common cause of reduced time at school,100,101 and has a significant impact on educational outcomes.102 Hence, it is important to not only manage symptoms but also provide strategies for coping with its effect on the young person and the family.63 The impact is amplified if these additional areas are not addressed and the transition to or from adolescence can be more complex. Parents also need assistance, as they are not only trying to manage the health issues but they may also not realize how important their role is in helping the young person navigate those tasks so that progress is not neglected or unduly delayed.101,102
This illness has been frequently disregarded by health professionals as there is no definitive laboratory test despite consistent symptomatology. This has been the source of much distress as young people frequently feel that they are either not believed or are dismissed.63,78 Nonetheless, it is recognized as a chronic illness impacting all aspects of a young person’s life. The experience of clinicians in managing it has been gained mostly by trial and error and listening to feedback from the young people themselves and their families.
A primer has been compiled by a group of clinicians from around the world pooling their experience.96 Careful data collection and long-term follow-up with regular feedback has informed practice.63,78,90 The following section will outline practical clinical management developed in response to feedback in not only assisting the young person with the specifics of ME/CFS, but helping them to gain some control in their life and support them in navigating the tasks of adolescence. These can be impacted by a chronic illness and have long-lasting effects. Young people recognized that ignoring some of these aspects significantly impeded their ability to re-engage once their symptoms improved.78 Hopefully, with increasing understanding of the underlying pathophysiology, specific treatments to target the pathology can shorten the natural history of this illness, but until then, assisting the young person (with family and educational support) to manage the condition, provides them with an opportunity to have a fulfilling life, even if for some, there remain some restrictions.
Principles of Management
Management consists of a careful initial assessment with an extensive history to confirm the presence of key symptoms, presence of any comorbidities and excluding possible alternative diagnoses. Symptom checklists are available to assist this process.6,96,103
Young people are very sensitive about whether they are being believed and accepted.
Provision of a diagnosis and explanation for most, is a great relief, as many young people report that they fear a hidden malignancy or other undisclosed serious illness.
Identification of the most troublesome symptom to manage to avoid polypharmacy allows them to see whether other symptoms either become more prominent or settle when the worst one is better controlled
Outline of a self-management plan allows them to plan how they balance educational, social, physical and enjoyable activities over the week that they review after each month.
Provision of regular, but not necessarily frequent, follow-up allows modification of both the plan and symptom management and review of whether more intensive interventions are required.
Initial Appointment
Key symptoms include post-exertional malaise, unrefreshing sleep and cognitive symptoms as well as additional somatic symptoms such as muscle or abdominal pain, headache and dizziness and orthostatic intolerance symptoms. Other conditions including school refusal, isolated significant depression or anxiety, eating disorders, Ehlers Danlos Syndrome, connective tissue disorders, fibromyalgia, allergic diseases, coeliac disease or endocrine disorders and somatisation disorder are specifically checked. An adolescent psychosocial (HEADSS) screen should be conducted where appropriate.104 Beighton score to assess hyperflexibility, and passive standing test for orthostatic intolerance with ME/CFS is recommended.1 School refusal can often be excluded if they can reassure you that they want to go to school, but cannot, as opposed to indicating that there is a problem going back to their current school. Depression can often be identified when they indicate that they do not feel like exercise but generally feel better after exercise, whereas ME/CFS patients would generally like to do it but feel worse afterwards. Depression and anxiety should be formally assessed if suspected.
Additional routine screening investigations include celiac screen, thyroid function and antinuclear antibody. Serology for EBV or cytomegalovirus (CMV) is often routinely assessed and if there is any likelihood of overseas or tropical infections or exposure from endemic areas, for example, Ross River Virus, Q fever (Coxiella burnetii), serology should be checked.
Feedback from young people indicates that following a diagnosis, it is helpful to rate the most troublesome symptom/s that they would like help with. It is important to hear their aspirations prior to becoming unwell and for them to describe current school attendance, their interests, and any previous participation in sport. This can inform the content and aims of their schedule. Information regarding the means of transport to school or activities, parental work schedule and the family situation and supports helps inform the logistics of implementing the plan. A brief explanation of current knowledge, a plan for managing the most severe symptoms, and an outline of a management plan that the young person could devise is provided.63,90,96 Although it is normal practice to have most of the consultation with adolescents alone, it is more common for them to request that their parent remains for most of the time. The reasons given relate to their difficulty with concentration and recall, and problems they have in relaying the information to their parents. In addition, parents appreciated being included in the active feedback component, as they have the majority of the responsibility for care.
Self-Management Plan Devised by the Young Person
Feedback from young people indicated that the management plan was helpful to minimize the impact of chronic illness while taking into account the specific challenges associated with ME/CFS.78 This plan included some proactive social contact, some academic input, some physical activity and a commitment to attend something enjoyable outside of home on a regular basis. They were not to leave any of these activities out, but there did not need to be an equal emphasis and some activities could incorporate several aspects, for example, social and academic, or social and enjoyable. It was important to plan these activities so that they did not precipitate excessive post-exertional malaise and adequate time for recovery was allowed.63,90 These tasks were to be balanced over a week taking into account their available energy. Hence, they needed to be able to sustain those weekly activities over the month before reviewing the plan and increasing some activity if they thought it was achievable. Otherwise, they would consider it the following month. Neglecting any of those areas resulted in significant additional hurdles to overcome, thus hampering their recovery. Young people reported that these hurdles could include social anxiety and gaps in social learning; not having a potentially satisfying or more lucrative, less physically demanding job due to reduced educational opportunities; needing to increase strength if deconditioned, or being unsure that they had acquired skills or resilience to face challenges in life.78 Learning to prioritize activities is a skill that is needed as developing adults. For young people with this condition, it is a useful skill to acquire and is important to help them navigate their management program.78 Young people could be given an outline of the range of possible variations for each of the activities to help them consider different options.
Although graded exercise therapy, as recommended from the PACE trial has been controversial due to the apparent disregard for post-exertional malaise,84–88 some physical activity is required to prevent significant de-conditioning from lack of use because they are so unwell. Feeling weak and fatigued can result from post-exertional malaise, or from deconditioning, and learning to balance either activities of daily living or pleasurable activities with sufficient recovery time is a significant challenge. Sometimes the activity may just be getting up to have a shower while seated, or going for a short walk, or for others, modifying a sport schedule. For some young people playing sport was their main social connection, and very important to them. Adjustments could be made. They could be moved to a less physically demanding team position. They could be excused from active training and just do skills training, or they could still be part of the team by playing for a few minutes.63,78,90,96
Social contact was important to ensure that the social learning that normally occurs during adolescence was not neglected. Feedback from many reported that ignoring it had contributed to their social anxiety and took several years of observing their peers before they felt comfortable socially. They reported that they struggled in knowing how to respond in different social situations and what behaviour is acceptable in different settings.78 This social contact could be as simple as having a friend visit for a defined period of time, meeting some friends at school or watching their team play sport. Others used communication over the internet as a starting point.
Remaining engaged in education was considered the best predictor of a good functional outcome at follow-up.63 It was regularly identified as equally important as any medical assistance as this was the area that caused the young person, their family and the school the most stress.63,78,100 Negotiating educational access at the appropriate level, amount, timing and format to enable them to remain engaged and progress to their goals is often the most challenging and time-consuming task for the clinician.78,96 Decisions regarding their education were informed by their aspirations prior to becoming unwell if they were still desired. Sometimes the severity of illness modified the aspirations as the physical demands of the career affected the choice of career, but at other times by modifying pathways to achieve the desired result, allowed hope to remain. This influenced subject choices and the minimum requirements to achieve that goal. Attending school for specific subjects was easier than attending for set times of the day especially if they were pre-requisites for their career and included teachers and subjects that they liked. Often there was a significant reduction in subjects, and this requires acceptance by the educational authorities to allow progression with peers. Trying to keep up with all subjects when missing most classes was a source of unnecessary stress, and this rarely succeeded.105,106 A planned timetable allowed more consistency and predictability for the teaching staff, students and families.105,106 Early in the illness, reading a story that they were already familiar with or about a hobby or interest may be all that they could manage. The COVID-19 pandemic has enabled many more resources and means of communication to be available as online teaching has become more mainstream. Nonetheless, it is recognized that the majority of learning that occurs during the first 4 years of secondary school is incidental social learning and if school cannot be attended to enable this to happen, then conscious efforts to engage socially outside of school are needed.78
The regular enjoyable activity outside of home was something that many struggled to accept as they needed to find something that was worth the effort but would not result in a prolonged recovery. They often worried that it would be viewed critically if they were not attending school frequently. They found it helpful to respond to criticism by indicating that this activity was “part of their program.” The purpose of having a regular commitment was to encourage decision-making, to attend consistently and to try something new. It was also to discourage prevarication about whether they felt well enough, whether they would cope or whether they could choose not to go. Only if they were too unwell to leave their bed did they not attend. It was considered important to have something enjoyable to look forward to regularly, and this was not something that they had even thought of in the context of coping with the illness.78
Following the visit, they were to make those decisions over the subsequent few weeks and discuss their plan with their parents. It was evident that even some children as young as 8 years could make a plan. No plans were the same, and it highlighted how much better they were devising an appropriate plan than one that might be prescribed by a clinician.90
Symptom Management
Only the most severe one or two symptoms were treated initially (Table 1). Young people reported that the importance of understanding their illness and having some control over their choices had helped with the severity of some symptoms.63,78 Also, treating one symptom such as sleep disturbance can reduce the severity of others. Difficulties with sleep initiation, frequent waking and disturbing nightmares or sleep phase shift can be actively managed with sleep hygiene techniques and melatonin or low dose tricyclic medications such as dothiepin or amitriptyline.96 If orthostatic intolerance was identified, fatigue, feelings of anxiety, concentration difficulties, complaints of headache, malaise, dizziness, nausea or sleep disturbance could be improved with simple measures. These included increasing salt and fluid intake, compression stockings and encouraging lower limb exercises and gentle exercise. Orthostatic intolerance has been shown to be associated with reduced blood flow to the brain,39 so management to stabilize the disorder can reduce the severity of cognitive symptoms. If non-pharmacological management was not sufficient, medications to modify heart rate and blood pressure were added and physical therapy introduced to increase lower limb muscle tone. Supervised gradual introduction of gentle exercise to improve cardiac reconditioning may initially be in a reclined position and then as tolerated using more upright posture.90,96
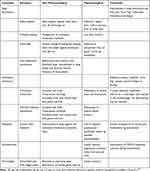 |
Table 1 Management of Common Symptoms in ME/CFS |
Despite the headache being unlike classic migraine, prophylactic medications such as pizotifen and cyproheptadine are anecdotally effective in reducing the severity of headache. Similarly, muscle pain and fibromyalgia can be helped by encouraging gentle exercise or physical therapy and reducing sleep disturbance. A more detailed outline of symptom management is available in the primer.96
Residual difficulties with concentration, depression, nausea, abdominal discomfort or persistent orthostatic symptoms were usually addressed after review and the implementation of the management plan. Dysmenorrhea can be particularly troublesome and may only respond to suppression of menstruation.
Follow-Up Visits
A review of their plan and whether the logistics were sustainable was usually scheduled at 6 weeks. Symptom management and any residual symptoms were assessed. Usually, there were additional queries about the illness to be addressed at this visit.
Once a decision had been made regarding the schedule for education, a specific education program to ensure maximum possible opportunity to participate is implemented. Documentation providing explanation, requests for extra support, special provision or special consideration is provided.96 Advocacy is often necessary. Sometimes this program requires a combination of Distance Education and school attendance for 1–2 subjects or attendance for a few classes with visiting teacher assistance. If necessary, the minimum requirements were negotiated to ensure the year level is passed so that they can progress with their peers. Negotiating what was most practical for student, family and teacher to achieve the desired outcome, without overloading the teaching staff, usually worked the best. The plan also assisted socially, as classmates knew when to expect a young person, and there were fewer comments about their absences. If there were, it was suggested that they could indicate that “they would be happy to change places with that person”. Educational strategies successfully used by Visiting Teachers for students with ME/CFS have been documented.105,106 Educational authorities have generally been cooperative in adapting programs to suit their needs as long as there was an explanation of the rationale, and documentation provided that satisfied their reporting requirements. Provisions for assessments such as flexibility in timing (usually not early in the day or not having several assessments required at once), extra time for writing and rest breaks and occasionally allowing access to food or drink were regularly granted. These requests were based on difficulties with recall under time pressure and concentration and processing difficulties. If adjustments to sport schedules were required, these were provided, and once coaches and staff understood the reasons for the requests, there was rarely a problem. There is much that can be done at school that makes an enormous difference to young people.96
Anxiety and panic attacks can be associated with orthostatic intolerance, and young people are often perplexed as they cannot identify why they should be feeling anxious. Others can recognize that their anxiety is related to being socially isolated or uncertainty around the illness or initially not having a diagnosis and thinking that it may be a malignancy or something similar. Some also have had bad experiences with over-doing activities, due to the post-exertional malaise, so that they are very anxious about initiating any participation again.78
Depression can be associated with ME/CFS and in many studies has not been identified as preceding the illness.107 Occasionally there was a family history of depression as a risk factor.63 Generally, the young people identified feeling “fed up and miserable” but when it was pointed out that it would be much more worrying if they were “happy” being at home, missing school, missing friends, not able to do any activity and “happy” being unwell, they acknowledged that that was probably a healthy response, and they felt they could cope with those feelings. They were asked to let their medical practitioner know if they felt miserable and no longer motivated rather than “fed up” and frustrated. Short courses of antidepressants often helped in that setting.63,78,96
Generally, 3-monthly reviews were arranged to assess progress, educational issues, symptom management and review of goals. Young people were seen more frequently if necessary. Occasionally young people were followed up by a local pediatrician.
The majority of young people could be managed by the clinician. Occasionally, if there were difficulties with a young person managing their plan, experiencing social isolation, family concerns, educational difficulties, or more commonly requiring more intensive assistance with orthostatic intolerance, an intensive self-management program over a 4-week period has been needed. Although the evidence for rehabilitation intervention is limited,93 feedback has indicated that an individualised rather than a standardized program has been more acceptable. A program from one center has been described in detail in Rowe et al.90 An inter-disciplinary team of physiotherapist, occupational therapist, psychologist and teacher work with a small group of young people on an individualised program. Over time, this program has been modified based on feedback and availability of resources and has predominantly functioned from outpatients. It has had more involvement of parents and had to adapt to online assistance during COVID lockdowns. Nonetheless, it has been helpful in supplementing what could be offered from outpatients.
Family Support
Navigating various developmental tasks during adolescence is difficult for most parents, but adding the complexities of chronic illness compounds their problem.101 Parents often need practical guidance as part of their management, especially when young people are so unwell that activities of daily living are a struggle. Sometimes allowance for time to catch up on these tasks is needed once the young person is well enough. During adolescence, it is expected that there is a progression in an increasing sense of independence and responsibility for their decisions and actions, ability to assess risk, to make future plans and a sense of self-worth. In addition, there is a development of peer relationships and sexual identity. With illness resulting in persistent dependence, reduced social interactions, reduced opportunity for decisions and potential for loss of hope for the future and depression, healthy progression can be jeopardized.
Parents can be conflicted as to whether they should be setting limits or making allowances; whether they should be advocating, defending or trusting their judgment or leaving young people to make mistakes. Many parents make additional work and leisure sacrifices to attend to the young person’s needs and this often adds significant stressors in the family. For many young people, ensuring they at least occasionally have meals with the family, and when well enough, have responsibility for some small chores that do not require much effort, can be helpful in reducing tensions with siblings.78
Younger patients often have concerns about what is actually wrong and whether they would recover. They may have increasing dependence, social anxiety, lack of basic literacy and numeracy skills due to absences and may experience significant prejudice due to lack of familiarity with the illness in this age group. The transition to secondary school can be particularly difficult if they have not been able to attend frequently enough to engage socially.
Parent support groups can be helpful. Clinicians recognize that they are not only caring for the young person but also recognize that parents are providing the majority of their care. It is not helpful if parents are not included in discussion of the management plan. It is also important that the negotiation with the school is not left up to the parents but assisted by the professionals. If there is a teacher liaison available, that is the most helpful in communicating with teachers, but medical support to ensure that teachers have documentation for their authorities is usually very much appreciated.
Feedback from Young People
Feedback from young people and their families has been welcomed and modified management. It should continue to inform management. They affirmed that being believed by clinicians, family and school staff, and feeling as if they had an advocate to help them navigate the education system, were crucial to for their general well-being and ability to cope.63,78 They reported feeling less out of control, helpless and hopeless due to being actively involved in lifestyle decisions. This management framework provided them with some control over their life again. Even if it took longer to achieve their aspirations or required modified plans, the support to pursue their aspirations gave hope. Continuing social contact helped ease their re-engagement into social life again.
Many indicated their frustration with family anxiety about pursuing “cures” in a myriad of alternative medical practices. Apart from sources providing advice for healthy diets, and provision of massage therapies for pain, there was minimal support for any.63
Many young people reported that while they were trying to manage the illness and school they did not welcome the suggestion of psychological assistance; however, frequently as they were recovering and becoming more socially engaged they welcomed the opportunity to work through some social issues and also needed to be reassured that if they had to deal with similar adversity again, they had the skills to cope with it. They noticed that they had often missed some opportunities for social learning during their time with illness, and they needed assurance that they did have those skills. A few, several years later, disclosed sexual abuse that occurred after they became unwell, which compounded their trauma, and they identified it as a significant contributor to their slower-than-expected recovery.
Others were more willing to accept that learning to cope and prioritize activities was useful. Many felt that there was a stigma around the illness as there was no obvious measurable pathology or visible disability. They were very sensitive to comments about it “being all in the mind” or “there must be a problem with school” or “in your family” or “you must be depressed”, etc..
If the outpatient program was not sufficient or there were additional family issues, an intensive interdisciplinary team program was reported as being helpful, especially for school liaison, assistance with planning and management of orthostatic intolerance.90
Conclusion
ME/CFS provides many challenges for both the clinician and the young person. In the light of a lack of evidence for underlying pathophysiology and specific interventions, apart from specific symptom management, there is much that can be done to support young people and their families dealing with the impact of chronic illness on the tasks of adolescence. Paying attention to social learning needs and educational support have been identified by young people as being of similar importance to medical management. Individualized plans with active input from young people are valued. For the few where additional support is needed, multidisciplinary teams can address psychological strategies for planning, coping and anxiety management; physical therapy, particularly if orthostatic intolerance has been difficult to manage; and occupational therapy for assistance with activities of daily living, as well as educational liaison.
Acknowledgments
The young people and their families that have actively participated in providing welcomed feedback regarding their management in this complex illness has been invaluable. Colleagues who have provided their insights and experience has been much appreciated.
Disclosure
The author reports no conflicts of interest in this work.
References
1. Rowe PC. Orthostatic intolerance and chronic fatigue syndrome: new light on an old problem. J Pediatr. 2002;140(4):387–389. PMID: 12006948. doi:10.1067/mpd.2002.124318
2. Holmes GP, Kaplan JE, Gantz NM, et al. Chronic fatigue syndrome: a working case definition. Ann Intern Med. 1988;108:387–389. PMID: 2829679. doi:10.7326/0003-4819-108-3-387
3. Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A. The chronic fatigue syndrome: a comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann Intern Med. 1994;121(12):953–959. doi:10.7326/0003-4819-121-12-199412150
4. Carruthers BM, van de Sande MI, De Meirleir KL, et al. Myalgic encephalomyelitis: international Consensus Criteria. J Intern Med. 2011;270(4):327–338. PMID: 21777306; PMCID: PMC3427890. doi:10.1111/j.1365-2796.2011.02428.x
5. Institute of Medicine. Committee on the diagnostic criteria for myalgic encephalomyelitis/chronic fatigue syndrome (10 February 2015), beyond myalgic encephalomyelitis/chronic fatigue syndrome: redefining an illness. Washington, DC: The National Academies Press; 2015. doi:10.17226/19012.
6. Jason LA, Jordan K, Miike T, et al. A pediatric case definition for myalgic encephalomyelitis and chronic fatigue syndrome. J Chronic Fatigue Syndr. 2006;13:1–44. doi:10.1300/J092v13n02_01
7. Jason LA, Barker A, Brown A. Pediatric myalgic encephalomyelitis/chronic fatigue syndrome. Rev Health Care. 2012;3(4):257–270. PMID: 24340168; PMCID: PMC3856907.
8. Rowe KS, Rowe KJ. Symptom patterns of children and adolescents with chronic fatigue syndrome. In: Singh NN, Ollendick TH, Singh AN, editors. International Perspectives on Child and Adolescent Mental Health. Vol. 2. New York: Elsevier Science; 2002:395–415.
9. Rowe KS, Rowe KJ. Symptom patterns and Psychological features of adolescents with chronic fatigue syndrome. J Paediatr Child Health. 2005;41(8):S9. August [Paediatrics and Child Health Division The Royal Australasian College of Physicians: Paediatric Abstracts presented at the Annual Scientific Meeting, May 2005: Paediatrics & Child Health Divisional Abstracts and Posters: 2005.
10. Beard G. Neurasthenia, or nervous exhaustion. Boston Med Surg J. 1869;3:217–221. doi:10.1056/NEJM18690429080130
11. Taylor RE. Death of neurasthenia and its psychological reincarnation: a study of neurasthenia at the National Hospital for the Relief and Cure of the Paralysed and Epileptic, Queen Square, London, 1870–1932. Br J Psychiatry. 2001;179(6):550–557. PMID: 11731361. doi:10.1192/bjp.179.6.550
12. Da Costa JM. On irritable heart; a clinical study of a form of functional cardiac disorder and its consequences; Age in two hundred cases. Result in 200 cases. Am J Med Sci. 1871;61(121):17–52.
13. Da Costa’s PO. syndrome or neurocirculatory asthenia. Heart. 1987;58:306–331. doi:10.1136/hrt.58.4.306
14. Klaas K, Burkholder D. Da Costa’s Syndrome and Postural Tachycardia Syndrome: a rose by any other name? Neurology Apr. 2017;88(16 Supplement):
15. Schooley RT. Chronic fatigue syndrome: a manifestation of Epstein-Barr virus infection? Curr Clin Top Infect Dis. 1988;9:126–146. PMID: 2855828.
16. Kreher JB, Schwartz JB. Overtraining syndrome: a practical guide. Sports Health. 2012;4(2):128–138. doi:10.1177/1941738111434406
17. Blattner R. Benign myalgic encephalomyelitis (Akureyri disease, Iceland disease). J Pediatr. 1956;49(4):504–506. PMID 13358047. doi:10.1016/S0022-3476(56)80241-2
18. Sigurdsson B, Siguryónsson J, Sigurdsson JHJ, Thorkelsson J, Gudmundsson KR. A d isease epidemic in Iceland Simulating Poliomyelitis. Am J Epidemiol. 1950;52(2):222–238. PMID: 14771044. doi:10.1093/oxfordjournals.aje.a119421
19. Hickie I, Davenport T, Wakefield D, et al. Post-infective and chronic fatigue syndromes precipitated by viral and non-viral pathogens: prospective cohort study. BMJ. 2006;333:575. PMID: 16950834; PMCID: PMC1569956. doi:10.1136/bmj.38933.585764.AE
20. White PD, Thomas JM, Amess J, Grover SA, Kangro HO, Clare AW. The existence of a fatigue syndrome after glandular fever. Psychol Med. 1995;25(5):907–916. PMID: 8588009. doi:10.1017/s0033291700037399
21. Katz BZ, Shiraishi Y, Mears CJ, Binns HJ, Taylor R. Chronic fatigue syndrome after infectious mononucleosis in adolescents. Pediatrics. 2009;124:189–193. PMID: 19564299; PMCID: PMC2756827. doi:10.1542/peds.2008-1879
22. Pedersen M, Tørre Asprusten T, Godang K, et al. Predictors of chronic fatigue in adolescents six months after acute Epstein-Barr virus infection: a prospective cohort study. Brain Behav Immun. 2019;75:94–100. PMID: 30261303. doi:10.1016/j.bbi.2018.09.023
23. The Medical Staff of The Royal Free Hospital. An outbreak of encephalomyelitis in the Royal Free Hospital Group, London, in 1955. Br Med J. 1957;2(5050):895–904. PMID: 13472002; PMCID: PMC1962472.
24. McEvedy CP, Beard AW. Royal Free epidemic of 1955: a reconsideration. Br Med J. 1970;1:7–11. doi:10.1136/bmj.1.5687
25. Ramsay AM. Hysteria and royal free disease. Br Med J. 1965;2(5469):1062. doi:10.1136/bmj.2.5469.1062-a
26. Johnson SK, DeLuca J, Natelson BH. Assessing somatization disorder in the chronic fatigue syndrome. Psychosom Med. 1996;58(1):50–57. PMID: 8677289. doi:10.1097/00006842-199601000-00008
27. Van Hoof E, De Becker P, De Meirleir K. Pediatric chronic fatigue syndrome and Munchausen-by-proxy. J Chronic Fatigue Syndr. 2006;13:45–53. doi:10.1300/J092v13n02_02
28. Taylor AK, Loades M, Brigden AL, Collin SM, Crawley E. ‘It’s personal to me’: a qualitative study of depression in young people with CFS/ME. Clin Child Psychol Psychiatry. 2017;22(2):326–340. PMID: 27742756; PMCID: PMC5405821. doi:10.1177/1359104516672507
29. Komaroff AL, Lipkin WI. Insights from myalgic encephalomyelitis/chronic fatigue syndrome may help unravel the pathogenesis of postacute COVID-19 syndrome. Trends Mol Med. 2021;27(9):895–906. PMID: 34175230; PMCID: PMC8180841. doi:10.1016/j.molmed.2021.06.002
30. Petracek LS, Suskauer SJ, Vickers RF, et al. Adolescent and Young Adult ME/CFS After Confirmed or Probable COVID-19. Front Med. 2021;8:668944. doi:10.3389/fmed.2021.668944
31. Owens B. How “long covid” is shedding light on postviral syndromes. BMJ. 2022;378:o2188. doi:10.1136/bmj.o2188
32. Owens B. How “long covid” is shedding light on postviral syndromes. BMJ. 2022;378–2188. doi:10.1136/bmj.o2188
33. Mackay A. A paradigm for post-covid-19 fatigue syndrome analogous to ME/CFS front. Front. Neurol. 2021;12: doi:10.3389/fneur.2021.701419.
34. Iacobucci G. Long covid: damage to multiple organs presents in young, low risk patients. BMJ. 2020;371:m4470. doi:10.1136/bmj.m4470
35. Rawal G, Yadav S, Kumar R. Post-intensive Care Syndrome: an Overview. J Transl Int Med. 2017;5(2):90–92. PMID: 28721340; PMCID: PMC5506407. doi:10.1515/jtim-2016-0016
36. van der Made CI, Netea MG, van der Veerdonk FL, Hoischen A. Clinical implications of host genetic variation and susceptibility to severe or critical COVID-19. Genome Med. 2022;14:96. doi:10.1186/s13073-022-01100-3
37. Ryan FJ, Hope CM, Masavuli MG, et al. Long-term perturbation of the peripheral immune system months after SARS-CoV-2 infection. BMC Med. 2022;20:26. doi:10.1186/s12916-021-02228-6
38. Paul BD, Lemle MD, Komaroff AL, Snyder SH. Redox imbalance links COVID-19 and myalgic encephalomyelitis/chronic fatigue syndrome. Proc Natl Acad Sci U S A. 2021;118(34):e2024358118. PMID: 34400495; PMCID: PMC8403932. doi:10.1073/pnas.2024358118
39. van Campen CLMC, Rowe PC, Visser FC. Orthostatic symptoms and reductions in cerebral blood flow in long-haul COVID-19 patients: similarities with myalgic encephalomyelitis/chronic fatigue syndrome. Medicina. 2022;58:1–18. PMID: 35056336; PMCID: PMC8778312. doi:10.3390/medicina58010028
40. Marx V. Scientists set out to connect the dots on long COVID. Nat Methods. 2021;18:449–453. doi:10.1038/s41592-021-01145-z
41. Azzolini E, Levi R, Sarti R, et al. Association between BNT162b2 vaccination and long COVID after infections not requiring hospitalization in health care workers. JAMA. 2022;328(7):676–678. PMID: 35796131; PMCID: PMC9250078. doi:10.1001/jama.2022.11691
42. Newsroom M. Long covid has resurfaced tensions over treatment of chronic fatigue syndrome. BMJ. 2021;24:373.
43. Knight S, Elders S, Rodda J, et al. Epidemiology of paediatric chronic fatigue syndrome in Australia. Arch Dis Child. 2019;104(8):733–738. PMID: 30798255. doi:10.1136/archdischild-2018-316450
44. Nacul L, Kingdon CC, Bowman EW, Curran H, Lacerda EM. Differing case definitions point to the need for an accurate diagnosis of myalgic encephalomyelitis/chronic fatigue syndrome. Fatigue. 2017;5(1):1–4. PMID: 29250461. doi:10.1080/21641846.2017.1273863
45. Norris T, Collin SM, Tilling K, et al. Natural course of chronic fatigue syndrome/myalgic encephalomyelitis in adolescents. Arch Dis Child. 2017;102:522–528. PMID: 28104625; PMCID: PMC5466925. doi:10.1136/archdischild-2016-311198
46. Johnston S, Brenu EW, Staines D, Marshall-Gradisnik S. The prevalence of chronic fatigue syndrome/ myalgic encephalomyelitis: a meta-analysi s. Clin Epidemiol. 2013;5:105–110. PMID: 23576883; PMCID: PMC3616604. doi:10.2147/CLEP.S39876
47. Lim EJ, Ahn YC, Jang ES, Lee SW, Lee SH, Son CG. Systematic review and meta-analysis of the prevalence of chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME). J Transl Med. 2020;18(1):100. PMID: 32093722. doi:10.1186/s12967-020-02269-0
48. Jordan KM, Huang CF, Jason LA, et al. Pediatric chronic fatigue syndrome in a community-based sample. J Chronic Fatigue Syndrome. 2006;13(2–3):75–78. doi:10.1300/J092v13n02_04
49. Sulheim D, Fagermoen E, Winger A, et al. Disease mechanisms and clonidine treatment in adolescent chronic fatigue syndrome: a combined cross-sectional and randomized clinical trial. JAMA Pediatr. 2014;168:351–360. doi:10.1001/jamapediatrics.2013.4647
50. Klimas NG, Broderick G, Fletcher MA. Biomarkers for chronic fatigue. Brain Behav Immun. 2012;26:1202–1210. PMID: 22732129; PMCID: PMC5373648. doi:10.1016/j.bbi.2012.06.006
51. Mensah FKF, Bansal AS, Ford B, Cambridge G. Chronic fatigue syndrome and the immune system: where are we now? Neurophysiol Clin. 2017;47:131–138. PMID: 28410877. doi:10.1016/j.neucli.2017.02.002
52. Katz BZ, Zimmerman D, Gorman MR, Mears CJ, Shiraishi Y, Taylor R. Normal salivary cortisol and NK cell function in adolescents with chronic fatigue syndrome following infectious mononucleosis. Arch Pediatr Infect Dis. 2013;1(5):211–216. doi:10.5812/pedinfect.13107
53. Montoya JG, Holmes TH, Anderson JN, et al. Cytokine signature associated with disease severity in chronic fatigue syndrome patients. Proc Natl Acad Sci USA. 2017;114(34):E7150–E7158. PMID: 28760971; PMCID: PMC5576836. doi:10.1073/pnas.1710519114
54. Giloteaux L, Goodrich JK, Walters WA, Levine SM, Ley RE, Hanson MR. Reduced diversity and altered composition of the gut microbiome in individuals with myalgic encephalomyelitis/chronic fatigue syndrome. Microbiome. 2016;4(1):30. PMID: 27338587; PMCID: PMC4918027. doi:10.1186/s40168-016-0171-4
55. Esfandyarpour R, Kashi A, Nemat-Gorgani M, Wilhelmy J, Davis RW. A nanoelectronics-blood-based diagnostic biomarker for myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Proc Natl Acad Sci U S A. 2019;116:10250–10257. PMID: 31036648; PMCID: PMC6535016. doi:10.1073/pnas.1901274116
56. Missailidis D, Annesley SJ, Fisher PR. Pathological mechanisms underlying myalgic encephalomyelitis/chronic fatigue syndrome. Diagnostics. 2019;9(3):E80. PMID: 31330791; PMCID: PMC6787592. doi:10.3390/diagnostics9030080
57. Bested AC, Marshall LM. Review of myalgic encephalomyelitis/chronic fatigue syndrome: an evidence-based approach to diagnosis and management by clinician s. Rev Environ Health. 2015;30:223–249. PMID: 26613325. doi:10.1515/reveh-2015-0026
58. Bou-Holaigah I, Rowe PC, Kan J, Calkins H. The relationship between neurally mediated hypotension and the chronic fatigue syndrome. JAMA. 1995;274:961–967. doi:10.1001/jama.1995.03530120053041
59. Sulheim D, Fagermoen E, Sivertsen ØS, Winger A, Wyller VB, Øie MG. Cognitive dysfunction in adolescents with chronic fatigue: a cross-sectional study. Arch Dis Child. 2015;100(9):838–844. PMID: 25791841; PMCID: PMC4552915. doi:10.1136/archdischild-2014-306764
60. Josev EK, Malpas CB, Seal ML, et al. Resting-state functional connectivity, cognition, and fatigue in response to cognitive exertion: a novel study in adolescents with chronic fatigue syndrome. Brain Imaging Behav. 2020;14(5):1815–1830. PMID: 31102168. doi:10.1007/s11682-019-00119-2
61. Ocon AJ, Messer ZR, Medow MS, Stewart JM. Increasing orthostatic stress impairs neurocognitive functioning in chronic fatigue syndrome with postural tachycardia syndrome. Clin Sci. 2012;122(5):227–238. PMID: 21919887; PMCID: PMC3368269. doi:10.1042/CS20110241
62. Pedersen M, Ekstedt M, Smastuen MC, et al. Sleep-wake rhythm disturbances and perceived sleep in adolescent chronic fatigue syndrome. J Sleep Res. 2017;26:595–601. PMID: 28470767. doi:10.1111/jsr.12547
63. Rowe KS. Long term follow up of young people with chronic fatigue syndrome attending a pediatric outpatient service. Front Pediatr. 2019;7:21. doi:10.3389/fped.2019.00021
64. Loades ME, Read R, Smith L, et al. How common are depression and anxiety in adolescents with chronic fatigue syndrome (CFS) and how should we screen for these mental health co-morbidities? A clinical cohort study. Eur Child Adolesc Psychiatry. 2021;30(11):1733–1743. PMID: 32964335; PMCID: PMC8558286. doi:10.1007/s00787-020-01646-w
65. Kesserwani H. Postural orthostatic tachycardia syndrome misdiagnosed as anxiety: a case report with a review of therapy and pathophysiology. Cureus. 2020;12(10):e10881. doi:10.7759/cureus.10881
66. Carter BD, Edwards JF, Kronenberger WG, Michalczyk L, Marshall GS. Case control study of chronic fatigue in pediatric patients. Pediatrics. 1995;95(2):179–186. PMID: 7838632.
67. Rimes KA, Goodman R, Hotopf M, Wessely S, Meltzer H, Chalder T. Incidence, prognosis, and risk factors for fatigue and chronic fatigue syndrome in adolescents: a prospective community study. Pediatrics. 2007;119(3):e603–e609. PMID: 17332180. doi:10.1542/peds.2006-2231
68. Krilov LR, Fisher M, Friedman SB, Reitman D, Mandel FS. Course and outcome of chronic fatigue in children and adolescents. Pediatrics. 1998;102(2 Pt 1):360–366. PMID: 9685439. doi:10.1542/peds.102.2.360
69. van der Werf SP, de Vree B, Alberts M, van der Meer JW, Bleijenberg G. Netherlands Fatigue Research Group Nijmegen. Natural course and predicting self-reported improvement in patients with chronic fatigue syndrome with a relatively short illness duration. J Psychosom Res. 2002;53(3):749–753. PMID: 12217448. doi:10.1016/s0022-3999(02)00324-0
70. Gill AC, Dosen A, Ziegler JB. Chronic fatigue syndrome in adolescents: a follow-up study. Arch Pediatr Adolesc Med. 2004;158(3):225–229. PMID: 14993080. doi:10.1001/archpedi.158.3.225
71. Adamowicz JL, Caikauskaite I, Friedberg F. Defining recovery in chronic fatigue syndrome: a critical review. Qual Life Res. 2014;23:2407–2416. doi:10.1007/s11136-014-0737-1
72. Kawatani J, Mizuno K, Shiraishi S, et al. Cognitive dysfunction and mental fatigue in childhood chronic fatigue syndrome--a 6-month follow-up study. Brain Dev. 2011;33(10):832–841. PMID: 21530119. doi:10.1016/j.braindev.2010.12.009
73. van Geelen SM, Bakker RJ, Kuis W, van de Putte EM. Adolescent chronic fatigue syndrome: a follow-up study. Arch Pediatr Adolesc Med. 2010;164(9):810–814. PMID: 20819962. doi:10.1001/archpediatrics.2010.145
74. Knoop H, Stulemeijer M, de Jong LW, Fiselier TJ, Bleijenberg G. Efficacy of cognitive behavioral therapy for adolescents with chronic fatigue syndrome: long-term follow-up of a randomized, controlled trial. Pediatrics. 2008;121(3):e619–e625. PMID: 18310181. doi:10.1542/peds.2007-1488
75. Bell DS, Jordan K, Robinson M. Thirteen-year follow-up of children and adolescents with chronic fatigue syndrome. Pediatrics. 2001;107(5):994–998. PMID: 11331676. doi:10.1542/peds.107.5.994
76. Rowe KS. 5-year follow-up of young people with Chronic Fatigue Syndrome following the double-blind randomised controlled intravenous gammaglobulin trial. J Chronic Fatigue Syndr. 1999;5(3/4):97–107. doi:10.1300/J092v05n03_08
77. Parslow R, Patel A, Beasant L, Haywood K, Johnson D, Crawley E. What matters to children with CFS/ME? A conceptual model as the first stage in developing a PROM. Arch Dis Child. 2015;100(12):1141–1147. PMID: 26453575; PMCID: PMC4680202. doi:10.1136/archdischild-2015-308831
78. Rowe K. Paediatric patients with myalgic encephalomyelitis/chronic fatigue syndrome value understanding and help to move on with their lives. Acta Paediatr. 2020;109(4):790–800. PMID: 31854020. doi:10.1111/apa.15054
79. Rowe KS. Double-blind placebo-controlled trial to assess the efficacy of intravenous gammaglobulin for the management of Chronic Fatigue Syndrome in Adolescents. J Psychiatr Res. 1997;31(1):133–147. PMID: 9201655. doi:10.1016/s0022-3956(96)00047-7
80. Lloyd A, Hickie I, Wakefield D, et al. A double-blind placebo-controlled trial of intravenous immunoglobulin therapy in patients with the Chronic Fatigue Syndrome. Am J Med. 1990;89:561–568.
81. Peterson PK, Shepard J, Macres M, et al. A controlled trial of intravenous immunoglobulin G in chronic fatigue syndrome. Am J Med. 1990;89:554–560.
82. Kim DY, Lee JS, Park SY, Kim SJ, Son CG. Systematic review of randomized controlled trials for chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME). J Transl Med. 2020;18(1):7. doi:10.1186/s12967-019-02196-9
83. Fluge O, Risa K, Lunde S, et al. B-Lymphocyte depletion in myalgic encephalopathy/chronic fatigue syndrome. An open-label Phase II study with rituximab maintenance treatment. PLoS One. 2015;10:e0129898. PMID: 26132314; PMCID: PMC4488509. doi:10.1371/journal.pone.0129898
84. White PD, Goldsmith KA, Johnson AL, et al. Comparison of adaptive pacing therapy, cognitive behaviour therapy, graded exercise therapy, and specialist medical care for chronic fatigue syndrome (PACE): a randomised trial. Lancet. 2011;377:823–836. PMID: 21334061; PMCID: PMC3065633. doi:10.1016/S0140-6736(11)60096-2
85. Wilshire CE, Kindlon T, Courtney R, et al. Rethinking the treatment of chronic fatigue syndrome—a reanalysis and evaluation of findings from a recent major trial of graded exercise and CBT. BMC Psychol. 2018;6(6). doi:10.1186/s40359-018-0218-3
86. Wise J. Reanalysis of PACE trial reignites row over chronic fatigue treatment. BMJ. 2016;354:i5230. pmid: 27686885. doi:10.1136/bmj.i5230
87. Torjesen I. Pressure grows on Lancet to review “flawed” PACE trial. BMJ. 2018;362. doi:10.1136/bmj.k3621
88. Shepherd CB. PACE. trial claims for recovery in myalgic encephalomyelitis/chronic fatigue syndrome—true or false? It’s time for an independent review of the methodology and results. J Health Psychol. 2017;22(9):1187–1191. PMID: 28805522. doi:10.1177/1359105317703786
89. Chalder T, Deary V, Husain K, Walwyn R. Family-focused cognitive behaviour therapy versus psycho-education for chronic fatigue syndrome in 11- to 18-year-olds: a randomized controlled treatment trial. Psychol Med. 2010;40(8):1269–1279. PMID: 19891804. doi:10.1017/S003329170999153X
90. Rowe K, Apple A, McDonald F. Self-management of chronic fatigue syndrome in adolescents. In: Heston TF, editor. Topics in Primary Care Medicine [Internet]. London: IntechOpen; 2020. doi:10.5772/intechopen.91413
91. Leveret J, Starbuck J, Chapple K, et al. Why should ACT work when CBT has failed? A study assessing Acceptability and Feasibility of Acceptance commitment Therapy (ACT) for paediatric patients the Chronic Fatigue Syndrome/Myalgic Encephalomyelitis (CFS/ME. BJ Psych Open. 2022;8(S1):S58–S58.
92. Whiting P, Bagnall AM, Sowden AJ, Cornell JE, Mulrow CD, Ramírez G. Interventions for the treatment and management of chronic fatigue syndrome. JAMA. 2001;286(11):1360–1368. PMID: 11560542.
93. Knight SJ, Scheinberg A, Harvey AR. Interventions in pediatric chronic fatigue syndrome/myalgic encephalomyelitis: a systematic review. J Adolesc Health. 2013;53(2):154–165. doi:10.1016/j.jadohealth.2013.03.009
94. Collard SS, Murphy J. Management of chronic fatigue syndrome/myalgic encephalomyelitis in a pediatric population: a scoping review. J Child Health Care. 2020;24(3):411–431. doi:10.1177/1367493519864747
95. CDC. Treatment of ME/CFS. Available from: https://www.cdc.gov/me-cfs/treatment/index.html.
96. Rowe PC, Underhill RA, Friedman KJ, et al. Myalgic encephalomyelitis/chronic fatigue syndrome diagnosis and management in young people: a primer. Front Pediatr. 2017;5:121. PMID: 28674681; PMCID: PMC5474682. doi:10.3389/fped.2017.00121
97. Rowe KS. Chronic fatigue syndrome. In: Kang M, Skinner SR, Sanci LA, Sawyer SM, editors. Youth Health and Adolescent Medicine. East Hawthorn, Vic: I P Communications; 2013:343–360.
98. Suris J-C, Viner R. The adolescent with a chronic condition. Part I: developmental issues. Arch Dis Child. 2004;89:938–942. PMID: 15383438; PMCID: PMC1719685. doi:10.1136/adc.2003.045369
99. Holmes AM, Deb P. The effect of chronic illness on the psychological health of family members. J Ment Health Policy Econ. 2003;6(1):13–22. PMID: 14578544.
100. Crawley E, Sterne JA. Association between school absence and physical function in paediatric chronic fatigue syndrome/myalgic encephalopathy. Arch Dis Child. 2009;94:752–756. PMID: 19001477. doi:10.1136/adc.2008.143537
101. Crawley EM, Emond AM, Sterne JAC. Unidentified Chronic Fatigue Syndrome/myalgic encephalomyelitis (CFS/ME) is a major cause of school absence: surveillance outcomes from school-based clinic s. BMJ Open. 2011;1(2):e000252. PMID: 22155938; PMCID: PMC3244656. doi:10.1136/bmjopen-2011-000252
102. Knight SJ, Politis J, Garnham C, Scheinberg A, Tollit MA. School functioning in adolescents with chronic fatigue syndrome. Front Pediatr. 2018. doi:10.3389/fped.2018.00302
103. Centers for Disease Control and Prevention, Myalgic encephalomyelitis/Chronic Fatigue Syndrome: Patient toolkit. Available from: https://www.cdc.gov/me-cfs/resources/patient-toolkit.html.
104. Goldenring JM, Cohen E. H.E.A.D.S.S.- getting into adolescent heads. Contemp Pediatr. 1998;5:75–90.
105. Rowe KS, Fitzgerald P. Educational strategies for chronically ill students: chronic fatigue syndrome. Aust Educ Dev Psychol. 1999;16(2):5–21.
106. Rowe K, Fitzgerald P, Higgins R, Anderson G, McLaughlin M, Brewin T. Educational strategies for Chronically ill students - with a special section on Chronic Fatigue Syndrome. J Issue. 1993;1:1–6. doi:10.18296/set.1003
107. Loades ME, Sheils EA, Crawley E. Treatment for paediatric chronic fatigue syndrome or myalgic encephalomyelitis (CFS/ME) and comorbid depression: a systematic review. BMJ Open. 2016;6(10):e012271. PMID: 27729349; PMCID: PMC5073581. doi:10.1136/bmjopen-2016-012271
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
