Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 13
Chronic cough as a novel phenotype of chronic obstructive pulmonary disease
Authors Koo HK, Park SW, Park JW, Choi HS , Kim TH , Yoon HK , Yoo KH , Jung KS, Kim DK
Received 11 October 2017
Accepted for publication 29 January 2018
Published 30 May 2018 Volume 2018:13 Pages 1793—1801
DOI https://doi.org/10.2147/COPD.S153821
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Hyeon-Kyoung Koo,1 Sung-Woo Park,2 Jeong-Woong Park,3 Hye Sook Choi,4 Tae-Hyung Kim,5 Hyoung Kyu Yoon,6 Kwang Ha Yoo,7 Ki-Suck Jung,8 Deog Kyeom Kim9
1Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Ilsan Paik Hospital, Inje University College of Medicine, Ilsan, Republic of Korea; 2Division of Allergy and Respiratory Medicine, Department of Internal Medicine, Soon Chun Hyang University Bucheon Hospital, Bucheon, Republic of Korea; 3Department of Pulmonary and Critical Care Medicine, Gachon University, Gil Medical Center, Incheon, Republic of Korea; 4Division of Pulmonary, Allergy, and Critical Care Medicine, Department of Internal Medicine, Hallym University Kangdong Sacred Heart Hospital, Seoul, Republic of Korea; 5Division of Pulmonary and Critical Care Medicine, Hanyang University Guri Hospital, Hanyang University College of Medicine, Guri, Republic of Korea; 6Department of Internal Medicine, The Catholic University of Korea, Yeouido St Mary’s Hospital, Seoul, Republic of Korea; 7Division of Pulmonary, Allergy and Critical Care Medicine, Department of Internal Medicine, Konkuk University School of Medicine, Seoul, Republic of Korea; 8Division of Pulmonary, Allergy, and Critical Care Medicine, Department of Medicine, Hallym University Sacred Heart Hospital, Hallym University College of Medicine, Anyang, Republic of Korea; 9Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Seoul Metropolitan Government-Seoul National University Boramae Medical Center, Seoul National University College of Medicine, Seoul, Republic of Korea
Background and purpose: Chronic cough can be a dominant symptom of chronic obstructive pulmonary disease (COPD), although its clinical impact remains unclear. The aim of our study was to identify phenotypic differences according to the presence of chronic cough or sputum and evaluate the impact of chronic cough on the risk of acute exacerbation of COPD (AECOPD).
Methods: In a nationwide COPD cohort including 1,613 COPD patients, patients with chronic cough only, those with sputum only, those with chronic bronchitis (CB), and those without cough and sputum were compared with regard to dyspnea, lung function, quality of life (QoL), and risk of AECOPD.
Results: The rates of chronic cough, chronic sputum, and both were 23.4%, 32.4%, and 18.2%, respectively. Compared with patients without chronic cough, those with chronic cough exhibited a lower forced expiratory volume in 1 second (% predicted) and diffusing capacity of the lungs for carbon monoxide (% predicted), more frequent AECOPD, more severe dyspnea, and worse QoL. Pulmonary function, dyspnea severity, and QoL worsened in the following order: without cough or sputum, with sputum only, with cough only, and with CB. Multivariate analyses revealed chronic cough as an independent risk factor for a lower lung function, more severe dyspnea, and a poor QoL. Moreover, the risk of future AECOPD was significantly associated with chronic cough (odds ratio 1.56, 95% CI 1.08–2.24), but not with chronic sputum.
Conclusion: Our results suggest that chronic cough should be considered as an important phenotype during the determination of high-risk groups of COPD patients.
Keywords: pulmonary disease, chronic obstructive, cough, exacerbation, severity
Introduction
Cough is a common symptom of chronic respiratory diseases such as chronic obstructive pulmonary disease (COPD). Several causes for chronic cough exist including COPD, and the concept of cough hypersensitivity syndrome1 has been introduced to explain the common mechanism of chronic cough. However, characteristics of chronic cough in COPD have not been well described; it may exhibit features different from those of cough hypersensitivity syndrome-associated cough. As evidence to support this hypothesis, one study found that cough frequency in COPD patients is associated with sputum production, smoking, and airway inflammation, and that these factors may appear to be more important than the sensitivity of the cough reflex.2 In this regard, sputum has been rather emphasized than cough in COPD patients, and a clinical phenotype characterized by prominent and persistent cough and sputum production for at least 3 months during each of two consecutive years has been defined as chronic bronchitis (CB). The CB phenotype is associated with worse respiratory symptoms, higher rate of acute exacerbation of COPD (AECOPD), and worse disease impact in COPD patients.3–8 However, chronic cough is not always accompanied by sputum, and it can occur as single manifestation of COPD.9 The clinical impact of chronic cough on COPD outcomes has not been well reported; therefore, it may be necessary to investigate how chronic cough affects COPD outcomes, including quality of life (QoL) and future risk of AECOPD, irrespective of presence or absence of sputum. The aim of our study was to identify phenotypic differences according to presence of chronic cough or sputum in COPD patients and evaluate the impact of chronic cough on the risk of future AECOPD.
Methods
Study population and data collection
We recruited patients enrolled in the KOrea COpd Subgroup Study (KOCOSS), which is an ongoing, multicenter cohort study of COPD that has included participants from 47 centers in South Korea since April 2012.10 Inclusion criteria were as follows: Korean patients aged >40 years and post-bronchodilator forced expiratory volume in 1 second (FEV1) to forced vital capacity ratio of <0.7. Spirometry and 6-minute walk distance test were performed according to standard techniques.11,12 At the first visit, information regarding the frequency and severity of exacerbations in the past 12 months; smoking status; patient-reported education levels; medications; and comorbidities were recorded. The modified Medical Research Council Dyspnea scale (mMRC)13,14 scores for dyspnea severity, scores for the COPD Assessment Test (CAT),15 and COPD-specific version of St George’s Respiratory Questionnaire (SGRQ-C)16 were assessed. All data were documented in case report forms completed by physicians or trained nurses, and patients were re-evaluated at regular 6-month intervals after the initial examination. The major exclusion criteria were as follows: asthma; other obstructive lung diseases including bronchiectasis; tuberculosis-destroyed lungs; inability to complete the spirometry; myocardial infarction or cerebrovascular events within the past 3 months; pregnancy; rheumatoid disease; malignancy; irritable bowel disease; and use of steroids for conditions other than AECOPD within 8 weeks before enrollment. Patients with recent (8 weeks before screening) exacerbation or other respiratory illness (such as upper respiratory infection or pneumonia) were excluded; however, patients who recovered from an exacerbation and had been stable for more than 8 weeks were included. Written informed consent was obtained from all of the study patients. Ethics approval for this study was obtained from the institutional review boards at each center, which are listed in the Supplementary material.
Definitions
COPD was defined and stratified by the Global Initiative for Obstructive Lung Disease (GOLD) criteria.17 KOCOSS includes following questionnaires to define CB: 1) Do you experience a cough most days, for at least 3 months per year? 2) Have you had cough for more than two consecutive years? 3) Do you produce sputum most days, for at least 3 months per year? and 4) Have you had sputum for more than two consecutive years? Chronic cough and sputum production were defined using these questions. If patient answered “yes” to question 1, then the subject was classified as having chronic cough. If patient answered “yes” to question 3, then they were classified into group with chronic sputum. If patient answered “yes” to 1 and 3, then the subject was defined as having CB. Patients who answered “I don’t know” and those who did not answer a question were excluded. AECOPD was defined as worsening of any respiratory symptom, such as increased sputum volume, purulence, and increased dyspnea, which required treatment with systemic corticosteroids, antibiotics, or both. Dyspnea was evaluated using the mMRC scale, which is a five-point scale with higher scores indicating more severe dyspnea.13,14 The health-related QoL was evaluated with CAT and SGRQ-C scores. CAT comprises eight items that are scored from 0 to 5, and higher score indicates more severe symptom.15 SGRQ-C is a 14-item questionnaire that provides a total score as well as scores for the following three components: symptoms, activities, and impacts.16 Total and component scores were calculated according to algorithms provided in the SGRQ-C instruction manual.18
Statistical analyses
Data are expressed as mean ± standard deviation, median (interquartile range [IQR]), or frequency distribution (%). For between-group comparisons, Student’s t-tests or analyses of variance were used to compare continuous variables and chi-square tests were used to compare categorical variables. Multivariate analyses were conducted using general linear regression. To compare the predictive power of future AECOPD in each model, area under the receiver operating characteristics curve was calculated. Data were analyzed using the STATA program (STATA 12.0 software, StataCorp LP, College Station, TX, USA). A P-value of <0.05 (two-sided P-values examined) was considered statistically significant.
Results
Characteristics of patients with chronic cough
At the time of analysis, a total of 1,613 COPD patients who met the inclusion criteria were enrolled; 1,380 (91.6%) were men and 434 (27.1%) were current smokers (Table 1). The median age of patients was 73 years (IQR, 67–78). GOLD stage 1 (FEV1 >80%), GOLD stage 2 (FEV1, >50% to ≤80%), GOLD stage 3 (FEV1, >30% to ≤50%), and GOLD stage 4 (FEV1 ≤30%) accounted for 138, 833, 527, and 115 patients, respectively. The median follow-up duration was 12.0 (IQR, 6.0–24.0) months. The mean FEV1 was 1.58 ± 0.55 L (% predicted, 59.2 ± 18.3). In total, 377 (23.4%) patients reported chronic cough, 523 (32.4%) reported chronic sputum, and 293 (18.2%) reported both symptoms (Figure 1). Compared with COPD patients without chronic cough, those with chronic cough included younger patients and more current smokers. Furthermore, patients with chronic cough experienced more frequent AECOPD and exhibited lower FEV1 (% predicted) and diffusing capacity of the lungs for carbon monoxide (DLCO; % predicted), more severe dyspnea as assessed using mMRC scale, and poorer QoL as assessed using SGRQ-C. The results of the 6-minute walk distance test were not different between groups (Table 1 and Figure 2). When the patients were classified according to the revised GOLD 2017 criteria, those with chronic cough were more assigned to subgroups B and D, which are more symptomatic subgroups (P<0.001; Figure 3).
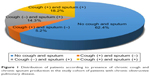  | Figure 1 Distribution of patients according to presence of chronic cough and chronic sputum production in the study cohort of patients with chronic obstructive pulmonary disease. |
The detailed clinical characteristics of COPD patients with chronic cough only, those with chronic sputum only, and those with CB are described in Table 2. COPD patients with chronic cough only were more common in current smokers compared with those without chough or sputum, similar to those with CB.
Impact of chronic cough on lung function, dyspnea, and QoL
Patients with cough only showed more severe airflow limitation during spirometry compared to patients with sputum only, which is a feature of patients with CB (Table 2). Multivariable analysis for FEV1 (% predicted) and DLCO (% predicted) were performed after adjusting for age, current smoking status, and amount of smoking; chronic cough remained a significant risk factor for a lower FEV1 and DLCO in COPD patients. However, sputum production was not found to be a significant factor for lower FEV1 and DLCO (Table 3). There was no significant interaction between cough and sputum with regard to FEV1 (P=0.33) and DLCO (P=0.78). Spirometry was followed up for 730 patients at 1 year later, and mean change of FEV1 was −0.04 ± 0.26 L. The mean changes of FEV1 were not different between groups according to presence of chronic cough (−0.04 ± 0.27 L vs −0.02 ± 0.23 L; P=0.28) or chronic sputum (−0.04 ± 0.26 L vs −0.03 ± 0.27 L; P=0.86).
Multivariate analyses for mMRC, CAT, and SGRQ scores were performed after adjusting for age, sex, body mass index, smoking status, history of previous exacerbation, and baseline FEV1 (% predicted). In each model, chronic cough was independently associated with poorer mMRC (P=0.003) and CAT scores (P<0.001) as well as poorer scores for all three components of SGRQ (P<0.001; Table 3). Although chronic sputum production was also associated with higher score for CAT (P=0.003) and symptom (P<0.001) and impact (P=0.04) components of SGRQ, the differences were not as prominent as those for chronic cough. Furthermore, chronic sputum production did not show an association with mMRC score (P=0.18) or score for activity component (P=0.32) of SGRQ (Table 3). There was no interaction between chronic cough and sputum with regard to mMRC (P=0.99), CAT (P=0.97), and SGRQ (P=0.22) scores.
Impact of chronic cough on the risk of future AECOPD
A total of 291 (18.1%) patients developed AECOPD at least once during the follow-up period. These included 15.5% patients without chronic cough or sputum, 13.3% with chronic sputum only, 17.8% with chronic cough only, and 23.1% with CB. Among them, 70 (24.1%) patients had experienced more than one exacerbation during follow-up, However, there was no significant difference in frequency of patients with multiple exacerbation according to presence of chronic cough (P=0.13) or chronic sputum (P=0.45). In univariate analyses, the risk of future AECOPD was associated with presence of chronic cough [odds ratio (OR), 1.52; P=0.004], but not with presence of chronic sputum (OR, 1.16; P=0.92). There was no interaction between chronic cough and sputum with regard to future exacerbation (P=0.16). Other factors related to future exacerbation included older age (OR, 1.04; P<0.001), previous history of exacerbation (OR, 1.95; P<0.001), and lower baseline FEV1 (% predicted; OR, 0.98; P<0.001). In multivariate analyses adjusted for age, smoking status, history of exacerbation, and baseline FEV1 (% predicted), chronic cough was independently associated with future exacerbation (OR 1.56, 95% CI, 1.08–2.24), whereas chronic sputum did not show any significant association (OR, 0.93; P=0.63; Table 4).
The area under the curve (AUC) for predicting future exacerbation was 0.640 when subgrouping was based on the 2011 GOLD guidelines (model 1) and 0.630 when subgrouping was based on the 2017 GOLD guidelines (model 2). When we added the variable of chronic cough while subgrouping according to the 2017 GOLD guidelines, the AUC increased to 0.653 (model 3), which was found to be the most predictive (Figure 4).
Discussion
In this nationwide cohort analysis, chronic cough in COPD patients was not a simple symptom; rather, it was an independent risk factor for lower FEV1 and DLCO, more severe dyspnea, worse QoL, and future exacerbation. Interestingly, chronic cough itself showed a more significant association with disease severity and prediction of poor outcomes compared with chronic sputum, and the degree of association was similar to that in patients with CB. These findings suggest that chronic cough, and not chronic sputum, could play a role in the effects of CB in COPD patients, and that it may be important to identify patients with chronic cough irrespective of sputum to find the high-risk group.
COPD is associated with chronic inflammatory processes in the airway and parenchyma. A cough reflex can be triggered by several inflammatory or mechanical changes in the airways.19 Furthermore, chronic cough was found to be associated with neutrophilic airway inflammation20,21 and cytokines, such as tumor necrosis factor-alpha or interleukin-8.21 Therefore, it could be postulated that chronic cough and COPD may share common pathophysiological pathways: airway inflammation. Thus, airway inflammation may be critical for chronic cough in COPD,22 and chronic cough could be prerequisite conditions for AECOPD.
Another hypothesis for explaining the association of chronic cough with higher risk of future AECOPD may be linked to transient receptor potential (TRP) channels, particularly transient receptor potential vanilloid 1 (TRPV1). TRPV1 may be a major molecular entity involved in the tussive response.23 The pivotal function of TRPV1 in cough response is to lower the threshold to cough, which is already reduced in COPD patients.24 TRP channels are associated with bronchoconstriction, airway hyper-responsiveness, and neutrophil activation. This TRP channel function may be altered in the presence of oxidative stress, inflammation, hypoxia, and mechanical stress.25 Therefore, these findings may also be a clue for associating chronic cough with an increased risk of future AECOPD.
Reportedly, 3.4%–22.0% adults in the general population26–35 and 14%–74% COPD patients are affected by CB.3–6 Moreover, some studies have reported a positive association between CB and poor COPD outcomes,3–8,34,35 whereas some have reported otherwise. Considering that the wide range of prevalence estimates for CB is because of different definitions for CB in each study, the studies should be reviewed in detail to elucidate the role of cough in the course of COPD. CB is classically defined as chronic cough and sputum production for 3 months a year for two consecutive years. However, various definitions, including one mentioning chronic sputum only, have been used in different studies, particularly in large cohorts. The prevalence of classic CB was 18.2% in the present study, while the prevalence of chronic sputum was up to 32.4%. In the Proyecto Latinoamericano de Investigación en Obstrucción Pulmonar (PLATINO) study,4 CB was defined as chronic sputum production, and the findings revealed more respiratory symptom, worse lung function, and poorer QoL in CB patients. However, the number of exacerbations was not significantly different between patients with chronic sputum and those without, although the proportion of exacerbation was higher in the chronic sputum group. Even in the Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints study,5 which is a larger study, CB was defined as chronic sputum production, and patients with chronic sputum exhibited a poorer QoL. However, there was no significant difference in FEV1 or the number of exacerbations, even though the study included more patients with advanced COPD compared with the PLATINO study. In The Genetic Epidemiology of COPD (COPDGene) study,6 CB was defined as chronic cough and sputum production. The findings revealed more exacerbations and poorer QoL in CB patients, as expected. These inconsistent effects of CB on COPD outcomes in previous studies may be explained by the results of our analysis, which may have been affected by the proportion of patients with chronic cough in each study.
This study could make a significant contribution to clinical practice. This is the first study, to the best of our knowledge, which compares the relative effects of chronic cough and chronic sputum on COPD outcomes and identifies the role of chronic cough in COPD. The findings suggest that assessment of chronic cough is important to evaluate disease severity and predict the future prognosis of COPD patients. Furthermore, this study used longitudinal data for analysis of the risk of future AECOPD. Most previous studies were cross-sectional and compared the rates of previous exacerbations by simple interviews, which is associated with the risk of recall bias.
This study also has several potential limitations. First, the proportion of patients with cough only was relatively smaller than that of patients with CB or sputum only. Second, we could not evaluate the risk of mortality, because of the lack of sufficient cases of death in the KOCOSS cohort. In addition, in KOCOSS, other common causes of chronic cough, including postnasal drip syndrome and esophageal reflux disease, were not ruled out. Third, in a certain portion of patients in our cohort, methacholine provocation test was performed to measure bronchial hyper-responsiveness; however, in most of cases, exclusion for asthma was determined by each physician’s clinical decision, and there could be possibility of existence of patients with asthma-COPD overlap. Fourth, since severity of cough or sputum production was not measured at our questionnaire and presence of cough or sputum was not followed as primary outcome, the changes of these phenotypes could not be evaluated in our study. Finally, because individual items of CAT and SGRQ were not analyzed, sensitivity analysis excluding items regarding cough and sputum could not be performed.
Conclusion
Chronic cough itself is associated with lower FEV1 and DLCO, more severe dyspnea, and worse QoL in COPD patients. Furthermore, it is an independent risk factor for future AECOPD. The symptom of chronic cough could be considered as a unique phenotype during determination of high-risk groups of COPD patients, particularly with regard to exacerbation. Further studies about the natural course and treatment outcomes of COPD patients with chronic cough are necessary.
Disclosure
The authors report no conflicts of interest in this work.
References
Chung KF. Chronic “cough hypersensitivity syndrome”: a more precise label for chronic cough. Pulm Pharmacol Ther. 2011;24(3):267–271. | ||
Chung KF. Advances in mechanisms and management of chronic cough: The Ninth London International Cough Symposium 2016. Pulm Pharmacol Ther. 2017;47:2–8. | ||
Burgel PR, Nesme-Meyer P, Chanez P, et al; Initiatives Bronchopneumopathie Chronique Obstructive (BPCO) Scientific Committee. Cough and sputum production are associated with frequent exacerbations and hospitalizations in COPD subjects. Chest. 2009;135(4):975–982. | ||
de Oca MM, Halbert RJ, Lopez MV, et al. The chronic bronchitis phenotype in subjects with and without COPD: the PLATINO study. Eur Respir J. 2012;40(1):28–36. | ||
Agusti A, Calverley PM, Celli B, et al; Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) investigators. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir Res. 2010;11:122. | ||
Kim V, Davey A, Comellas AP, et al; COPDGene® Investigators. Clinical and computed tomographic predictors of chronic bronchitis in COPD: a cross sectional analysis of the COPDGene study. Respir Res. 2014;15:52. | ||
Lindberg A, Sawalha S, Hedman L, Larsson LG, Lundbäck B, Rönmark E. Subjects with COPD and productive cough have an increased risk for exacerbations and death. Respir Med. 2015;109(1):88–95. | ||
Vestbo J, Prescott E, Lange P. Association of chronic mucus hypersecretion with FEV1 decline and chronic obstructive pulmonary disease morbidity. Copenhagen City Heart Study Group. Am J Respir Crit Care Med. 1996;153(5):1530–1535. | ||
Koo HK, Jeong I, Lee SW, et al. Prevalence of chronic cough and possible causes in the general population based on the Korean National Health and Nutrition Examination Survey. Medicine (Baltimore). 2016;95(37):e4595. | ||
Lee JY, Chon GR, Rhee CK, et al. Characteristics of patients with chronic obstructive pulmonary disease at the first visit to a pulmonary medical center in Korea: the KOrea COpd subgroup study team cohort. J Korean Med Sci. 2016;31(4):553–560. | ||
Standardization of Spirometry, 1994 Update. American Thoracic Society. Am J Respir Crit Care Med. 1995;152(3):1107–1136. | ||
ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–117. | ||
Jones PW, Adamek L, Nadeau G, Banik N. Comparisons of health status scores with MRC grades in COPD: implications for the GOLD 2011 classification. Eur Respir J. 2013;42(3):647–654. | ||
Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest. 1988;93(3):580–586. | ||
Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34(3):648–654. | ||
Meguro M, Barley EA, Spencer S, Jones PW. Development and validation of an improved, COPD-specific version of the St. George Respiratory Questionnaire. Chest. 2007;132(2):456–463. | ||
Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management and Prevention of COPD; 2017. Available from: www.goldcopd.com/guidelines-global-strategyfor-diagnosis-management.html. Accessed March 1, 2017. | ||
Jones PW, Forde Y. St George’s Respiratory Questionnaire for COPD Patients (SGRQ-C) Manual. London: St George’s University of London; 2008. | ||
Chung KF, Pavord ID. Prevalence, pathogenesis, and causes of chronic cough. Lancet. 2008;371(9621):1364–1374. | ||
Niimi A, Torrego A, Nicholson AG, Cosio BG, Oates TB, Chung KF. Nature of airway inflammation and remodeling in chronic cough. J Allergy Clin Immunol. 2005;116(3):565–570. | ||
Jatakanon A, Lalloo UG, Lim S, Chung KF, Barnes PJ. Increased neutrophils and cytokines, TNF-alpha and IL-8, in induced sputum of non-asthmatic patients with chronic dry cough. Thorax. 1999;54(3):234–237. | ||
Chung KF. Advances in mechanisms and management of chronic cough: The Ninth London International Cough Symposium 2016. Pulm Pharmacol Ther. 2017;47:2–8. | ||
Geppetti P, Materazzi S, Nicoletti P. The transient receptor potential vanilloid 1: role in airway inflammation and disease. Eur J Pharmacol. 2006;533(1–3):207–214. | ||
Wong CH, Morice AH. Cough threshold in patients with chronic obstructive pulmonary disease. Thorax. 1999;54(1):62–64. | ||
Abbott-Banner K, Poll C, Verkuyl JM. Targeting TRP channels in airway disorders. Curr Top Med Chem. 2013;13(3):310–321. | ||
von Hertzen L, Reunanen A, Impivaara O, Mälkiä E, Aromaa A. Airway obstruction in relation to symptoms in chronic respiratory disease – a nationally representative population study. Respir Med. 2000;94(4):356–363. | ||
Cerveri I, Accordini S, Verlato G, et al; European Community Respiratory Health Survey (ECRHS) Study. Variations in the prevalence across countries of chronic bronchitis and smoking habits in young adults. Eur Respir J. 2001;18(1):85–92. | ||
Janson C, Chinn S, Jarvis D, Burney P. Determinants of cough in young adults participating in the European Community Respiratory Health Survey. Eur Respir J. 2001;18(4):647–654. | ||
Huchon GJ, Vergnenègre A, Neukirch F, Brami G, Roche N, Preux PM. Chronic bronchitis among French adults: high prevalence and underdiagnosis. Eur Respir J. 2002;20(4):806–812. | ||
Lundbäck B, Lindberg A, Lindström M, et al; Obstructive Lung Disease in Northern Sweden Studies. Not 15 but 50% of smokers develop COPD? Report from the obstructive lung disease in Northern Sweden studies. Respir Med. 2003;97(2):115–122. | ||
Miravitlles M, de la Roza C, Morera J, et al. Chronic respiratory symptoms, spirometry and knowledge of COPD among general population. Respir Med. 2006;100(11):1973–1980. | ||
de Marco R, Accordini S, Cerveri I, et al. Incidence of chronic obstructive pulmonary disease in a cohort of young adults according to the presence of chronic cough and phlegm. Am J Respir Crit Care Med. 2007;175(1):32–39. | ||
Harmsen L, Thomsen SF, Ingebrigtsen T, et al. Chronic mucus hypersecretion: prevalence and risk factors in younger individuals. Int J Tuberc Lung Dis. 2010;14(8):1052–1058. | ||
Pelkonen M, Notkola IL, Nissinen A, Tukiainen H, Koskela H. Thirty-year cumulative incidence of chronic bronchitis and COPD in relation to 30-year pulmonary function and 40-year mortality: a follow-up in middle-aged rural men. Chest. 2006;130(4):1129–1137. | ||
Martinez CH, Kim V, Chen Y, et al; COPDGene Investigators. The clinical impact of non-obstructive chronic bronchitis in current and former smokers. Respir Med. 2014;108(3):491–499. |
Supplementary material
Ethics approval for this study was obtained from the institutional review boards (IRBs) at each center: Seoul National University Hospital IRB, Catholic Medical Center Central IRB, Yonsei University Wonju College of Medicine IRB, Severance Hospital IRB, Soon Chun Hyang University Cheonan Hospital IRB, Ajou University Hospital IRB, Hallym University Dongtan Sacred Heart Hospital IRB, Hallym University Chuncheon Sacred Heart Hospital IRB, Hallym University Pyeongchon Sacred Heart Hospital IRB, Hanyang University Guri Hospital IRB, Konkuk University Hospital IRB, Konkuk University Chungju Hospital IRB, Hallym University Kangdong Sacred Heart Hospital IRB, Hallym University Kangnam Sacred Heart Hospital IRB, Seoul National University Boramae Medical Center IRB, Korea University Guro Hospital IRB, Korea University Anam Hospital IRB, Dongguk University Gyeongju Hospital IRB, Dong-A University Hospital IRB, Gachon University Gil Medical Center IRB, Gangnam Severance Hospital IRB, Kyung Hee University Hospital at Gangdong IRB, Kangbuk Samsung Hospital IRB, Kangwon National University Hospital IRB, Kyungpook National University Hospital IRB, Gyeongsang National University Hospital IRB, Pusan National University Hospital IRB, Soon Chun Hyang University Bucheon Hospital IRB, Seoul National University Bundang Hospital IRB, CHA Bundang Medical Center, CHA University IRB, Asan Medical Center IRB, Inje University Ilsan Paik Hospital IRB, Eulji General Hospital IRB, Samsung Medical Center IRB, Ulsan University Hospital IRB, Soon Chun Hyang University Seoul Hospital IRB, Yeungnam University Hospital IRB, Ewha Womans University Mok-dong Hospital IRB, Inha University Hospital IRB, Chonbuk National University Hospital IRB, and Jeju National University Hospital IRB.
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.







