Back to Journals » Journal of Pain Research » Volume 11
Chronic 17β-estradiol pretreatment has pronociceptive effect on behavioral and morphological changes induced by orofacial formalin in ovariectomized rats
Authors Fejes-Szabó A, Spekker E, Tar L, Nagy-Grócz G, Bohár Z, Laborc KF, Vécsei L, Párdutz A
Received 11 March 2018
Accepted for publication 18 June 2018
Published 25 September 2018 Volume 2018:11 Pages 2011—2021
DOI https://doi.org/10.2147/JPR.S165969
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Erica Wegrzyn
Annamária Fejes-Szabó,1 Eleonóra Spekker,2 Lilla Tar,3 Gábor Nagy-Grócz,1,4 Zsuzsanna Bohár,1,2 Klaudia Flóra Laborc,2,5 László Vécsei,1,2 Árpád Párdutz2
1MTA-SZTE Neuroscience Research Group, Szeged, Hungary; 2Department of Neurology, Faculty of Medicine, Albert Szent-Györgyi Clinical Centre, University of Szeged, Szeged, Hungary; 3Department of Neurology, University of Ulm, Ulm, Germany; 4Faculty of Health Sciences and Social Studies, University of Szeged, Szeged, Hungary; 5Molecular and Behavioral Neuroscience Institute, University of Michigan, Ann Arbor, MI, USA
Background: The prevalence of craniofacial pain disorders show sexual dimorphism with generally more common appearance in women suggesting the influence of estradiol, but the exact cause remains unknown. The common point in the pathogenesis of these disorders is the activation of trigeminal system. One of the animal experimental models of trigeminal activation is the orofacial formalin test, in which we investigated the effect of chronic 17β-estradiol pretreatment on the trigeminal pain-related behavior and activation of trigeminal second-order neurons at the level of spinal trigeminal nucleus pars caudalis (TNC).
Methods: Female Sprague Dawley rats were ovariectomized and silicone capsules were implanted subcutaneously containing cholesterol in the OVX group and 17β-estradiol and cholesterol in 1:1 ratio in the OVX+E2 group. We determined 17β-estradiol levels in serum after the implantation of capsules. Three weeks after operation, 50 µL of physiological saline or 1.5% of formalin solution was injected subcutaneously into the right whisker pad of rats. The time spent on rubbing directed to the injected area and c-Fos immunoreactivity in TNC was measured as the formalin-induced pain-related behavior, and as the marker of pain-related neuronal activation, respectively.
Results: The chronic 17β-estradiol pretreatment mimics the plasma levels of estrogen occurring in the proestrus phase and significantly increased the formalin-induced pain-related behavior and neuronal activation in TNC.
Conclusion: Our results demonstrate that the chronic 17β-estradiol treatment has strong pronociceptive effect on orofacial formalin-induced inflammatory pain suggesting modulatory action of estradiol on head pain through estrogen receptors, which are present in the trigeminal system.
Keywords: c-Fos, headache, pain, sexual dimorphism, trigeminal system
Introduction
The perception of intensity of pain or the responses to painkillers show differences between the sexes.1,2 Lower tolerance for pain, greater ability to discriminate painful sensations, lower pain thresholds and higher pain ratings can be observed in women.1–3 Moreover, the localization of pain according to body parts can amplify this sex-related difference, because this discrepancy is more pronounced in the case of craniofacial pain, which is usually more common in women. For example, temporomandibular disorders, mainly manifested in paroxysmal pain in the masticatory muscles and temporomandibular joints,4 are three times more prevalent in women.5 Also, more women than men suffer from trigeminal neuralgia,6,7 which is characterized by recurrent unilateral brief electric shock-like pains, abrupt in onset and termination, limited to the distribution of one or more divisions of the trigeminal nerve and triggered by innocuous stimuli.8 Significant sex-related differences can be observed among the primary headache disorders as well. Women also have higher prevalence of tension-type headache than men,9 and migraine is three times more common in females compared to males.10 In contrast, cluster headache is five times more frequent in men than women.11
These data indicate that sex hormones may influence the development of trigeminal pain conditions, which is underlined by the observations, that migraine without aura usually starts after menarche, tends to be related to menstrual cycle and ameliorates during pregnancy and after menopause in women.12 In addition, the appearance of migraine without aura is thought to be related to the fall in estrogen concentrations prior to menstruation,12,13 while in migraine with aura an increase in attack frequency can be observed related to high estrogen levels, eg, pregnancy.14 Similarly, the marked female predominance appears only after puberty in the case of tension-type headache as well.15
Animal research data also show the presence of the sexual dimorphism and the modulatory effect of sex hormones on the orofacial pain;16 moreover, site-specific effect of sex hormones on nociception was detected in rats as well.17 These experimental data might give useful information concerning the molecular mechanisms underlying the sex differences in pain conditions, but they are rather controversial.
Concerning the possible mechanisms, these sex-related differences in craniofacial pain disorders suggest that trigeminal neurons are sensitive to sex hormones, which can modulate their function. Hormonal receptors are present in both the trigeminal ganglion and the spinal trigeminal nucleus pars caudalis (TNC) providing the molecular basis for direct modulatory action on the peripheral and central sensitization in the trigeminal system.18–21 At present, however, the exact mechanisms underlying sex-related differences in the prevalence of these craniofacial pain conditions remain obscure.
To get further data on the role of estradiol in the sex-related trigeminal nociception, we investigated trigeminal pain-related behavior and c-Fos immunoreactivity – a morphological marker of trigeminal activation – in rats with stable low and stable high estrogen levels in the orofacial formalin test.
Materials and methods
Animals
Fifty-five female Sprague Dawley rats weighing 150–250 g were used. The animals were raised and housed under standard laboratory conditions (in an air-conditioned, humidity-controlled and ventilated room), with drinking water and regular rat chow available ad libitum on a 12 h- 12 h dark–light cycle. The procedures used in this study followed the guidelines of the International Association for the Study of Pain and the directive of the European Economic Community (86/609/ECC). They were approved by the Committee of Animal Research at the University of Szeged (I-74-12/2012) and the Scientific Ethics Committee for Animal Research of the Protection of Animals Advisory Board (XXIV/352/2012). All efforts were made to minimize the number of animals used and their suffering.
Ovariectomy
The animals were ovariectomized under deep chloral hydrate (0.4 g/kg body weight, catalog ID: 23100; Sigma-Aldrich, St. Louis, MO, USA) anesthesia administered intraperitoneally. Prior to surgery, rats’ back were shaved with electric clippers and furs were removed completely. Cutasept was applied to the shaved area to disinfect the skin. Ovariectomy was preceded by a midline dorsal skin incision, 3 cm long, approximately half way between the middle of the back and the base of the tail. 1.5 cm long peritoneal incisions were made bilaterally. After access into the peritoneal cavity, the ovary and associated fat were easily found, and exteriorized by gentle retraction. Ligature of the blood vessels was also performed. The connection between the Fallopian tube and the uterine horn was cut and the ovaries were removed. Afterward, the animals were randomly divided into two groups: 1) In the OVX group, the rats had two 15 mm long silastic capsule (3.18 mm outer diameter and 1.57 mm inner diameter, catalog ID: 508–008; Dow Corning, Midland, Michigan, USA) filled with cholesterol (15 mg, catalog ID: C8667; Sigma-Aldrich) as control. 2) In the OVX+E2 group, the animals received two 15 mm long silastic capsule filled with an 1:1 mixture of 17β-estradiol (7.5 mg, catalog ID: 75262; Fluka, Sigma-Aldrich) and cholesterol (7.5 mg). Capsules were inserted subcutaneously in the interscapular region. After implantation of capsules, peritoneal cavity and skin were closed with absorbable sutures. High degree of aseptic procedure was maintained throughout the operation. Surgical instruments were sterilized in 70% ethanol. During and after the surgery, animals were placed on heating plate and covered with paper in order to avoid hypothermia. The analgesia and attenuation of inflammation were provided by subcutaneous (sc) administration of carprofen (5 mg/kg body weight) three times: once before the operation and twice after the surgery (24 and 48 hours).
Measurement of estradiol concentration
17β-estradiol concentration of serum was measured in both groups (n=5). The blood samples were taken weekly from the tail vein for 5 weeks. The serum was cleared from cellular components of the blood by centrifugation at 12,000 rpm for 10 minutes at 4°C and stored at –80°C until use. The concentrations were measured by using Estradiol EIA Kit (catalog ID: 582251; Cayman Chemical Company, Ann Arbor, MI USA) based on the guidelines of the manufacturer.
Behavioral test
Both groups (OVX and OVX+E2) of animals were divided further into two subgroups (n=10–12 per subgroup): In the OVX-Phys and OVX+E2-Phys subgroups, the animals received a sc injection of 50 µL physiological saline administered via a 26-gauge needle into the right whisker pad after 3 weeks of recovery following the ovariectomy. In the OVX-Form and OVX+E2-Form subgroups, the rats were injected with sc 50 µL 1.5% formalin solution (containing 0.55% formaldehyde) diluted in physiological saline via a 26-gauge needle into the right whisker pad. According to Clavelou et al, this concentration is the most appropriate to detect changes in pain-related behavior of rats.22
The testing procedures were performed during the light phase (between 8 a.m. and 2 p.m.) in a quiet room. The test box was a 30×30×30 cm glass terrarium with mirrored walls. For the off-line analysis of rubbing activity directed to the whisker pad, the behavior of the individually tested rats was recorded with a video camera (Logitech HD Webcam C615; Logitech Inc., Newark, NJ, USA) situated 1 m above the terrarium. After 10 minutes habituation in the test box, the whisker pads of the rats were injected with formalin or physiological saline and the animals were replaced immediately back in the chamber for 45 minutes. The rats did not receive any food or water during the observation period. The test box was cleaned and decontaminated after each animal. An observer blind to the experimental procedures analyzed the recorded videos. The 45-minute recording period was divided into 15×3 minutes blocks and the total time (number of seconds) spent on rubbing directed to the injected area with the ipsilateral fore- or hindpaw was measured in each block and defined as the nociceptive score for that block. The grooming activity of physiological saline-injected animals was used as control based on an earlier study.22
c-Fos immunohistochemistry
Four hours after the formalin or physiological saline injection, the rats were perfused transcardially with 100 mL of phosphate-buffered saline, followed by 500 mL of 4% paraformaldehyde in phosphate buffer under deep chloral hydrate anesthesia. The medullary segment containing the TNC between +1 and –5 mm from the obex was removed, postfixed overnight for immunohistochemistry in the same fixative and cryoprotected (10% sucrose for 2 hours, 20% sucrose until the blocks sank and 30% sucrose overnight). Before sectioning, each segment was marked with a small incision on the ventral and left (contralateral) side of the tissue block, allowing side discrimination during the quantification process; 30 µm transverse cryostat sections were cut through the rostrocaudal axis from the beginning of the TNC and were serially collected in wells containing cold PBS. Each well contained every tenth section at 0.3 mm intervals along the rostrocaudal axis (15 levels [sections]/well). The free-floating sections were rinsed in PBS and immersed in 0.3% H2O2 in PBS for 30 minutes to suppress endogenous peroxidase activity. After several rinses in PBS containing 1% Triton X-100 (PBST), sections were incubated at room temperature overnight in PBST containing rabbit anti-rat c-Fos polyclonal antibody (catalog ID: sc-52, RRID: AB_2106783; Santa Cruz Biotechnology Inc., Dallas, TX, USA) at a dilution of 1:2000. The immunohistochemical reaction was visualized by using Vectastain Elite Avidin-Biotin Kits (catalog ID: PK6101; Vector Laboratories, Burlingame, CA, USA). Briefly, the sections were incubated at room temperature for 2 hours in PBST containing goat anti-rabbit biotinylated secondary antibody. After several rinses in PBST, and incubation at room temperature for 2 hours in PBST containing avidin and biotinylated horseradish peroxidase, the immunohistochemical labeling was visualized with 3,3′-diaminobenzidine intensified with nickel ammonium sulphate. The specificity of the immune reactions was checked by omitting the primary antiserum.
The counting of immunoreactive (IR) cells in the TNC was performed by an observer blinded to the experimental procedures under the 10× objective of a Nikon Optiphot-2 light microscope in every tenth transverse section in each animal. Before the counting, the location of each section along the rostrocaudal axis and the location of the TNC on each medullary section were determined by means of The Rat Brain in Stereotaxic Coordinates Atlas.23 The c-Fos neurons with obvious specific nuclear staining were counted in the TNC both ipsilaterally and contralaterally to the formalin or physiological saline injection.
Statistical analysis
For statistical comparison of 17β-estradiol concentration of serum in the two groups (OVX and OVX+E2), we used two-way repeated-measures ANOVA. Pairwise comparisons of group means were based on the estimated marginal means with Sidak adjustment for multiple comparisons.
In the behavioral study, we compared the nociceptive responses in two time periods as described by Clavelou et al.22 The first 3 minutes (block 1) are characterized by intensive rubbing activity and defined as the first phase. Following a relatively relaxed period, the rubbing intensifies again between about 12 and 45 minutes (second phase) and remains high for a longer period of time (blocks 5–15), which is defined as the second phase. For the comparison of the rubbing activities between subgroups (OVX-Phys, OVX-Form, OVX+E2-Phys and OVX+E2-Form) in the first and second phase, we used one-way ANOVA followed by the Tamhane post hoc test.
The number of c-Fos-IR neurons in the various subgroups (OVX-Phys, OVX-Form, OVX+E2-Phys and OVX+E2-Form) were compared at each level of 0.3 mm (15 levels) along the rostrocaudal axis by using two-way repeated-measures ANOVA. There was no significant difference in the number of c-Fos-IR neurons between the contra- and ipsilateral sides in rats injected with sc physiological saline and the contralateral sides in animals injected with sc formalin (OVX-Form and OVX+E2-Form); therefore, the data obtained from the contralateral sides of the subgroups injected with sc formalin (OVX-Form and OVX+E2-Form) were used as controls in the statistical analysis. Pairwise comparisons of subgroup means were based on the estimated marginal means with Sidak adjustment for multiple comparisons.
All tests were two-sided, and probability levels P<0.05 were considered to be statistically significant. Group values are reported as mean ± standard error of the mean (SEM).
Statistical analysis of measurements was carried out with IBM SPSS Statistics, version 20 software (IBM Corporation, Armonk, NY, USA).
Results
Estradiol concentration
Following the ovariectomy, in both groups, an approximate steady state status in serum concentration of 17β-estradiol was maintained for 5 weeks with an average value of 25.93 pg/mL in the OVX group and 64.55 pg/mL in the OVX+E2 group (Figure 1). In OVX+E2 group, the ovariectomized female rats received two silastic capsules containing in all 15 mg 17β-estradiol, which chronic pretreatment keeps the serum concentration of 17β-estradiol at significantly higher level compared with the OVX group (***P<0.001; Figure 1). Although we did not find significant difference between the serum levels measured weekly in OVX+E2 group, a tendency of lower level in the fifth week can be observed (Figure 1). This result suggests that serum concentration of 17β-estradiol began to decrease in the fifth week similarly to data published in an earlier work.24 Since a stable serum concentration was found from second to fourth week (Figure 1), we examined the potential modulating effect of chronic 17β-estradiol pretreatment in the third week following the ovariectomy and implantation of capsules. At this time point, the average estradiol concentration in the serum was 25.01 pg/mL in OVX group and 61.29 pg/mL in OVX+E2 group (Figure 1).
Nociceptive response
The behavioral pattern following orofacial injection of formalin observed in the rats is in accordance with previous findings.22,25,26 After the formalin injection, the rats immediately withdrew their heads, often accompanied by vocalization. Following their return to the observation box, the rats started to rub their whisker pad continuously and intensely with the ipsilateral forepaw accompanied often by the contralateral forepaw, and occasionally scraped the perinasal area with the ipsilateral hindpaw after a quiet period of ~20 seconds. This period, referred to as the first phase, lasted ~3–4 minutes, and was followed by a quiescent period of 9–10 minutes, separating the first phase from the second phase (Figure 2). The second phase was characterized by less intense, but continuous rubbing of the face, predominantly with the ipsilateral forepaw consorted often by the contralateral forepaw as well. This tonic phase lasted ~30–33 minutes (Figure 2). In both subgroups injected with formalin (OVX-Form and OVX+E2-Form), the biphasic pain-related behavioral pattern can be observed (Figure 2). In the OVX+E2-Form subgroup, this pattern was clearly more pronounced than that in the OVX-Form subgroup (Figure 2), and such behavior was not detected at all in the OVX-Phys and OVX+E2-Phys subgroups, where the animals displayed very little rubbing activity (Figure 2).
As a result of the statistical analysis of the two phases, we found that the face rubbing activity in the OVX-Form and OVX+E2-Form subgroups was significantly higher during both the first (*P<0.01; ***P<0.001) and the second phase (***P<0.001) than in the OVX-Phys and OVX+E2-Phys subgroups (Figure 2).
Data obtained from OVX-Form and OVX+E2-Form subgroups show that chronic 17β-estradiol pretreatment significantly increased the nociceptive behavior in both phases (#P<0.05; ###P<0.001; Figure 2).
c-Fos in the TNC
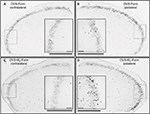
Microscopic examination of the immunostained transverse sections of medulla containing the TNC revealed c-Fos immunoreactivity in the nuclei of the neurons. In the OVX-Form and OVX+E2-Form subgroups, unilateral sc formalin injection produced an increase in the number of c-Fos-IR neurons in the dorsal, superficial area of the ipsilateral TNC when compared with the non-treated contralateral side (Figure 3). This increase was significant at all levels along the rostrocaudal axis, in accordance with the somatotopic representation (*P<0.05; **P<0.01; ***P<0.001;+ P<0.05;++ P<0.01;+++ P<0.001; Figure 4).
In the OVX+E2-Form subgroup, the effect of formalin on the number of c-Fos-IR neurons seems to be similar, but more pronounced than that in the OVX-Form subgroup (Figure 3). The results of statistical analysis show that this chronic 17β-estradiol-induced increase in the formalin-related activation of the second-order trigeminal neurons is significant at several levels of the TNC along the rostrocaudal axis (#P<0.05; ##P<0.01; ###P<0.001; Figure 4). On the contralateral sides of the TNCs, there were no significant differences either between the subgroups or between the different levels along the rostrocaudal axis (Figure 4).
Discussion
Although, there is a clear female predominance in craniofacial pain disorders suggesting the modulatory role of sex hormones, there are only relatively few and conflicting studies, which investigated their influence on the trigeminal nociception. In the present experiments, we examined the effect of chronic, stable high 17β-estradiol level in serum on trigeminal pain and trigeminal activation in orofacial formalin test of rat.
The source of estradiol found in OVX rats is probably extragonadal.27 On the other hand, the used chronic estradiol pretreatment resulted in an average serum 17β-estradiol level of 61.29 pg/mL in OVX+E2 group of rats, which is comparable with the value of serum concentration of estradiol in cycling rats during the proestrus phase, when the estrogen concentration is at its peak level.28–31
Our data show that the chronic 17β-estradiol treatment was pronociceptive in orofacial formalin test compared with the control, ovariectomized, female rats. The effect of estradiol was shown in both the first and the second phase of orofacial formalin test, where the first phase is caused by the direct chemical stimulation of the nociceptors by the formalin solution, while the second phase is the result of peripheral inflammation.32 Furthermore, this chronic estradiol treatment enhanced the formalin-induced trigeminal activation at the level of second-order trigeminal neurons located in the TNC, as reflected by the increased c-Fos immunoreactivity, which is one of the anatomical markers of the pain-induced neuronal activity.33
The molecular basis for estrogen to directly regulate the pain transmission at the level of trigeminal system is mediated by estrogen receptors, which has three known types: estrogen receptor alpha (ERα), ERβ and G-protein-coupled estrogen receptor (or G-protein-coupled receptor-30 [GPR30]). These receptors are present in the trigeminal system. ERα can be observed in 22% of primary trigeminal neurons of rat, where it is found mainly in nuclei of cells with larger diameter and in cytoplasm of smaller neurons.34 Satellite glia in the trigeminal ganglion of rat also express ERα.35 ERβ is also present in both small to large neurons in the Gasserian ganglion of rat, but not in the satellite glia.35 GPR30 receptor can be observed in 35% of neurons in trigeminal ganglion of rat and shows cytoplasmatic localization mainly in small diameter neurons with unmyelinated axons, but it is present in neurons with myelinated axons with a broad range of cell sizes, too.34 ERα and GPR30 are colocalized in 10% of primary trigeminal neurons of rat.34 In superficial laminae of rat TNC, ERα and ERβ proteins are co-expressed by neurons,18 and in this area, ERα was shown to be present in nociceptive-responsive neurons.36 GPR30 IR cells were also localized in the mouse TNC.37 In human TNC, ERα immunostaining was found in the nucleus and cytoplasm of neurons and glial cells and in the nerve fibers; ERβ was detected in the cytoplasm of neuronal cells.38 Experimental data show that the modulation of these receptors results in well-defined changes of trigeminal pain processing. In temporomandibular joint inflammation induced by Complete Freund’s Adjuvant (CFA), estradiol potentiated the effect in dose-dependent manner and the blocking of estrogen receptors by an antagonist was able to evolve anti-inflammatory action.39 It was also shown that estrogen receptors located in different parts of the trigeminal system might mediate pronociceptive responses to estrogen. In the same animal model, estrogen receptor stimulation by specific agonists enhanced the secondary mechanical allodynia and the authors found in the trigeminal ganglion an increased immunoreactivity of the activated extracellular signal-regulated kinase, which is a specific marker of pain-induced activation of nociceptor.34 The involvement of trigeminal ganglion in this process was shown in primary trigeminal cultures: microarray and protein activity assays also demonstrated the estrogen-induced activation of ERK.40
Effects of estrogen may manifest through two different pathways: a slow genomic and a rapid non-genomic mechanism41,42 and both pathways play important role in regulation of trigeminal pain processing. By influencing the transcription of certain genes (mitogen-activated protein kinase-1, interleukin-1 receptor type I, bradykinin B2 receptor, GABA transporter protein, GABA A receptor subunit a6, opioid receptor-like 1 receptor, purinoreceptor P2X3, transient receptor potential vanilloid 1 and neuropeptide Y) with potential relevance to craniofacial pain, long-lasting changes were reported in different cells.35,40,43–46 Estrogen can activate intracellular signaling pathways via non-genomic, membrane-mediated mechanisms also, which may occur within seconds or minutes.40,47–50 These cellular mechanisms affect numerous processes, which are essential in trigeminal pain perception including the function of endogenous antinociceptive system,51,52 the modulation of the excitability of TNC neurons.53 In other studies, alteration in the activation mechanisms,54 in the neuronal firing activity48 and in the glutamatergic neurotransmission55,56 was demonstrated. On the other hand, estrogen-dependent changes were reported in the expression of several factors such as calcium/calmodulin-dependent protein kinase II α, calcitonin gene-related peptide or serotonin, as well. These molecules are playing important role during pain processing.57–59
Our present data on the pronociceptive effect of estradiol are supported by earlier studies. In ovariectomized rats, estradiol valerate replacement, administered as a single, sc injection, increased primary (in masseter muscle) and secondary (in whisker pad) facial allodynia after CFA-induced inflammation of masseter muscle mediated by ERK activation in the trigeminal ganglion.49 Kou et al39 reported that in ovariectomized rats 17β-estradiol, subcutaneously administered for 10 days, potentiated the inflammation and exacerbated the pain-induced decrease in the food intake in temporomandibular joint inflammation model. The inflammation was induced by intraarticular injection of CFA and the effect was dose-dependent. The authors discuss the possibility of estrogen effect through the nuclear factor-κB (NF-κB) pathway inducing the enhancement of the DNA-binding activity of NF-κB and the increased transcription of its target genes in the synovial membrane. In another experiment, a single sc injection of 17β-estradiol, administered 48 hours before the testing, worsened the thermal hyperalgesia in orofacial inflammation caused by sc injected carrageenan in ovariectomized rats, which may be caused by the decreased α2-adrenoceptor-mediated inhibition of nociception and hyperalgesia.60 High 17β-estradiol for 2 days, mimicking the plasma levels of estrogen in proestrus, significantly increased the duration of pain-related behavior (eye wipe test induced by capsaicin) and the activation of trigeminal neurons in TNC indicated by c-Fos immunoreactivity. The authors conclude that the effect may be in part due to the estrogen-dependent increases in mRNA of transient receptor potential vanilloid 1 and anoctamin 1 in TNC, which have important role in trigeminal pain.46
The complexity of the possible mechanisms involved may explain the conflicting data on the effect of estrogen on trigeminal pain and trigeminal system. Antinociceptive effect of single sc estradiol replacement administered 18–24 hours prior to the nociceptive testing was shown by Flores et al61 on facial pain-related behavior induced by intracisternally injected N-methyl-d-aspartic acid. In other experiments, pronociceptive effect of low serum level of estrogen was reported in rats in the temporomandibular joint inflammation,50,62,63 in the orofacial formalin model,17 in the basic facial mechanical pain threshold45 or after ligature of the masseter tendon.64 There are also results, however, which show that serum level of estrogen do not have any effect on trigeminal pain for example in the eye wipe test after ten-day long pretreatment with daily sc 17β-estradiol-3-benzoate54 and in the masseter inflammation in normally cycling female and male rats.51 Similarly, estradiol benzoate, given subcutaneously 48 hours prior to the nociceptive testing, had no effect in rats on facial pain-related behavior induced by intracisternally injected NMDA in males, in ovariectomized females and in normally cycling females tested at proestrus or diestrus stages.65
Conclusion
We can conclude that chronic 17β-estradiol pretreatment was able to significantly influence both the formalin-induced nociceptive behavior and the c-Fos expression at the level of trigeminal system suggesting its pronociceptive effect on the trigeminal pain and the potentiation of activation of TNC neurons. For the first time, we maintained continuously high 17β-estradiol serum level for a relatively longer period (21 days) in ovariectomized female rats, excluding the effect of other sexual hormones in the orofacial formalin test. The estrogen receptors are present in all key areas of trigeminal nociception, including the trigeminal ganglion and the TNC, and we hypothesize that the hormone may influence the neuronal processes induced by pain through the increase of mRNA of transient receptor potential vanilloid 1 and anoctamin 1 in TNC, or modulation of the NF-κB pathway or activation of ERK in the trigeminal ganglion.
Acknowledgments
We thank Mrs Valéria Vékony for the histotechnical assistance. This work was supported by the Hungarian Brain Research Program (grant no.: KTIA_13_NAP-A-III/9) and the GINOP-2.3.2-15-2016-00034. Dr. Árpád Párdutz was supported by the Bolyai Scholarship Programme of the Hungarian Academy of Sciences.
Author contributions
The specific contributions of the authors to this research were as follows: AFSz participated in the design and implementation of experiments, collected data for statistical analysis, interpreted the data and wrote the manuscript. ES, LT and GNG participated in the design and in the implementation of the experiments and in the critical revision of the manuscript. ZB participated in the design of the experiments and in the critical revision of the manuscript. KFL participated in the critical revision of the manuscript. LV participated in the design of the experiments, in the critical revision of the manuscript and in the final approval of the version to be published. ÁP participated in the design of the experiments, the interpretation of the data and the writing and critical revision of the manuscript. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Walker JS, Carmody JJ. Experimental pain in healthy human subjects: gender differences in nociception and in response to ibuprofen. Anesth Analg. 1998;86(6):1257–1262. | ||
Feine JS, Bushnell MC, Miron D, Duncan GH. Sex differences in the perception of noxious heat stimuli. Pain. 1991;44(3):255–262. | ||
Wise EA, Price DD, Myers CD, Heft MW, Robinson ME. Gender role expectations of pain: relationship to experimental pain perception. Pain. 2002;96(3):335–342. | ||
McNeill C, Mohl ND, Rugh JD, Tanaka TT. Temporomandibular disorders: diagnosis, management, education, and research. J Am Dent Assoc. 1990;120(3):253–263. | ||
LeResche L. Epidemiology of temporomandibular disorders: implications for the investigation of etiologic factors. Crit Rev Oral Biol Med. 1997;8(3):291–305. | ||
Katusic S, Beard CM, Bergstralh E, Kurland LT. Incidence and clinical features of trigeminal neuralgia, Rochester, Minnesota, 1945-1984. Ann Neurol. 1990;27(1):89–95. | ||
Mueller D, Obermann M, Yoon MS, et al. Prevalence of trigeminal neuralgia and persistent idiopathic facial pain: a population-based study. Cephalalgia. 2011;31(15):1542–1548. | ||
Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia. 2013;33(9):629–808. | ||
Rasmussen BK, Jensen R, Schroll M, Olesen J. Epidemiology of headache in a general population--a prevalence study. J Clin Epidemiol. 1991;44(11):1147–1157. | ||
Lipton RB, Stewart WF, Diamond S, Diamond ML, Reed M. Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache. 2001;41(7):646–657. | ||
Lieba-Samal D, Wöber C. Sex hormones and primary headaches other than migraine. Curr Pain Headache Rep. 2011;15(5):407–414. | ||
Sacco S, Ricci S, Degan D, Carolei A. Migraine in women: the role of hormones and their impact on vascular diseases. J Headache Pain. 2012;13(3):177–189. | ||
Somerville BW. Estrogen-withdrawal migraine. I. Duration of exposure required and attempted prophylaxis by premenstrual estrogen administration. Neurology. 1975;25(3):239–244. | ||
MacGregor EA. Oestrogen and attacks of migraine with and without aura. Lancet Neurol. 2004;3(6):354–361. | ||
Laurell K, Larsson B, Eeg-Olofsson O. Prevalence of headache in Swedish schoolchildren, with a focus on tension-type headache. Cephalalgia. 2004;24(5):380–388. | ||
Cairns BE. The influence of gender and sex steroids on craniofacial nociception. Headache. 2007;47(2):319–324. | ||
Pajot J, Ressot C, Ngom I, Woda A. Gonadectomy induces site-specific differences in nociception in rats. Pain. 2003;104(1–2):367–373. | ||
Bereiter DA, Cioffi JL, Bereiter DF. Oestrogen receptor-immunoreactive neurons in the trigeminal sensory system of male and cycling female rats. Arch Oral Biol. 2005;50(11):971–979. | ||
Gupta S, McCarson KE, Welch KM, Berman NE. Mechanisms of pain modulation by sex hormones in migraine. Headache. 2011;51(6):905–922. | ||
Lee KS, Asgar J, Zhang Y, Chung MK, Ro JY. The role of androgen receptor in transcriptional modulation of cannabinoid receptor type 1 gene in rat trigeminal ganglia. Neuroscience. 2013;254:395–403. | ||
Lee KS, Zhang Y, Asgar J, et al. Androgen receptor transcriptionally regulates μ-opioid receptor expression in rat trigeminal ganglia. Neuroscience. 2016;331:52–61. | ||
Clavelou P, Dallel R, Orliaguet T, Woda A, Raboisson P. The orofacial formalin test in rats: effects of different formalin concentrations. Pain. 1995;62(3):295–301. | ||
Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6th ed. Amsterdam, Boston:: Academic Press/Elsevier; 2007. | ||
Mannino CA, South SM, Inturrisi CE, Quinones-Jenab V. Pharmacokinetics and effects of 17beta-estradiol and progesterone implants in ovariectomized rats. J Pain. 2005;6(12):809–816. | ||
Clavelou P, Pajot J, Dallel R, Raboisson P. Application of the formalin test to the study of orofacial pain in the rat. Neurosci Lett. 1989;103(3):349–353. | ||
Raboisson P, Dallel R. The orofacial formalin test. Neurosci Biobehav Rev. 2004;28(2):219–226. | ||
Zhao H, Tian Z, Hao J, Chen B. Extragonadal aromatization increases with time after ovariectomy in rats. Reprod Biol Endocrinol. 2005;3:6. | ||
Edwards HE, Burnham WM, Mendonca A, Bowlby DA, MacLusky NJ. Steroid hormones affect limbic afterdischarge thresholds and kindling rates in adult female rats. Brain Res. 1999;838(1–2):136–150. | ||
Menjívar M, Cárdenas M, Ortiz G, Pedraza-Chaverrí J. Fertility diminution in female rats with experimental chronic nephrosis. Biol Reprod. 2000;63(5):1549–1554. | ||
Kaneko K, Aoki H, Furuichi T, Hatori S, Tanimoto H, Kawakami S. Influence of uterine inflammation on the estrous cycle in rats. J Reprod Dev. 2004;50(3):361–367. | ||
Faccio L, Da Silva AS, Tonin AA, et al. Serum levels of LH, FSH, estradiol and progesterone in female rats experimentally infected by Trypanosoma evansi. Exp Parasitol. 2013;135(1):110–115. | ||
Tjølsen A, Berge OG, Hunskaar S, Rosland JH, Hole K. The formalin test: an evaluation of the method. Pain. 1992;51(1):5–17. | ||
Harris JA. Using c-fos as a neural marker of pain. Brain Res Bull. 1998;45(1):1–8. | ||
Liverman CS, Brown JW, Sandhir R, McCarson KE, Berman NE. Role of the oestrogen receptors GPR30 and ERalpha in peripheral sensitization: relevance to trigeminal pain disorders in women. Cephalalgia. 2009;29(7):729–741. | ||
Puri J, Bellinger LL, Kramer PR. Estrogen in cycling rats alters gene expression in the temporomandibular joint, trigeminal ganglia and trigeminal subnucleus caudalis/upper cervical cord junction. J Cell Physiol. 2011;226(12):3169–3180. | ||
Amandusson A, Blomqvist A. Estrogen receptor-alpha expression in nociceptive-responsive neurons in the medullary dorsal horn of the female rat. Eur J Pain. 2010;14(3):245–248. | ||
Hazell GG, Yao ST, Roper JA, Prossnitz ER, O’Carroll AM, Lolait SJ. Localisation of GPR30, a novel G protein-coupled oestrogen receptor, suggests multiple functions in rodent brain and peripheral tissues. J Endocrinol. 2009;202(2):223–236. | ||
Fenzi F, Rizzzuto N. Estrogen receptors localization in the spinal trigeminal nucleus: an immunohistochemical study in humans. Eur J Pain. 2011;15(10):1002–1007. | ||
Kou XX, Wu YW, Ding Y, et al. 17β-estradiol aggravates temporomandibular joint inflammation through the NF-κB pathway in ovariectomized rats. Arthritis Rheum. 2011;63(7):1888–1897. | ||
Puri V, Puri S, Svojanovsky SR, et al. Effects of oestrogen on trigeminal ganglia in culture: implications for hormonal effects on migraine. Cephalalgia. 2006;26(1):33–42. | ||
Heldring N, Pike A, Andersson S, et al. Estrogen receptors: how do they signal and what are their targets. Physiol Rev. 2007;87(3):905–931. | ||
Srivastava DP, Woolfrey KM, Penzes P. Insights into rapid modulation of neuroplasticity by brain estrogens. Pharmacol Rev. 2013;65(4):1318–1350. | ||
Puri V, Cui L, Liverman CS, et al. Ovarian steroids regulate neuropeptides in the trigeminal ganglion. Neuropeptides. 2005;39(4):409–417. | ||
Flores CA, Shughrue P, Petersen SL, Mokha SS. Sex-related differences in the distribution of opioid receptor-like 1 receptor mRNA and colocalization with estrogen receptor mRNA in neurons of the spinal trigeminal nucleus caudalis in the rat. Neuroscience. 2003;118(3):769–778. | ||
Yu LH, Li N, Liu CY, Ma B. Estrogen altered facial mechanical pain threshold and trigeminal P2X3 receptor expression. Neuro Endocrinol Lett. 2011;32(6):811–815. | ||
Yamagata K, Sugimura M, Yoshida M, et al. Estrogens Exacerbate Nociceptive Pain via Up-Regulation of TRPV1 and ANO1 in Trigeminal Primary Neurons of Female Rats. Endocrinology. 2016;157(11):4309–4317. | ||
Tashiro A, Okamoto K, Bereiter DA. Chronic inflammation and estradiol interact through MAPK activation to affect TMJ nociceptive processing by trigeminal caudalis neurons. Neuroscience. 2009;164(4):1813–1820. | ||
Tashiro A, Okamoto K, Bereiter DA. Rapid estrogenic effects on TMJ-responsive brainstem neurons. J Dent Res. 2012;91(2):210–214. | ||
Liverman CS, Brown JW, Sandhir R, Klein RM, McCarson K, Berman NE. Oestrogen increases nociception through ERK activation in the trigeminal ganglion: evidence for a peripheral mechanism of allodynia. Cephalalgia. 2009;29(5):520–531. | ||
Fávaro-Moreira NC, Torres-Chávez KE, Fischer L, Tambeli CH. Peripheral estradiol induces temporomandibular joint antinociception in rats by activating the nitric oxide/cyclic guanosine monophosphate signaling pathway. Neuroscience. 2009;164(2):724–732. | ||
Niu KY, Zhang Y, Ro JY. Effects of gonadal hormones on the peripheral cannabinoid receptor 1 (CB1R) system under a myositis condition in rats. Pain. 2012;153(11):2283–2291. | ||
Tashiro A, Okamoto K, Bereiter DA. Morphine modulation of temporomandibular joint-responsive units in superficial laminae at the spinomedullary junction in female rats depends on estrogen status. Eur J Neurosci. 2008;28(10):2065–2074. | ||
Flake NM, Bonebreak DB, Gold MS. Estrogen and inflammation increase the excitability of rat temporomandibular joint afferent neurons. J Neurophysiol. 2005;93(3):1585–1597. | ||
Diogenes A, Patwardhan AM, Jeske NA, et al. Prolactin modulates TRPV1 in female rat trigeminal sensory neurons. J Neurosci. 2006;26(31):8126–8136. | ||
Gazerani P, Dong X, Wang M, Kumar U, Cairns BE. Sensitization of rat facial cutaneous mechanoreceptors by activation of peripheral N-methyl-d-aspartate receptors. Brain Res. 2010;1319:70–82. | ||
Bereiter DA, Benetti AP. Amino acid release at the spinomedullary junction after inflammation of the TMJ region in male and female rats. Pain. 2006;126(1–3):175–183. | ||
Multon S, Pardutz A, Mosen J, et al. Lack of estrogen increases pain in the trigeminal formalin model: a behavioural and immunocytochemical study of transgenic ArKO mice. Pain. 2005;114(1–2):257–265. | ||
Pardutz A, Hoyk Z, Varga H, Vecsei L, Schoenen J. Oestrogen-modulated increase of calmodulin-dependent protein kinase II (CamKII) in rat spinal trigeminal nucleus after systemic nitroglycerin. Cephalalgia. 2007;27(1):46–53. | ||
Pardutz A, Multon S, Malgrange B, Parducz A, Vecsei L, Schoenen J. Effect of systemic nitroglycerin on CGRP and 5-HT afferents to rat caudal spinal trigeminal nucleus and its modulation by estrogen. Eur J Neurosci. 2002;15(11):1803–1809. | ||
Nag S, Mokha SS. Activation of the trigeminal α2-adrenoceptor produces sex-specific, estrogen dependent thermal antinociception and antihyperalgesia using an operant pain assay in the rat. Behav Brain Res. 2016;314:152–158. | ||
Flores CA, Wang XM, Zhang KM, Mokha SS. Orphanin FQ produces gender-specific modulation of trigeminal nociception: behavioral and electrophysiological observations. Neuroscience. 2001;105(2):489–498. | ||
Kramer PR, Bellinger LL. The effects of cycling levels of 17beta-estradiol and progesterone on the magnitude of temporomandibular joint-induced nociception. Endocrinology. 2009;150(8):3680–3689. | ||
Fischer L, Torres-Chávez KE, Clemente-Napimoga JT, et al. The influence of sex and ovarian hormones on temporomandibular joint nociception in rats. J Pain. 2008;9(7):630–638. | ||
Kramer PR, Bellinger LL. Infusion of Gabrα6 siRNA into the trigeminal ganglia increased the myogenic orofacial nociceptive response of ovariectomized rats treated with 17β-estradiol. Neuroscience. 2014;278:144–153. | ||
Nag S, Mokha SS. Activation of alpha2-adrenoceptors in the trigeminal region produces sex-specific modulation of nociception in the rat. Neuroscience. 2006;142(4):1255–1262. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.