Back to Journals » Journal of Inflammation Research » Volume 16
Chonggu Granules Improve Cartilage Matrix Metabolism in Knee Osteoarthritis via the miR-148a-3p/Wnt/β-Catenin Pathway
Authors Cheng L, Huang C, Li M, Shang S, Chen J, Tang Z
Received 2 August 2023
Accepted for publication 27 September 2023
Published 20 October 2023 Volume 2023:16 Pages 4751—4762
DOI https://doi.org/10.2147/JIR.S428582
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Lili Cheng,1 Chuanbing Huang,2 Ming Li,2 Shuangshuang Shang,2 Junjie Chen,1 Zhongfu Tang1
1The First Clinical Medical College, Anhui University of Traditional Chinese Medicine, Hefei, Anhui Province, 230038, People’s Republic of China; 2Department of Rheumatology and Immunology, First Affiliated Hospital of Anhui University of Traditional Chinese Medicine, Hefei, Anhui Province, 230038, People’s Republic of China
Correspondence: Chuanbing Huang, Department of Rheumatology and Immunology, First Affiliated Hospital of Anhui University of Traditional Chinese Medicine, Hefei, Anhui Province, 230038, People’s Republic of China, Tel +86-15156986428, Email [email protected]
Purpose: This study aims to explore the effect and underlying mechanism of Chonggu Granules (CGG) in knee osteoarthritis (KOA) in rats.
Methods: A papain-induced KOA model was established in rats. The pathological alterations of extracellular matrix in rat cartilage tissues were observed through hematoxylin and eosin (H&E) staining, followed by Mankin score for quantitative scoring. The ultrastructure of cartilage extracellular matrix was examined under a transmission electron microscopy (TEM). ELISA was used to measure the levels of IL-6, TNF-α, and IL-1β in rat serum. Immunofluorescence was performed for assessing the levels of MMP-3, MMP-13, and Col2al in rat cartilage. Western blot was used to identify the protein expressions of wnt1, GSK-3β, β-catenin, and Aggrecan in rat cartilage. The mRNA relative expressions of miR-148a-3p, wnt1, β-catenin, and GSK-3β in rat cartilage were detected by RT-PCR. Luciferase reporter gene was used to detect the target genes of miR-148a-3p.
Results: CGG significantly improved articular cartilage tissue and extracellular matrix metabolism compared to the model group as indicated by H&E, Mankin score, and TEM data. Moreover, low, medium, and high doses of CGG reduced the levels of IL-6, TNF-α, IL-1β, MMP-3, and MMP-13 in serum to varying degrees but increased the levels of Col2al and Aggrecan. Mechanistically, CGG targeted wnt1 by increasing the expression of miR-148a-3p in a dose-dependent manner, thereby downregulating the mRNA and protein expressions of β-catenin in cartilage tissue and upregulating the mRNA and protein expressions of GSK-3β.
Conclusion: CGG may control the miR-148a-3p/wnt/β-catenin signaling pathway to decrease the levels of its downstream target genes MMP-13 and MMP-3, increase the expressions of Col2al and Aggrecan, and downregulate the contents of inflammatory cytokines IL-6, TNF-α, and IL-1β, thereby improving the metabolism of cartilage extracellular matrix and alleviating the degeneration of articular cartilage in KOA.
Keywords: knee osteoarthritis, cartilage extracellular matrix metabolism, chonggu granules, miR-148a-3p, Wnt/β-catenin signaling pathway
Introduction
Knee osteoarthritis (KOA) is the most common degenerative joint disease that causes significant physical disability among the middle-aged and elderly population, which brings certain economic burden to both family members and society.1 The major pathological hallmarks of KOA include subchondral sclerosis, synovial inflammation, and osteophyte hyperplasia, etc., resulting in corresponding clinical manifestations such as swelling, pain, rigidity, and limited joint activity in patients.2 KOA has a multifactorial etiology involving multiple risk factors such as genetic factors, aging, obesity, synovitis, inflammatory responses, autoimmunity, joint shape and dysplasia, and trauma, among which aging is the most prominent risk factor.3 Statistics show that the number of KOA patients in China has soared from 26.1 million 30 years ago to 61.2 million at present, meaning that 3–4 out of every 100 Chinese people suffer from OA, which has become one of the public health problems of great concern.4 However, the specific pathogenesis of KOA has not been fully elucidated. Currently, the treatment methods for KOA in clinical practice can only alleviate patient pain, which can be divided into non-surgical treatments and surgical treatments. Non-surgical treatments include education of patients to improve their lifestyle, physical therapy, non-steroidal anti-inflammatory drugs, corticosteroids, and other drug treatments, while surgical treatment is primarily joint replacement. Unfortunately, these treatment methods have their own limitations in clinical practice and their efficacy is not satisfactory.5 Therefore, there is an urgent need to find new treatment methods or drugs to improve the outcomes of KOA patients.
Chonggu Granule (CGG) is formulated by Huang Chuanbing, chief physician of our hospital, CGG is composed of Eucommia and Davalliaceae as monarch drug, Achyranthes and parasitic loranthus as ministerial drug, Homalomena occulta, Herba Pyrolae and cuttle-bone as assistant drug. Together, CGG has the effect of tonifying liver and kidney, strengthening tendons and bones and relieving pain. According to the clinical experience of syndrome differentiation and treatment, it can inhibit inflammation, improve extracellular matrix (ECM) metabolism of cartilage, and improve bone metabolism. For example, preliminary clinical research6 has found that CGG can significantly improve the clinical symptoms of KOA patients, reduce the levels of C-reactive protein (CRP), interleukin (IL)-1β, and tumor necrosis factor-α (TNF-α), reduce the scores for traditional Chinese medicine (TCM) syndromes, reduce the disease activity, and improve the quality of life of patients. Chen7 has suggested that CGG may reduce the degradation and destruction of ECM by promoting the expressions of proteoglycan and bone morphogenetic protein (BMP), facilitating the synthesis of ECM, and inhibiting the expressions of matrix metalloproteinase-13 (MMP-13) and serum cartilage oligomeric matrix protein (COMP). Zhu et al8 have found that the possible mechanism of CGG in increasing carboxy-terminal propeptide of type II procollagen (PIICP) and osteocalcin (BGP) and decreasing amino-terminal propeptide of type I procollagen (β-CTX) may be to reduce the level of inflammatory factors and improve bone metabolism. However, the exact mechanism of CGG in ECM metabolism remains unclear.
MicroRNAs (miRNAs) are small non-coding RNAs known to regulate 60% of human genes posttranscriptionally9 and have been demonstrated to participate in a wide range of biological processes since their discovery in 1993. More significantly, aberrant miRNA expression has been associated with various human diseases, including KOA.10 A vast number of clinical researches in recent years have revealed11 that miRNAs control the production of collagen and MMPs and have critical roles in the regulation mechanism of ECM metabolism in KOA cartilage. MiR-148a-3p, formerly known as miR-148a, has previously been found to function as an oncogene or tumor suppressor in carcinogenesis. Moreover, emerging studies have revealed the involvement of miR-148a in the occurrence and development of KOA, and miR-148a-3p dysregulation has a direct impact on cellular inflammation, ECM stability, as well as cell proliferation and apoptosis.12
Wnt/β-catenin is a well-known component of the Wnt signaling system, which can control proliferation, differentiation, regeneration, senescence, and apoptosis in embryonic development and disease pathogenesis.13 The canonical Wnt/β-catenin pathway has a negative regulatory effect on cartilage matrix metabolism in KOA.14 Wnt/β-catenin pathway activation triggers the production of MMPs and other proteases, leading to the decomposition of proteoglycan matrix and upsetting the equilibrium of ECM degradation.15
The regulatory relationship between miR-148a and Wnt/β-catenin signaling pathway has already been confirmed. MiRNAs function as novel molecular regulators responsible for the expressions of target genes in the Wnt/β-catenin signaling.16 Meanwhile, miR-148a overexpression can directly target Wnt1 and promote its translocation to the cytoplasm and nuclear membrane. Downregulation of miR-148a activates the Wnt/β-catenin signaling pathway. After activation of Wnt1, β-catenin accumulates in the cytoplasm and membrane and transfers to the nucleus, which then regulates the transcription of its downstream molecules. Data from luciferase gene assays by Zhang et al17 showed that Wnt1 was a direct target of miR-148 and Wnt1 overexpression was inversely proportional to miR-148 expression. However, the interaction and regulatory mechanisms of miR-148a-3p and Wnt/β-catenin in ECM metabolism of KOA cartilage have not been widely studied.
Herein, we used CGG to intervene in an animal model of KOA to detect its effects on miR-148a-3p /Wnt/β-catenin signaling and ECM metabolism, as well as to further investigate the effects of CGG on KOA rats at the tissue and molecular levels, which shall confer a theoretical foundation and support for clinical application of CGG in the treatment of KOA.
Materials and Methods
Animals and Groups
Thirty-six male Sprague-Dawley (SD) rats (weighing 2.5–3.0 kg, aged 8–9 weeks old) were purchased from Beijing Vital River Experimental Animal Technology Co. [Eligibility Number: SCXK (Jing) 2021–0011]. All animals were provided with adequate ambient temperature and humidity, as well as standard drinking water and feed. After 1 week of adaptive feeding, the rats were randomized into 6 groups: blank group (n = 6), model control group (n = 6), CGG low-dose group (n = 6), CGG medium-dose group (n = 6), CGG high-dose group (n = 6), and positive control group (n = 6). The control rats were injected with 0.15 mL (0.9%) of saline in the right knee cavity, and the rats in the remaining groups were established as OA model rats. After successfully modeling and adaptive feeding for 1 week, the blank group and model control group received intragastric administration of normal saline (0.9%). The positive control group was given 0.135g/kg−1 * d−1 of glucosamine hydrochloride (approval number: H20060647, Jiangsu Zhengda Qingjiang Pharmaceutical Co., LTD., China) intragastrically.
CGG was provided by Pharmacy of Traditional Chinese Medicine of the First Affiliated Hospital of Anhui University of Chinese Medicine, and it is composed of 9g Davalliaceae, and 10g Eucommia, 10g Achyranthes, 10g parasitic loranthus, 10g Homalomena occulta, 10g Herba Pyrolae and 10g cuttle-bone with a dosage form of granule. The CGG low-dose group, medium-dose group, and high-dose group were intragastrically given 6.2g/kg−1 · d−1, 12.4g/kg−1 · d−1, and 24.8g/kg−1 · d−1 of CGG every day, respectively. Each group was dosed continuously for 4 weeks. This study was approved by the Ethics Committee of Anhui University of Traditional Chinese Medicine (approval number: 2022123). All experiments were conducted in accordance with the National Institutes of Health’s Guidelines for the Care and Use of Laboratory Animals.
Establishment of a Rat Model of KOA
KOA rat model was established as previously reported.18,19 Briefly, all 30 animals were anesthetized by intraperitoneal injection of 3% sodium pentobarbital (Beijing propbs Biotechnology Co., LTD., China) at a dose of 3mg/100g after 12 h of fasting. After satisfactory anesthesia, the rat skin was routinely prepared and sterilized at 1cm around the knee joint. The knee joint of the rat was bent at 45° and perforated along the intercondylar fossa. With the outer edge of the patella tendon under the patella as the tip of the needle, 0.2mL of papain solution (Purity 800u/mg, CAS#9001-73-4, No. S10011, Shanghai yuanye Bio-Technology Co., Ltd, China) was slowly injected into the joint cavity. We strictly followed the above procedures three times a week (on the 1st, 4th, and 7th days). After three injections, it was observed that all 30 rats had swelling of bilateral knee joints, delayed movement, and obvious claudication.
Measurement of IL-6, IL-1β, and TNF-α Levels by Enzyme-Linked Immunosorbent Assay (ELISA)
First, the blood was collected from the abdominal aorta and left at room temperature for 20 min. After natural coagulation, the blood was centrifuged at 2500 r/min for 20 min to discard the precipitate and collect the serum supernatant. The serum was added to ELISA plates (50 µL per well) and incubated at 37°C for 30 min. After sealing the plates with membrane, the concentrations of IL-6 (YM0646Ra, Wuhan Genemai Technology Co., Ltd. China), IL-1β (JYM0419Ra, Wuhan Genemai), and TNF-α (JYM0635Ra, Wuhan Genemai) in the peripheral blood of rats were detected in strict accordance with the instructions of ELISA kits.
Hematoxylin and Eosin (H&E) Staining and Mankin Score
After drug administration, the tibia platform and femoral condyle cartilage were taken for pathological observation. The cartilage tissues of rats in each group were fixed in 4% paraformaldehyde (BL539A, Biosharp, China) for 24 h. Subsequently, they were decalcified, embedded in paraffin, and cut into 5μm sections. After routine deparaffinization and dehydration, the sections were immersed in hematoxylin for 5 min followed by eosin for 5 min. Finally, the sections were sealed with neutral gum and observed under a microscope. Following H&E staining, the sections were evaluated using the Mankin scoring system from three aspects: cartilage structure, chondrocytes, and tideline damage severity. The total score ranged from 0 to 14, with the higher score indicating greater severity.
Detection of the Expressions of MMP-3, MMP-13, and Col2al by Immunofluorescence (IF)
The cartilage tissues of rats in each group were detected by IF. The cartilage tissue sections were washed with xylene and ethanol and then repaired with antigens at high pressure. Afterward, the cartilage tissue sections were rinsed with phosphate buffer saline (PBS) containing 0.05% (v/v) Tween-20 (PBST) and cultured in an incubator at 37°C with 0.5% TritonX-100 (B025, Ebiogo, China) and goat serum blocking solution. The goat serum was discarded and the primary antibodies MMP3 (1:200, bs-0413R, Bioss, China), MMP13 (1:200, bs-0575R, Bioss) and Col2aI (1:200, bs-0578R, Bioss) were introduced 60 min before incubation respectively. The sections were then removed, rinsed three times with PBS-T, and infused with the IF secondary antibody (goat anti-rabbit IgG (FITC)/1:400) for 30 min in a 37°C incubator in the dark. Following three times of PBS-T rinsing, the sections were finally sealed with the anti-fluorescence quenching sealing agent.
Detection of the Protein Expressions of wnt1, Glucose Synthase Kinase-3β (GSK-3β), β-Catenin, and Aggrecan by Western Blot (WB)
The cartilage tissue samples were isolated with radio-immunoprecipitation assay (RIPA) cell lysate and the supernatant was removed, followed by the addition of buffer, electrophoresis, and membrane transfer. Then the protein membrane was rinsed with Western washing solution for 5 min and added with the Western blocking solution. Dilution incubation and washing were performed according to the instructions for primary antibody incubation including Wnt1 (1:1000, ab1525, Abcam, USA), GSK3β (1:2000, bs-0023R, Bioss), and β-catenin (1:1000, 8480S, CST, USA), Aggrecan (1:2000, ab3778, Abcam, USA) and secondary antibody incubation. Finally, the enhanced chemiluminescence (ECL, 340958, Thermo, USA) kit was used for protein detection according to the instructions.
Detection of the mRNA Transcription Levels of miR-148a-3p, wnt1, GSK-3β, and β-Catenin by RT-PCR
The cartilage tissues of rats in each group were ground into powder by adding liquid nitrogen. The total RNA was extracted using TRIzol reagent (Life Technologies, USA) and subjected to reverse transcriptase lysis and amplification reactions. PCR reactions were performed after cDNA synthesis using PrimeScript™RT reagent Kit with gDNA Eraser (RR047A, TaKaRa, Japan) and Novostart SYBR qPCR SuperMix Plus (E096-01B, Novoprotein, USA) according to the kit instructions. The PCR products were semi-quantitatively analyzed by gel electrophoresis using the Gelpro32 gel image analysis software. Relative quantification was performed using the 2−ΔΔCt method with β-actin as the internal reference. Each experiment was repeated three times (Table 1).
 |
Table 1 Gene Detection Index Primer |
Detection of the Target Gene of miR-148a-3p by Luciferase Reporter Gene
The Rno-Wnt1 target plasmid was constructed and thoroughly mixed with Rno-miR-148a-3p /Negative. Control (N.C). Then, the Dulbecco’s modified Eagle’s medium (DMEM) was mixed with the transfection reagent. The two were mixed to form a transfection mixture, added to fresh medium, and cultured at 37°C in 5% CO2. Subsequently, the cells were collected for analysis. The 96-well plates were added with 100uL of Luciferase Assay Reagent II (LAR II) working solution (Luciferase Assay Reagent, Progema) and 20uL of cell lysate, and mixed 2–3 times with a pipette gun. The Firefly luciferase value was measured and recorded as the internal reference value. After adding 100uL of Stop&Glo® Reagent (Luciferase Assay Reagent, Progema), the mixture was mixed with a pipet gun 2–3 times, and the Renilla luciferase value was measured and recorded as the luminescence value of reporter gene.
Statistical Analysis
SPSS statistical software 26.0 (IBM Corp.) was applied for statistical analysis and GraphPad Prism software 8.2 (GraphPad Software) was used to capture images. The measurement data conforming to normal distribution were expressed as mean ± standard deviation, and the decimal place was reserved. One-way analysis of variance (ANOVA) was used to evaluate the differences between groups, with P < 0.05 indicating a statistically significant difference.
Results
Histopathological Changes in Articular Cartilage and Mankin Sore
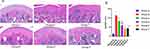
As shown in Figure 1A, in group A, the articular cartilage had smooth surface and complete morphology, and chondrocytes were arranged neatly and evenly in the matrix with clear layers. Moreover, the chondrocytes were evenly stained outside the matrix. In group B, there were obvious defects on the articular cartilage surface, with a large amount of deformation and disordered arrangement of chondrocytes, a significant reduction in the number of chondrocytes, and irregular distribution. A large amount of degraded cartilage matrix could be seen, and uneven staining was observed in the cartilage matrix. Compared with the model group, the group C showed a slightly less smooth surface of the articular cartilage, with an increase in the number of chondrocytes but a slightly irregular arrangement. Compared with the model group, the group D presented smooth articular cartilage surface with complete morphology, regular arrangement and uniform distribution of chondrocytes in the matrix, and a large number of visible normal chondrocytes, moreover, the cartilage matrix was fully stained and the tide line was slightly incomplete. In group E, there was a significant increase in chondrocytes in the articular cartilage, with most chondrocytes showing a normal appearance and slightly uneven staining of the ECM. Compared with the model group, the cartilage surface of group F returned to normal, with a significant increase in the number of chondrocytes and uneven staining of the ECM of chondrocytes.
To observe the changes in cartilage tissues in each group more accurately, the Mankin score was used for quantitative evaluation. As shown in Figure 1B, the Mankin score in the model group was significantly higher than that in the blank group, indicating the successful establishment of the early KOA model. After CGG treatment, the Mankin scores in all drug groups were decreased in comparison with the model group. In addition, the CGG medium-dose group had the lowest Mankin score and the CGG high-dose group had similar Mankin score to the glucosamine group, suggesting that CGG significantly improved KOA.
Effects of CGG on the Expressions of Pro-Inflammatory Factors in Knee Cartilage Tissues of KOA Rats
As shown in Figure 2, the levels of IL-6 (Figure 2A), TNF-α (Figure 2B), andIL-1β (Figure 2C) in the model control group were significantly higher than those in the normal group (P < 0.01), and the levels of IL-6, IL-1β, and TNF-α in the model group were higher than those in other treatment groups to varying degrees (P < 0.01). The CGG medium-dose group had the lowest levels of IL-6 and IL-1β, followed by glucosamine hydrochloride group (P < 0.05). However, there was no significant difference in TNF-α levels between the two groups (P < 0.05).
Effects of CGG on the Expressions of ECM-Related Markers in Knee Cartilage Tissues of KOA Rats
As shown in Figure 3, the protein levels of MMP-3 and MMP-13 in the model control group were significantly higher than those in the blank group, while the protein level of Col2al in the model control group was significantly lower than that in the blank group. Compared with the model group, the protein levels of MMP-3 and MMP-13 in the other four treatment groups were significantly decreased, while the protein level of Col2al was increased. The comparison between two groups revealed that the CGG medium-dose group showed the most significant decrease in MMP-3 and MMP-13 protein levels, and the increase in Col2al was similar to that of the glucosamine hydrochloride group. The improvement of articular cartilage damage by CGG in KOA rats may be related to the regulation of cartilage matrix metabolism.
Effects of CGG on the Expressions of Wnt/β-Catenin Signaling Pathway-Related Proteins in Knee Cartilage Tissues of KOA Rats
As shown in Figure 4E, there are results of Western blot of Aggrecan, GSK3β, wnt1 and β-catein in knee cartilage of rats in each group. And in Figure 4, the relative protein expressions of wnt1 (Figure 4A) and β-catenin (Figure 4B) were the highest and the protein expressions of GSK-3β (Figure 4C) and Aggrecan (Figure 4D) were the lowest in cartilage tissues of the model control group, with significant differences compared to the normal group (P < 0.01). Compared with the model group, the protein expressions of wnt1 and β-catenin in each treatment group were decreased, while the protein expressions of GSK-3β and Aggrecan were increased. The protein expressions of wnt1 and β-catenin were the lowest (P < 0.05) while the protein expressions of GSK-3β and Aggrecan were the highest (P < 0.05) in the CGG medium-dose group.
Effects of CGG on the Expressions of miR-148a-3p/Wnt/β-Catenin Signaling Pathway-Related mRNAs in Knee Cartilage Tissues of KOA Rats
As shown in Figure 5, compared with the blank group, the mRNA levels of wnt1 (Figure 5B) and β-catenin (Figure 5D) in the model control group were significantly increased, and the mRNA levels of miR-148a-3p (Figure 5A) and GSK-3β (Figure 5C) were significantly decreased (P < 0.01). Compared with the model group, the CGG low-, medium-, and high-dose groups and the glucosamine hydrochloride group exhibited significantly decreased mRNA levels of wnt1 and β-catenin and increased mRNA levels of miR-148a-3p and GSK-3β (P < 0.01). Compared with the glucosamine hydrochloride group, the relative mRNA levels of wnt1 and β-catenin were notably reduced in the CGG medium-dose group, while the relative mRNA levels of miR-148a-3p and GSK-3β were elevated (P < 0.01).
There Was a Targeted Regulatory Relationship Between miR-148a-3p and wnt1
To verify whether there is a targeting relationship between miR-148a-3p and wnt1, we performed dual-luciferase reporter assay. As shown in Figure 6A, the binding sequence between miR-148a-3p and wnt1 was predicted by bioinformatics. The luciferase reporter plasmids containing the wild-type (WT1-WT) and mutant (WT1-MUT) of miR-148a-3p were constructed, respectively, and the plasmids contained the predicted binding sites of miR-148a-3p. As shown in Figure 6B, compared with the NC mimic group, Rno-miR-148a-3p significantly down-regulated the expression of luciferase in Rno-Wnt1-wt, indicating that there was a binding effect between miR-148a-3p and wnt1. After mutation, Rno-miR-148a-3p failed to down-regulate the luciferase expression of Rno-Wnt1-mut compared with the NC group, indicating that the mutation was successful.
Discussion
KOA is a chronic progressive disease with a high disability rate and multifactorial etiology, one of the main pathological causes for KOA is the disorder of ECM synthesis and metabolism.20 CGG was formulated by Huang Chuanbing, chief physician of the Rheumatology and Immunology Department of First Affiliated Hospital of Anhui University of Traditional Chinese Medicine, according to many years of rich clinical experience and theoretical basis. CGG has a high clinical application rate and receives favorable responses among patients.
To further explore the possible mechanism of CGG in KOA, we established a rat model of KOA by papainase injection and observed the changes in the cartilage tissues of the knee joint. The main reason for adopting papanin injection lies in the decomposition function of papanin on proteoglycans in cartilage matrix, which leads to the degradation of ECM to insult cartilage damage. Further, cartilage damage stimulates synovial inflammation, and in turn, the secreted inflammatory mediators can inhibit the synthesis of collagen fibers and proteoglycans in ECM, thus forming a vicious circle, which has similar properties to human OA.21
A key link in the occurrence and development of OA is the abnormal metabolism of cartilage ECM, which is mainly caused by the imbalance of synthesis and degradation of ECM components such as collagen type II (Col2) and agglutinin (ACAN). Cartilage ECM as an important part of articular cartilage is a complex network structure composed of water, collagen, and proteoglycan (Col2 and ACAN). The homeostasis of cartilage ECM is crucial for maintaining the normal function of chondrocytes and responding promptly to changes in the external environment.22 Therefore, the imbalance of ECM metabolism can impair the supporting effect of cartilage ECM on chondrocytes and cartilage structure, disturbing intercellular signal transduction, and affecting cell behaviors such as proliferation, differentiation, and migration.
H&E staining results showed that except for the blank group, all five groups of rats had varying degrees of degeneration and damage to the knee joint cartilage, suggesting that the experimental model was successfully established. In the model group, the articular cartilage surface was damaged significantly, with obvious cracks, disordered arrangement of chondrocytes, massive necrotic chondrocytes, cartilage matrix dissolution, and unclear boundary of each layer. After CGG administration, the articular cartilage surface damage of rats was milder than that of the model control group. Although the surface of the articular cartilage was not yet smooth, there was a certain degree of repair performance, with regular arrangement of chondrocytes and varying degrees of local recovery in the cartilage matrix. Therefore, it is preliminarily determined that CGG may contribute to improving cartilage ECM degradation and alleviating cartilage injury.
The most significant biochemical changes in KOA cartilage are the loss of proteoglycan and Col2. The expression of MMP-13 and MMP-3 proteins is positively correlated with the severity of cartilage degeneration. Studies have also shown that IL-1β, TNF-α, and IL-6 control the degradation of the articular cartilage matrix, making them the main targets for treatment.23 In this study, we found that the expressions of COL2a1 and Aggrecan in cartilage tissues of KOA rats were significantly lower than those in the blank group, while the expressions of MMP-3 and MMP-13, as well as the levels of IL-6, IL-1βm and TNF-α were significantly elevated compared to the blank group. The intervention of CGG resulted in varying degrees of increased levels of COL2a1 and Aggrescan proteins in each group of rats compared to the model group, as well as decreased levels of MMP-3, MMP-13, and pro-inflammatory factors. Consistently, Cong et al24 found that T-614 slowed KOA progression by reducing serum levels of IL-6 and TNF-α and inhibiting the secretion of serum MMP-13, thereby controlling the degradation of articular cartilage matrix. Briefly, CGG promote the synthesis of Col2 and Aggrescan, reduce the production MMPs, suppress the release of pro-inflammatory factors, thereby balancing the ECM and alleviating cartilage damage.
The Wnt protein family is a group of morphogens associated with embryonic bone formation, tissue repair, fibrosis, and joint homeostasis.25 Accumulating studies have pointed out that the wnt/β-catenin pathway regulates the balance of ECM in OA. The canonical Wnt pathway triggers signal transmission into cells by regulating β-catenin levels and β-catenin subcellular localization. Wnt/β-catenin is a key signaling pathway in the pathogenesis of OA, and overexpression of β-catenin stimulates the production of relevant MMPs.26 Tamamura Y et al15 confirmed that Wnt/β-catenin participated in the pathogenesis of OA and promoted the expressions of different proteins related to cartilage matrix degradation, including MMP-13, ADAMTS-4, ADAMTS-5, and core-binding factor α1. Bai et al27 found that MMP expression levels were strongly associated with the Wnt/β-catenin signaling pathway. Inhibition of β-catenin reduced the expressions MMPs and ADAMTS-5, thereby reducing articular cartilage damage in animal OA models. All of these findings suggest that ECM degradation can be mainly attributed to the Wnt/β-catenin pathway, which leads to cartilage destruction and OA progression. Targeting this pathway may be a promising strategy to treat or prevent OA.
In the model group, there was a significant increase in gene and protein levels of wnt1 and catenin in cartilage while GSK-3β was significantly reduced, suggesting that the wnt1/β-catenin signaling pathway was activated, which led to cartilage matrix degradation and ultimately articular cartilage degradation. Notably, the protein and gene expression profiles were reversed after addition of CGG. Taken together, we demonstrated that CGG could modulate the wnt1/β-catenin signaling pathway, promote phosphorylation of GSK-3β, and reduce nuclear β-catenin levels, thereby inhibiting the production of MMPs and ultimately affecting the pathology of articular cartilage matrix.
MiR-148a-3p, formerly known as miR-148a, has been identified as an oncogene or tumor suppressor in tumorigenesis. In recent years, numerous studies have shown that miR-148a regulates the initiation and progression of OA disease, and miR-148a expression directly affects cell inflammation, ECM stability, and cell proliferation and apoptosis.12 Vonk LA et al28 found that miR-148a expression in OA cartilage was 10 times lower than that in normal cartilage, and real-time fluorescence quantitative PCR also confirmed that miR-148a expression in OA cartilage was 9 times lower than that in normal cartilage. Overexpression of miR-148a had no effect on COL1a1 expression, but increased COL2a1 expression and decreased COL10a1, MMP13, and ADAMTS5 expressions, suggesting that overexpression of miR-148a may improve ECM metabolism and repair damaged cartilage. This is consistent with our findings that miR-148a-3p expression was significantly reduced in the model group and could be reversed through CGG intervention.
Other emerging evidence highlights16 miRNAs as novel molecular regulators for the expressions of target genes in Wnt/β-catenin signaling. Furthermore, miR-148a overexpression directly targets Wnt1 and promotes its localization to cytoplasmic and nuclear membranes. Downregulation of miR-148a activates the Wnt/β-catenin signaling pathway. The activation of wnt1 leads to the translocation of β-catenin accumulated in the cytoplasmic membrane to the nucleus to regulate the transcription of downstream molecules. Luciferase reporter gene detection also confirmed the target relationship between miR-148a-3p and wnt1. Similarly, Zhang et al17 found that Wnt1 overexpression was inversely proportional to miR-148 expression. Wnt1 inhibited cell growth but induced apoptosis and inhibited invasion, which was consistent with the effects of miR-148b overexpression. Overexpression of miR-148 significantly inhibited Wnt1 expression, thereby inhibiting the Wnt/β-catenin signaling. These data suggest the regulation of miR-148a on β-catenin. Peng et al29 demonstrated that miR-148a negatively regulated the protein expressions of β-catenin and MMP-9. Similarly, Jiang et al30 confirmed that overexpression of miR-148a reduced the mRNA and protein levels of Wnt1, and also inhibited the key components of the Wnt/β-catenin pathway.
Conclusion
By increasing the expression of miR-148a-3p in cartilage tissues and targeting the wnt1/β-catenin pathway, CGG can inhibit the secretion of MMPs, promote the synthesis of Col2 and Aggrecan, improve the metabolism of cartilage ECM, reduce cartilage inflammation, and improve the prognosis of rats with KOA.
Ethical Statement
This study was approved by the Ethics Committee of Anhui University of Traditional Chinese Medicine (approval number: 2022123). All experiments were conducted in accordance with the National Institutes of Health’s Guidelines for the Care and Use of Laboratory Animals.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was awarded the Key project of Natural Science Research Foundation for Universities in Anhui Province (KJ2020A0395), Collaborative Innovation Program of Universities in Anhui Province (No. GXXT-2021-085), Traditional Chinese Medicine Inheritance and Innovation Development Research Project of Anhui Red Cross Foundation (No. 2021ZYZD3), University-level Exploratory Research Project of Anhui University of Traditional Chinese Medicine in 2021 (2021zxts49), and Key Project of Health Research of Anhui Province in 2022 (AHWJ2022a005).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kan HS, Chan PK, Chiu KY, et al. Non-surgical treatment of knee osteoarthritis. Hong Kong Med J. 2019;25(2):127–133. doi:10.12809/hkmj187600
2. Michael JW, Schluter-Brust KU, Eysel P. The epidemiology, etiology, diagnosis, and treatment of osteoarthritis of the knee. Dtsch Arztebl Int. 2010;107(9):152–162. doi:10.3238/arztebl.2010.0152
3. Lespasio MJ, Piuzzi NS, Husni ME, Muschler GF, Guarino A, Mont MA. Knee Osteoarthritis: a Primer. Perm J. 2017;21:16–183. doi:10.7812/TPP/16-183
4. Li MC, Leng XM. Small symptoms, big burden: an overview of the epidemiology of osteoarthritis. Chin J Clinical Immunol Allergy. 2012;16(3):331.
5. Hussain SM, Neilly DW, Baliga S, Patil S, Meek R. Knee osteoarthritis: a review of management options. Scott Med J. 2016;61(1):7–16. doi:10.1177/0036933015619588
6. Cheng HC, Tang ZF. Clinical observation on the treatment of 30 cases of knee osteoarthritis with liver and kidney deficiency with Chonggu granules. Rheumatism and Arthritis. 2021;10(12):7–10+15.
7. Xueshan C. Effect of Chonggu granule on knee osteoarthritis with liver-kidney deficiency and its influence on cartilage matrix metabolism. Anhui Univ Traditional Chine Med. 2022.
8. Shu PL, Li YF, Fu WL, Huang CB. Clinical efficacy of Chonggu granule in the treatment of knee osteoarthritis with liver and kidney deficiency and its effect on bone metabolism indexes. Clin Efficacy Chonggu. 2023;42(01):31–35.
9. Mendell JT. MicroRNAs: critical regulators of development, cellular physiology and malignancy. Cell Cycle. 2005;4(9):1179–1184. doi:10.4161/cc.4.9.2032
10. Soyocak A, Kurt H, Ozgen M, Turgut Cosan D, Colak E, Gunes HV. miRNA-146a, miRNA-155 and JNK expression levels in peripheral blood mononuclear cells according to grade of knee osteoarthritis. Gene. 2017;627:207–211. doi:10.1016/j.gene.2017.06.027
11. Li SH, Wu QF. MicroRNAs target on cartilage extracellular matrix degradation of knee osteoarthritis. Eur Rev Med Pharmacol Sci. 2021;25(3):1185–1197. doi:10.26355/eurrev_202102_24821
12. Jones SW, Watkins G, Le Good N, et al. The identification of differentially expressed microRNA in osteoarthritic tissue that modulate the production of TNF-alpha and MMP13. Osteoarthritis Cartilage. 2009;17(4):464–472. doi:10.1016/j.joca.2008.09.012
13. Shang X, Boker KO, Taheri S, Hawellek T, Lehmann W, Schilling AF. The Interaction between microRNAs and the Wnt/beta-Catenin Signaling Pathway in Osteoarthritis. Int J Mol Sci. 2021;22(18). doi:10.3390/ijms22189887
14. Yu H, Liu Y, Yang X, et al. Strontium ranelate promotes chondrogenesis through inhibition of the Wnt/beta-catenin pathway. Stem Cell Res Ther. 2021;12(1):296. doi:10.1186/s13287-021-02372-z
15. Tamamura Y, Otani T, Kanatani N, et al. Developmental regulation of Wnt/beta-catenin signals is required for growth plate assembly, cartilage integrity, and endochondral ossification. J Biol Chem. 2005;280(19):19185–19195. doi:10.1074/jbc.M414275200
16. Dong Z, Jiang H, Jian X, Zhang W. Change of miRNA expression profiles in patients with knee osteoarthritis before and after celecoxib treatment. J Clin Lab Anal. 2019;33(1):e22648. doi:10.1002/jcla.22648
17. Zhang L, Cheng H, Yue Y, Li S, Zhang D, He R. H19 knockdown suppresses proliferation and induces apoptosis by regulating miR-148b/WNT/beta-catenin in ox-LDL -stimulated vascular smooth muscle cells. J Biomed Sci. 2018;25(1):11. doi:10.1186/s12929-018-0418-4
18. Yao N, Chen GC, Lu YY, et al. Bushen Qiangjin capsule inhibits the Wnt/α-catenin pathway to ameliorate papain-induced knee osteoarthritis in rat. J Traditional Chine Med. 2021;41(6):935–942. doi:10.19852/j.cnki.jtcm.2021.06.010
19. Liu J, Liu S, Pan W, Li Y. Wogonoside attenuates the articular cartilage injury and the infiltration of Th1/Th2-type cytokines in papain-induced osteoarthritis in rat model via inhibiting the NF-κB and ERK1/2 activation. Immunopharmacol Immunotoxicol. 2021;43(3):343–352. doi:10.1080/08923973.2021.1913503
20. Xie ZH. Research progress of traditional Chinese medicine intervention for osteoarthritis based on Wnt/β-catenin signaling pathway. Chine J Osteoporosis. 2018;24(05):664–670.
21. Murat N, Karadam B, Ozkal S, Karatosun V, Gidener S. [Quantification of papain-induced rat osteoarthritis in relation to time with the Mankin score]. Acta Orthop Traumatol Turc. 2007;41(3):233–237.
22. Hodgkinson T, Kelly DC, Curtin CM, O’Brien FJ. Mechanosignalling in cartilage: an emerging target for the treatment of osteoarthritis. Nat Rev Rheumatol. 2022;18(2):67–84. doi:10.1038/s41584-021-00724-w
23. Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7(1):33–42. doi:10.1038/nrrheum.2010.196
24. Cong S, Meng Y, Wang L, Sun J. T-614 attenuates knee osteoarthritis via regulating Wnt/beta-catenin signaling pathway. J Orthop Surg Res. 2021;16(1):403. doi:10.1186/s13018-021-02530-2
25. Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149(6):1192–1205. doi:10.1016/j.cell.2012.05.012
26. van den Bosch MH, Blom AB, van de Loo FA, et al. Brief Report: induction of Matrix Metalloproteinase Expression by Synovial Wnt Signaling and Association With Disease Progression in Early Symptomatic Osteoarthritis. Arthritis Rheumatol. 2017;69(10):1978–1983. doi:10.1002/art.40206
27. Bai M, Ge L, Chen H, Jin Q. Calcitonin protects rat chondrocytes from IL-1beta injury via the Wnt/beta-catenin pathway. Exp Ther Med. 2019;18(3):2079–2085. doi:10.3892/etm.2019.7806
28. Vonk LA, Kragten AH, Dhert WJ, Saris DB, Creemers LB. Overexpression of hsa-miR-148a promotes cartilage production and inhibits cartilage degradation by osteoarthritic chondrocytes. Osteoarthritis Cartilage. 2014;22(1):145–153. doi:10.1016/j.joca.2013.11.006
29. Peng L, Liu Z, Xiao J, et al. MicroRNA-148a suppresses epithelial-mesenchymal transition and invasion of pancreatic cancer cells by targeting Wnt10b and inhibiting the Wnt/beta-catenin signaling pathway. Oncol Rep. 2017;38(1):301–308. doi:10.3892/or.2017.5705
30. Jiang Q, He M, Ma MT, et al. MicroRNA-148a inhibits breast cancer migration and invasion by directly targeting WNT-1. Oncol Rep. 2016;35(3):1425–1432. doi:10.3892/or.2015.4502
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.